Abstract
Background
Dual-mobility acetabular cups have been marketed with the purported advantages of reduced dislocation rates and improvements in ROM; however, the relative efficacies of these designs in terms of changing joint stability via ROM and dislocation distance have not been thoroughly evaluated.
Questions/purposes
In custom computer simulation studies, we addressed the following questions: (1) Do variations in component geometry across dual-mobility designs (anatomic, modular, and subhemispheric) affect the posterior horizontal dislocation distances? (2) How do these compare with the measurements obtained with standard hemispheric fixed bearings? (3) What is the effect of head size on posterior horizontal dislocation distances for dual-mobility and standard hemispheric fixed bearings? (4) What are the comparative differences in prosthetic impingement-free ROM between three modern dual-mobility components (anatomic, modular, and subhemispheric), and standard hemispheric fixed bearings?
Methods
CT scans of an adult pelvis were imported into computer-aided design software to generate a dynamic three-dimensional model of the pelvis. Using this software, computer-aided design models of three dual-mobility designs (anatomic, modular, and subhemispheric) and standard hemispheric fixed bearings were implanted in the pelvic model and the posterior horizontal dislocation distances measured. Hip ROM simulator software was used to compare the prosthetic impingement-free ROMs of dual-mobility bearings with standard hemispheric fixed-bearing designs.
Results
Variations in component design had greater effect on posterior horizontal dislocation distance values than increases in head size in a specific design (p < 0.001). Anatomic and modular dual-mobility designs were found to have greater posterior horizontal dislocation distances than the subhemispheric dual-mobility and standard hemispheric fixed-bearing designs (p < 0.001). Increasing head sizes increased posterior horizontal dislocation distances across all designs (p < 0.001). The subhemispheric dual-mobility implant was found to have the greatest prosthetic impingement-free ROM among all prosthetic designs (p < 0.001; R2 = 0.86).
Conclusions
The posterior horizontal dislocation distances differ with the individual component geometries of dual-mobility designs, with the anatomic and modular designs showing higher posterior horizontal dislocation distances compared with subhemispheric dual-mobility and standard hemispheric fixed-bearing designs.
Clinical Relevance
Static, three-dimensional computerized simulation studies suggest differences that may influence the risk of dislocation among components with varying geometries, favoring anatomic and modular dual-mobility designs. Clinical studies are needed to confirm these observations.
Introduction
Dislocation continues to be a challenging problem after THA, with reported rates of instability after primary THA typically varying between 2% and 3% [8, 11, 13, 17, 22, 28]. It is also a common cause for revision THAs, with recurrent dislocations accounting for nearly 22% of 51,345 revisions in a review of the National Inpatient Sample Database from October 1, 2005, through 2006 [5]. Numerous factors such as acetabular and femoral component malposition, inadequate soft tissue tension, abductor deficiency, neuromuscular conditions, and various other patient-related factors have been associated with increased risks of dislocation. Several implant-related features such as component geometries, femoral head diameter, head-neck ratio, and the extent of prosthetic impingement-free ROM also affect the risk of dislocation [6, 10, 12, 14, 20, 21, 23]. The use of small-diameter cups with small head sizes or large-diameter components with reduced head-shell ratios may increase the risk of dislocation even further [26]. Various newer designs with subtle modifications of component geometries such as extended posterior liners, bipolar devices, constrained liners, and, more recently, a newer generation of dual-mobility designs have been offered by manufacturers to potentially reduce these dislocation risks. Previous studies have shown that changes in component design may play a considerable role in the prevention of dislocations after THA [2, 12, 20, 21].
Dual-mobility devices were proposed for use in THAs more than three decades ago and have potential advantages of improved stability and increased prosthetic impingement-free ROM [3, 7, 9, 24, 25, 30]. Their use historically may have been limited owing to concerns regarding increased wear of soft-on-hard articulations [16]. However, there has been a recent resurgence in the use of these devices owing to improvements in the wear resistance of a new generation of highly cross-linked polyethylene bearings that may reduce particulate wear [15]. In dual-mobility designs, a standard-diameter femoral head (22 or 28 mm) is snap-fit into a large polyethylene head, which in turn articulates with a polished metal acetabular shell. This effectively increases the femoral head diameter and leads to an increase in the jump distance required for the femoral head to be unseated from the acetabular cup. Historically, the dual-mobility components were cylindrospheric (ie, deeper than a pure hemisphere). However, more recently, some modified designs (subhemispheric, anatomic, and modular) have been marketed with the purported advantages of improving prosthetic impingement-free ROM without a corresponding change in the jump distance. It has been shown that three-dimensional (3-D) jump distance (posterior horizontal dislocation distance) is an accurate in vitro measure of hip stability and may predict the risk of dislocation for various component designs [20]. In brief, posterior horizontal dislocation distance is the minimum distance the femoral head must travel laterally before it is tangential to the rim of the acetabular component. A larger posterior horizontal dislocation distance indicates a potentially more stable construct. