Abstract
Plant species that have a vernalization requirement exhibit variation in the ability to “remember” winter – i.e., variation in the stability of the vernalized state. Studies in Arabidopsis have demonstrated that molecular memory involves changes in the chromatin state and expression of the flowering repressor FLOWERING LOCUS C, and have revealed that single-gene differences can have large effects on the stability of the vernalized state. In the perennial Arabidopsis relative Arabis alpina, the lack of memory of winter is critical for its perennial life history. Our studies of flowering behavior in the model grass Brachypodium distachyon reveal extensive variation in the vernalization requirement, and studies of a particular Brachypodium accession that has a qualitative requirement for both cold exposure and inductive day length to flower reveal that Brachypodium can exhibit a highly stable vernalized state.
Keywords: vernalization, flowering, Brachypodium, epigenetics, life history
INTRODUCTION
Specific timing of flowering is an important adaptive trait that ensures flowering occurs when conditions are favorable. In many plant species, flowering takes place during a particular time of year in response to the sensing of seasonal cues, such as changing day lengths and/or temperature. One adaptation to the seasonal changes that occur in temperate climates has been the evolution of a vernalization response (Amasino, 2010; Andrés and Coupland, 2012). Vernalization is the process by which exposure to the prolonged cold of winter results in the ability to flower in the next growing season (Chouard, 1960). Cold exposure alone is typically not sufficient to induce flowering, but it often must be coupled to an additional inductive cue such as the increasing day lengths experienced during spring and summer months (Lang, 1952). Although satisfying the vernalization requirement often permits flowering after exposure to inductive cues, the key adaptive value of a vernalization requirement is that it prevents flowering in the fall season, thus ensuring that flowering does not commence as winter begins (Amasino, 2010).
CONCEPT OF MEMORY
An interesting component of the vernalization response in some plant species is that the acquisition of the ability to flower from cold exposure is mitotically stable after plants resume active growth in warm conditions (Amasino, 2004). This “memory of winter” is readily demonstrated in species for which there is a qualitative requirement for an additional cue to flower, such as inductive photoperiods, after the cold requirement has been satisfied. One of the first studies on the stability of the vernalization state (or thermoinduced state as it was sometimes referred to) was done with a biennial strain of henbane (Hyoscyamus niger) that has an obligate requirement for a vernalizing cold exposure followed by long days (LD); i.e., vernalized henbane plants will not flower when grown in non-inductive short days (SD). In henbane, the vernalized state is “remembered” because vernalized plants grown in SD for long periods of time readily flower after they are shifted to inductive LD (Lang and Melchers, 1947).
MOLECULAR BASIS OF THE MEMORY OF WINTER
A considerable amount of information regarding the molecular nature of the vernalization pathway is known from studies in the eudicot model Arabidopsis thaliana (Brassicaceae; Kim et al., 2009; Amasino, 2010; Song et al., 2013). The vernalization requirement is largely due to the expression of a MADS-box-containing transcription factor, FLOWERING LOCUS C (FLC), which is an effective flowering repressor (Michaels and Amasino, 1999; Sheldon et al., 1999). The level of FLC expression is determined by an extensive regulatory network that includes components involved in small RNA metabolism (Swiezewski et al., 2009; Heo and Sung, 2011; Song et al., 2013) as well as a protein complex that appears to have evolved specifically for FLC activation (the FRIGIDA complex; Choi et al., 2011; Lee and Amasino, 2013). The FLC protein prevents flowering at a molecular level by binding to the promoters of specific genes and blocking their expression (Helliwell et al., 2006; Searle et al., 2006); these genes include FT, which encodes the mobile “florigen” signalin leaves as well as SUPPRESSOR OF CONSTANS 1 (SOC1) and FD in meristems. FD, FT, and SOC1 encode proteins that activate a suite of floral homeotic genes such as APETALA1 (AP1) that specify floral organs (Kim et al., 2009).
Vernalization results in the silencing of FLC expression (Michaels and Amasino, 1999). During winter, exposure to prolonged cold results in a polycomb-like, chromatin-modifying complex initiating the modification of FLC chromatin, transforming it from an active euchromatic state into a stably repressed heterochromatic state that remains repressed for the rest of the life cycle (for reviews see Amasino, 2010; Andrés and Coupland, 2012; Zografos and Sung, 2012; Song et al., 2013). During cold, a unique, cold-specific polycomb component known as VERNALIZATION INSENSITIVE 3 (VIN3) is induced; the VIN3 protein is necessary for polycomb-mediated FLC silencing (Sung and Amasino, 2004; Wood et al., 2006; De Lucia et al., 2008). The polycomb complex adds methyl groups to histone 3 (H3) at lysine 27 (K27) residues to form trimethylated H3 (H3K27me3), and increased H3K27me3 of FLC chromatin appears to be one of the first chromatin changes accompanying FLC silencing (Bastow et al., 2004; Sung and Amasino, 2004; Finnegan and Dennis, 2007; De Lucia et al., 2008; Angel et al., 2011). Vernalization-mediated FLC silencing is also associated with increased lysine 9 (K9) trimethylation at H3 (H3K9me3; Sung et al., 2006a,b), and H3K9me3 appears to be required for the memory of winter at FLC.
The H3K27me3 modification at FLC spreads and persists after the cold exposure is over (Finnegan and Dennis, 2007; De Lucia et al., 2008; Angel et al., 2011), and H3K9me3 is likely to spread as well. Because the vernalized state and FLC chromatin modification persist through mitotic cell divisions in meristem cells after cold treatment ends, it is reasonable to think of vernalization as an environmentally induced epigenetic switch (Amasino, 2004; Schmitz and Amasino, 2007). Although FLC repression is maintained throughout the plant’s life cycle, the repressed state of FLC becomes reset to an active state in the following generation, resulting in the re-establishment of the vernalization requirement (Amasino, 2004; Schmitz and Amasino, 2007). The stable repression of FLC is consistent with the annual life history of Arabidopsis which involves the conversion of all shoot meristems to flowering which maximizes the number of progeny in a single cycle of reproduction (Amasino, 2009).
TO HAVE OR NOT TO HAVE MEMORY
In contrast with annual plants such as Arabidopsis, perennials live for many years and flower repeatedly throughout their lives. For perennials to persist for multiple growth cycles, it is critical that not all of the shoot meristems become irreversibly floral; rather, some meristems need to be reserved for next season’s growth (Amasino, 2009; Turck and Coupland, 2013). Recently, aspects of the molecular basis of the perennial life history trait have been studied in Arabis alpina, a relative of Arabidopsis in the Brassicaceae (Wang et al., 2009; Bergonzi et al., 2013; Turck and Coupland, 2013). Like many accessions of Arabidopsis, A. alpina also requires vernalization in order to flower. However, unlike Arabidopsis, in A. alpina vernalization does not result in the flowering of all shoot meristems (Wang et al., 2009). Only certain meristems (those that were most actively growing before cold exposure commenced) produce flowers, whereas other meristems produce only vegetative shoots following cold exposure (Wang et al., 2009).
In A. alpina, not all shoot meristems become floral at least in part because vernalization is “forgotten.” In many (and probably all) Brassicaceae, a vernalization requirement results from flowering repression mediated by FLC or an FLC ortholog. In A. alpina, the FLC ortholog is known as PERPETUAL FLOWER1 (PEP1; Wang et al., 2009). The PEP1 expression pattern is similar to that of FLC before and during cold; specifically, it is highly expressed prior to cold exposure and is down-regulated during cold exposure. However, a key difference is that after cold exposure ends, FLC repression is maintained in Arabidopsis, whereas PEP1 repression is forgotten: after cold exposure ends, PEP1 mRNA levels begin to rise and eventually reach pre-vernalization levels (Wang et al., 2009). Furthermore, although the repressive chromatin mark, H3K27me3, increases during cold, it does not persist after cold exposure ends (Wang et al., 2009). Thus, the difference in the stability of chromatin modifications in PEP1 versus FLC contributes to the perennial versus annual life history trait of A. alpina and Arabidopsis. As discussed below, single-gene mutations in Arabidopsis can result in PEP1-like behavior at the FLC locus including a transient, cold-specific increase in H3K27me3 (Sung and Amasino, 2004).
This perennial strategy “works” in A. alpina because in this species (as well as in Arabidopsis) once flowering commences it is irreversible and no longer subject to FLC/PEP1-mediated repression. Some of the shoot meristems of A. alpina become irreversibly committed to flowering before post-vernalization PEP1 levels rise, whereas the post-vernalization resumption of PEP1 expression appears to prevent flowering in other meristems thus “reserving” those meristems for the next growing season (Wang et al., 2009). Irreversible FLC-independent flowering results, at least in part in Arabidopsis, from a positive feedback loop involving the floral meristem-identity genes LEAFY and APETALA1 which activate each other’s expression (Liljegren et al., 1999), and the feedback loop is not subject to FLC repression.
GENETIC VARIATION FOR MEMORY
Interestingly, the stability of the vernalized, repressed state of FLC in Arabidopsis is easily perturbed through single-gene mutations (Levy et al., 2002; Sung et al., 2006a; Schmitz et al., 2008; Kim et al., 2009). For example, in vrn1, prmt5, or lhp1 mutants, cold-mediated FLC repression is transient similar to that in A. alpina – i.e., FLC is repressed during cold exposure, but expression rises after plants resume growth in warmer conditions (Levy et al., 2002; Sung et al., 2006a; Schmitz et al., 2008). VRN1, PRMT5, and LHP1 do not encode components of the polycomb complex; rather, they encode other types of chromatin-modifying proteins involved in epigenetically “locking in” the repressed state of FLC such that polycomb-initiated repression becomes mitotically stable in warm conditions (Kim et al., 2009). This mitotic stability appears to require an increased level of H3K9 trimethylation at FLC, as well as H3K27 trimethylation (Bastow et al., 2004; Sung and Amasino, 2004; Sung et al., 2006a,b). Thus, a single-gene change can determine the difference between a memory of winter or lack thereof, and it might be expected that families of plants in addition to the Brassicaceae would contain vernalization-requiring members that had a memory of winter and members that did not depending upon the life history strategies they have evolved.
MEMORY IN A GRASS MODEL
Vernalization-requiring species exist in many plant groups spanning angiosperm diversification (Preston and Sandve, 2013). There are additional examples of species (or varieties within a species) in whichthe vernalized state is not stable in non-inductive SD conditions such as sugar beet (Caryophyllales; Margara, 1960), primrose (Myrtales; Chouard, 1960), carrot (Apiales; Bernier et al., 1981; Bernier, 1988), wheat (Poales; Evans, 1987), and Cheiranthus (Brassicales; Barendse, 1964). Thus, many plant species that have a vernalization requirement do not have the ability to “remember” prior cold.
We sought to determine if the small, temperate grass Brachypodium distachyon has the ability to “remember” prior cold. Recently, we and others have characterized natural variation in the vernalization response in many Brachypodium accessions, and we found considerable variation in flowering behavior, ranging from accessions that exhibit rapid flowering without prior cold exposure in inductive LD to accessions that have an obligate vernalization requirement (Schwartz et al., 2010; Ream et al., 2012, 2014; Colton-Gagnon et al., 2013; Tyler et al., 2014). Furthermore, among the accessions that have an obligate vernalization requirement, the amount of cold needed to saturate the vernalization response ranges from 2 weeks to greater than 16 weeks (Ream et al., 2014). With respect to the requirement for inductive photoperiods, none of the Brachypodium accessions tested flower after several months of outgrowth in SD (8-h day length) even if vernalized extensively (Ream et al., 2014) i.e., like biennial henbane, many Brachypodium accessions have an obligate requirement for both vernalization and inductive photoperiods in order to flower. Thus, there are Brachypodium accessions that can be used to investigate whether or not Brachypodium can remember prior cold with an experimental design similar to the classic work first done in henbane by Lang and Melchers (1947).
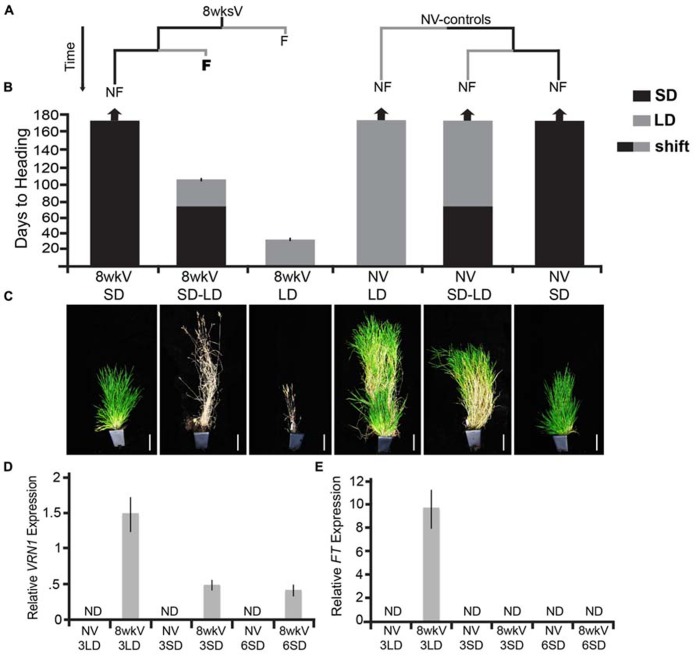
To determine if the vernalized state is mitotically stable in Brachypodium, we grew vernalized plants in non-inductive photoperiods before shifting to inductive photoperiods (experimental design is outlined in Figure 1A). Briefly, we first vernalized the accession Bd29-1 as imbibed seed for 8 weeks, which is a saturating vernalization treatment (Ream et al., 2014). After the vernalization treatment, we placed the vernalized seeds (and non-vernalized controls at the same stage of development) in either non-inductive SD or inductive 20-h LD. Some of the vernalized as well as non-vernalized plants grown in SD for 70 days were then shifted to LD. The shift of the vernalized plants from SD to LD revealed that the vernalized state was robustly maintained in SD because the time to flowering after the shift to LD was the same as that for plants directly moved from cold exposure into inductive photoperiods (Figure 1B). Specifically, the vernalized plants directly moved into LD as well as the vernalized plants first moved into SD flowered in fewer than 30 days forming only four leaves in inductive LD prior to flowering (Figure 1B, leaf data not shown). This indicates that, as is the case in henbane, there are accessions of Brachypodium in which the vernalization response is mitotically stable and that vernalization provides only the competence to flower, given none of the SD-only controls flowered during the duration of the experiment.
FIGURE 1.
The vernalization response is mitotically stable in Brachypodium distachyon (Bd29-1). (A) Plants were exposed to either 8 weeks of cold to saturate the vernalization response (8wksV) or were non-vernalized (NV-controls). Both the 8wksV and NV treated plants were placed either into inductive 20-h long days (LD; indicated by the gray bar) or non-inductive 8-h short days (SD; indicated by the black bar). After 70 days, some of the plants that had been in SD were shifted into LD, and, as a control the remainder of the SD-grown plants were kept in SD for the duration of the experiment. Only vernalized plants flowered in LD indicated by the letter F (flower); plants that did not flower are indicated by NF (non-flowering). (B) Days to heading was measured as the number of days to first spikelet emergence as done in Ream et al. (2014). Note that time during vernalization treatment is excluded. Arrows at the top of the bar graph indicate plants that did not flower for the duration of the experiment (170 days). Black bars represent SD-grown plants; gray bars represent LD-grown plants and bars with black and gray represent those plants that were first exposed to SD for 70 days followed by a shift into LD (SD–LD). Bars represent the days to heading average of six plants (experiment was repeated with similar results). (C) Photographs of representative plants at the end of experiment. Plants were grown and scored as described in Ream et al. (2014). Scale bar = 9 cm. (D,E) VRN1 and FT expression in a newly expanded 3rd leaf and 6th leaf of Bd29-1. Imbibed seeds in soil were exposed to either a saturating cold treatment (5°C for 8 weeks) or no cold. At the end of cold treatment, non-vernalized and cold-treated plants were grown in SD and LD for ~3 weeks (3LD, 3SD) and SD for an additional ~6 weeks (6SD) at 22°C during the light phase and 18°C during the dark phase until plants reached the third leaf stage and the sixth leaf stage (Note vernalized 29-1 in LD had flowered by 5 weeks so did not sample 6LD). VRN1 and FT transcript levels were determined byRT-qPCR as described in Ream et al. (2014) and normalized to UBIQUITIN-CONJUGATING ENZYME18. ND denotes no expression detected. Bars represent the average of four biological replicates ± standard deviation (three leaves per replicate).
There are several controls for this experiment. One is to shift non-vernalized plants from SD to LD. The reason for this control is that short days are able to substitute for vernalization in some accessions of Brachypodium (Schwartz et al., 2010; Ream et al., 2014) as well as in accessions of other grass species such as wheat and rye (Purvis and Gregory, 1937; Evans, 1987; Heide, 1994). However, the Bd29-1 accession was chosen for this study because growth in SD does not have any effect on the vernalization requirement (Figure 1B). An additional control is the growth of both non-vernalized and vernalized plants in SD or LD for the duration of the experiment. None of the SD-only control plants or the LD-non-vernalized controls flowered during the duration of the experiment [170 days; Figure 1A; several SD-only control plants were also dissected after 170 days of growth and all meristems were vegetative (data not shown)]. The SD-only controls demonstrate that indeed growth in SDs does not permit flowering even in vernalized plants. The LD-only controls were chosen to ensure that the robust flowering observed in the vernalized plants was indeed due to the prior vernalization treatment and not simply due to the age of the plant when shifted into inductive LD. As expected for a species with a monocarpic life history, vernalized plants grown in LD senesced rapidly after seed fill whereas non-vernalized controls were green and still actively producing leaves throughout the duration of the experiment (Figures 1B,C).
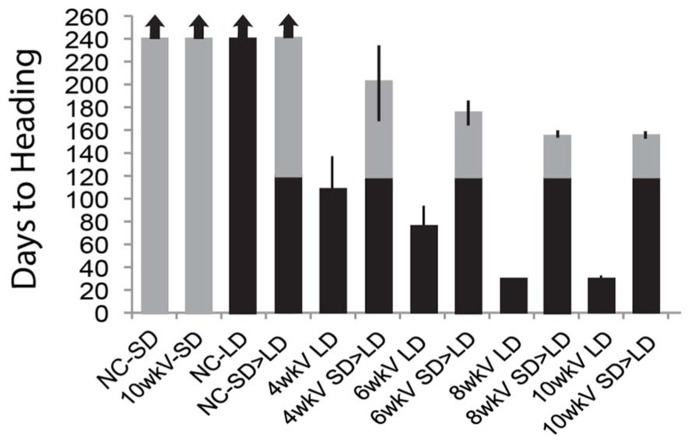
We also determined whether the quantitative aspect of the memory of vernalization is maintained in SD – i.e., is there a memory of the duration of cold exposure in Brachypodium when prolonged growth in SD separates cold exposure from a shift into inductive LD? Accordingly, imbibed seeds of Bd29-1 were exposed to varying lengths of cold (4, 6, 8, and 10 weeks), and then transferred to SD for 120 days prior to transfer to inductive LD (Figure 2; note this “memory test” is longer than the 70-day SD treatment presented in Figure 1). Cold exposures of 4 and 6 weeks are sub-saturating for vernalization in Bd29-1 (Ream et al., 2014). In this study, controls were similar to those presented in Figure 1: vernalized plants did not flower in SD and growth in continuous LD without prior vernalization also did not result in flowering illustrating the obligate nature of the vernalization requirement. A key control was the transfer of plants directly to inductive LD after cold exposure, which enabled a comparison of the efficacy of different durations of cold exposure with and without an interlude between cold exposure and photoperiodic induction of flowering. Even sub-saturating durations of cold exposure were effectively “remembered” during this long SD interlude as shown by the similar time to flowering after exposure to LD commenced in the plants exposed to 4 or 6 weeks of cold and then shifted immediately or after 120 days to LD (Figure 2). Saturating cold exposures of 8 and 10 weeks were also fully “remembered” during the 120-day SD treatment (Figure 2).
FIGURE 2.
Increasing duration of cold exposure causes increasingly rapid flowering in Brachypodium regardless of whether or not prolonged growth in SD separates cold exposure from inductive LD. Plants were vernalized for 4, 6, 8, and 10 weeks then placed into either SD for 120 days followed by a shift into LD or placed directly into LD. Flowering time was measured as days to heading upon emergence of first spikelet, and the time of cold exposure (vernalization treatment) was excluded. Arrows indicate plants that did not flower at end of experiment (240 days). Bars represent the days to heading average of six plants (experiment was repeated with similar results). LD = 20-h long days (gray bars), SD = 8-h short days (black bars), SD > LD = shift after growth in SD for 120 days into LD (black and gray bars). Plants were grown and scored as described in Ream et al. (2014).
Information about the vernalization systems in Pooideae has largely been derived from studies of existing allelic variation in wheat and barley. Such studies are consistent with a flowering model in which three genes, VERNALIZATION1 (VRN1), VERNALIZATION2 (VRN2), and VERNALIZATION3 (VRN3) form a regulatory loopin leaves that responds to vernalization and photoperiod (Greenup et al., 2009; Distelfeld and Dubcovsky, 2010). VRN3 is orthologous to FT (Yan et al., 2006) and hereafter will be referred to as FT. Prior to cold exposure, VRN2 represses FT expression and thus prevents flowering, whereas during and after cold VRN2 expression decreases thus permitting flowering (Yan et al., 2004; Sasani et al., 2009; Distelfeld and Dubcovsky, 2010). Therefore, VRN2 occupies a position in the flowering “circuitry” analogous to that of FLC (both are inhibitors of flowering that are repressed by cold), although VRN2 encodes a protein not related to FLC – it is a CCT domain-containing transcription factor and part of the type VI CO-like family of genes (Yan et al., 2004). Unlike, FLC in which chromatin-level suppression is the basis of memory, VRN2 suppression is not likely to be the primary event in memory for two reasons. One is that no changes in chromatin marks have been observed around the VRN2 locus during or after cold (Oliver et al., 2009). The other is that in Brachypodium VRN2 mRNA levels are the same before and after cold exposure and this expression pattern is not consistent with VRN2 acting as part of an evolutionarily conserved memory system in grasses (Ream et al., 2014).
VRN1, however, does exhibit cold-mediated chromatin changes. VRN1 is a repressor of VRN2 and it is up-regulated in leaves by cold exposure and its increased expression is maintained in warm post-vernalization conditions (Yan et al., 2004; Sasani et al., 2009; Distelfeld and Dubcovsky, 2010; Chen and Dubcovsky, 2012). The activation of VRN1 by cold is accompanied by a decrease in the repressive chromatin modification H3K27 methylation and an increase in activating H3K4 methylation in a presumed regulatory region of its first intron (Oliver et al., 2009, 2013). The level of VRN1 expression is proportional to the amount of cold experienced and thus correlates with the quantitative nature of the vernalization response in wheat, barley and Brachypodium (Yan et al., 2003; Sasani et al., 2009; Ream et al., 2014). Furthermore, the “henbane-like” behavior of certain Brachypodium accessions such as 29-1 enabled us to determine that increased VRN1 expression is maintained after cold exposure regardless of whether or not the plants are shifted to inductive photoperiods (Figure 1D). That VRN1 expression is maintained after cold exposure in SD – conditions in which FT is not expressed and flowering does not occur – demonstrates that vernalization causes a stable VRN1 “on state” that is independent of FT expression (Figures 1D,E). This day length-independent stability of the on state of VRN1 after cold exposure is consistent with a role for stable VRN1 activation to contribute to the memory of the vernalized state in Brachypodium. That VRN1 mRNA levels are higher in LD than in SD after vernalization is likely to be a result of FT enhancing VRN1 expression as shown in other cereals (Yan et al., 2006; Sasani et al., 2009; Shimada et al., 2009; Distelfeld and Dubcovsky, 2010).
There are other candidates for genes that may have a role in the memory of winter in grasses. For example, Huan et al. (2013) recently identified several hundred genes in Brachypodium for which the expression patterns changed during cold and the changes are maintained after 7 days post cold. It will be interesting to determine which Brachypodium genes maintain vernalization-mediated expression changes during a “long-term memory test” of prolonged exposure to SD after vernalization. Recently FLC-like genes have been identified in monocots, but whether or not these FLC-like genes have a role in flowering in the grass lineage remains to be experimentally determined (Ruelens et al., 2013). There remains much to learn about the molecular basis of vernalization in temperate grasses.
AUTHOR CONTRIBUTIONS
Daniel P. Woods, Richard M. Amasino, and Thomas S. Ream designed the experiments. Daniel P. Woods performed the experiments. Daniel P. Woods and Richard M. Amasino wrote and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank members of the Amasino lab for useful discussions. This research is supported by the College of Agricultural and Life Sciences and the Graduate School of the University of Wisconsin, and by grants from the Great Lakes Bioenergy Research Center (DOE BER office of Science DE-FCO2-07ER64494), the NSF (IOS-1258126), and a National Institutes of Health sponsored pre-doctoral training fellowship to the University of Wisconsin Genetics Training Program for Daniel P. Woods. Thomas S. Ream was a Gordon and Betty Moore Foundation Fellow of the Life Sciences Research Foundation.
REFERENCES
- Amasino R. (2004). Vernalization, competence, and the epigenetic memory of winter. Plant Cell 16 2553–2559 10.1105/tpc.104.161070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. (2009). Floral induction and monocarpic versus polycarpic life histories. Genome Biol. 10 228 10.1186/gb-2009-10-7-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. (2010). Seasonal and developmental timing of flowering. Plant J. 61 1001–1013 10.1111/j.1365-313X.2010.04148.x [DOI] [PubMed] [Google Scholar]
- Andrés F., Coupland G. (2012). The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13 627–639 10.1038/nrg3291 [DOI] [PubMed] [Google Scholar]
- Angel A., Song J., Dean C., Howard M. (2011). A polycomb-based switch underlying quantitative epigenetic memory. Nature 476 105–108 10.1038/nature10241 [DOI] [PubMed] [Google Scholar]
- Barendse G. W. M. (1964). Vernalization in Cheiranthus allionii Hort. Meded. Landbouwhogeschool Wageningen 64 1–64 [Google Scholar]
- Bastow R., Mylne J. S., Lister C., Lippman Z., Martienssen R. A., Dean C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167 10.1038/nature02269 [DOI] [PubMed] [Google Scholar]
- Bergonzi S., Albani M. C., Loren van Themaat E. V., Nordstrom K. J. V., Wang R., Schneeberger K., et al. (2013). Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 340 1094–1097 10.1126/science.1234116 [DOI] [PubMed] [Google Scholar]
- Bernier G. (1988). The control of floral evocation and morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 175–219 10.1146/annurev.pp.39.060188.001135 [DOI] [Google Scholar]
- Bernier G., Kinet J.-M., Sachs R. M. (1981). The Physiology of Flowering. Boca Raton, FL: CRC Press [Google Scholar]
- Chen A., Dubcovsky J. (2012). Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 8:e1003134 10.1371/journal.pgen.1003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kim J., Hwang H. J., Kim S., Park C., Kim S. Y., et al. (2011). The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23 289–303 10.1105/tpc.110.075911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard P. (1960). Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 11 191–238 10.1146/annurev.pp.11.060160.001203 [DOI] [Google Scholar]
- Colton-Gagnon K., Ali-Benali M. A., Mayer B. F., Dionne R., Bertrand A., Do Carmo S., et al. (2013). Comparative analysis of the cold acclimation and freezing tolerance capacities of seven diploid Brachypodium distachyon accessions. Ann. Bot. 10.1093/aob/mct283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia F., Crevillen P., Jones A. M. E., Greb T., Dean C. (2008). A PHD- polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 105 16831–16836 10.1073/pnas.0808687105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A., Dubcovsky J. (2010). Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol. Genet. Genomics 283 223–232 10.1007/s00438-009-0510-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. T. (1987). Short day induction of inflorescence initiation in some winter wheat varieties. Funct. Plant Biol. 14 277–286 10.1071/PP9870277 [DOI] [Google Scholar]
- Finnegan E. J., Dennis E. S. (2007). vernalization-induced trimethylation of histone H3 Lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr. Biol. 17 1978–1983 10.1016/j.cub.2007.10.026 [DOI] [PubMed] [Google Scholar]
- Greenup A., Peacock W. J., Dennis E. S., Trevaskis B. (2009). The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann. Bot. 103 1165–1172 10.1093/aob/mcp063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide O. M. (1994). Control of flowering and reproduction in temperate grasses. New Phytol. 128 347–362 10.1111/j.1469-8137.1994.tb04019.x [DOI] [PubMed] [Google Scholar]
- Helliwell C. A., Wood C. C., Robertson M., James Peacock W., Dennis E. S. (2006). The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 46 183–192 10.1111/j.1365-313X.2006.02686.x [DOI] [PubMed] [Google Scholar]
- Heo J. B., Sung S. (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331 76–79 10.1126/science.1197349 [DOI] [PubMed] [Google Scholar]
- Huan Q., Mao Z., Zhang J., Xu Y., Chong K. (2013). Transcriptome-wide analysis of vernalization reveals conserved and species-specific mechanisms in Brachypodium. J. Integr. Plant Biol. 55 696–709 10.1111/jipb.12050 [DOI] [PubMed] [Google Scholar]
- Kim D.-H., Doyle M. R., Sung S., Amasino R. M. (2009). Vernalization: winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25 277–299 10.1146/annurev.cellbio.042308.113411 [DOI] [PubMed] [Google Scholar]
- Lang A., Melchers G. (1947). Vernalisation und Devernalisation bei einer zweijährigen Pflanze. Z. Naturforsch. 2b 444–449 10.1007/BF01872507 [DOI] [Google Scholar]
- Lang A. (1952). Physiology of flowering. Annu. Rev. Plant. Physiol. 3 265–306 10.1146/annurev.pp.03.060152.001405 [DOI] [Google Scholar]
- Lee J., Amasino R. M. (2013). Two FLX family members are non-redundantly required to establish the vernalization requirement in Arabidopsis. Nat. Commun. 4 1–9 10.1038/ncomms3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y. Y., Mesnage S., Mylne J. S., Gendall A. R., Dean C. (2002). Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297 243–246 10.1126/science.1072147 [DOI] [PubMed] [Google Scholar]
- Liljegren S., Gustafson-Brown C., Pinyopich A., Ditta G., Yanofsky M. (1999). Interaction among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11 1007–1018 10.1105/tpc.11.6.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margara J. (1960). Recherches sur le de terminisme de l’e longation et de la floraison dans le genre Beta. Ann. Amelioration Plantes 10 362–471 [Google Scholar]
- Michaels S. D., Amasino R. M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956 10.1105/tpc.11.5.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. N., Deng W., Casao M. C., Trevaskis B. (2013). Low temperatures induce rapid changes in chromatin state and transcript levels of the cereal VERNALIZATION1 gene. J. Exp. Bot. 64 2413–2422 10.1093/jxb/ert095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. N., Finnegan E. J., Dennis E. S., Peacock W. J., Trevaskis B. (2009). Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc. Natl. Acad. Sci. U.S.A. 106 8386–8391 10.1073/pnas.0903566106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. C., Sandve S. R. (2013). Adaptation to seasonality and the winter freeze. Front. Plant Sci. 4:167 10.3389/fpls.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis O. N., Gregory F. G. (1937). Studies in vernalisation of cereals. Ann. Bot. 1 1–26 [Google Scholar]
- Ream T. S., Woods D. P., Amasino R. M. (2012). The molecular basis of vernalization in different plant groups. Cold Spring Harb. Symp. Quant. Biol. 77 105–115 10.1101/sqb.2013.77.014449 [DOI] [PubMed] [Google Scholar]
- Ream T. S., Woods D. P., Schwartz C. J., Sanabria C. P., Mahoy J. A., Walters E. M., et al. (2014). Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 164 694–709 10.1104/pp.113.232678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelens P., de Maagd R. A., Proost S., Theissen G., Geuten K., Kaufmann K. (2013). FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat. Commun. 4 1–8 10.1038/ncomms3280 [DOI] [PubMed] [Google Scholar]
- Sasani S., Hemming M. N., Oliver S. N., Greenup A., Tavakkol-Afshari R., Mahfoozi S., et al. (2009). The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J. Exp. Bot. 60 2169–2178 10.1093/jxb/erp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R. J., Amasino R. M. (2007). Vernalization: a model for investigating epigenetics and eukaryotic gene regulation in plants. Biochim. Biophys. Acta 1769 269–275 10.1016/j.bbaexp.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Schmitz R. J., Sung S., Amasino R. M. (2008). Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 105 411–416 10.1073/pnas.0710423104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Doyle M. R., Manzaneda A. J., Rey P. J., Mitchell-Olds T., Amasino R. M. (2010). Natural variation of flowering time and vernalization responsiveness in Brachypodium distachyon. Bioenerg. Res. 3 38–46 10.1007/s12155-009-9069-3 [DOI] [Google Scholar]
- Searle I., He Y., Turck F., Vincent C., Fornara F., Kröber S., et al. (2006). The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 20 898–912 10.1101/gad.373506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C. C., Burn J. E., Perez P. P., Metzger J., Edwards J. A., Peacock W. J., et al. (1999). The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11 445–458 10.1105/tpc.11.3.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Ogawa T., Kitagawa S., Suzuki T., Ikari C., Shitsukawa N., et al. (2009). A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J. 58 668–681 10.1111/j.1365-313X.2009.03806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Irwin J., Dean C. (2013). Remembering the prolonged cold of winter minireview. Curr. Biol. 23 R807–R811 10.1016/j.cub.2013.07.027 [DOI] [PubMed] [Google Scholar]
- Sung S., Amasino R. M. (2004). Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427 159–164 10.1038/nature02195 [DOI] [PubMed] [Google Scholar]
- Sung S., He Y., Eshoo T. W., Tamada Y., Johnson L., Nakahigashi K., et al. (2006a). Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 38 706–710 10.1038/ng1795 [DOI] [PubMed] [Google Scholar]
- Sung S., Schmitz R. J., Amasino R. M. (2006b). A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 20 3244–3248 10.1101/gad.1493306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiezewski S., Liu F., Magusin A., Dean C. (2009). Cold-induced silencing by long antisense transcripts of an Arabidopsis polycomb target. Nature 462 799–802 10.1038/nature08618 [DOI] [PubMed] [Google Scholar]
- Turck F., Coupland G. (2013). Natural variation in epigeneticgene regulation and its effects on plant developmental traits. Evolution 68 620–631 10.1111/evo.12286 [DOI] [PubMed] [Google Scholar]
- Tyler L., Fangel J. U., Fagerström A. D., Steinwand M. A., Raab T. K., Willats W. G., et al. (2014). Selection and phenotypic characterization of a core collection of Brachypodium distachyon inbred lines. BMC Plant Biol. 14:25 10.1186/1471-2229-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Farrona S., Vincent C., Joecker A., Schoof H., Turck F., et al. (2009). PEP1 regulates perennial flowering in Arabis alpina. Nature 459 423–427 10.1038/nature07988 [DOI] [PubMed] [Google Scholar]
- Wood C. C., Robertson M., Tanner G., Peacock W. J., Dennis E. S., Helliwell C. A. (2006). The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. U.S.A. 103 14631–14636 10.1073/pnas.0606385103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Fu D., Li C., Blechl A., Tranquilli G., Bonafede M., et al. (2006). The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. U.S.A. 103 19581–19586 10.1073/pnas.0607142103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Blechl A., Tranquilli G., Ramakrishna W., SanMiguel P., et al. (2004). The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303 1640–1644 10.1126/science.1094305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Tranquilli G., Helguera M., Fahima T., Dubcovsky J. (2003). Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. U.S.A. 100 6263–6268 10.1073/pnas.0937399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zografos B. R., Sung S. (2012). Vernalization-mediated chromatin changes. J. Exp. Bot. 63 4343–4348 10.1093/jxb/ers157 [DOI] [PubMed] [Google Scholar]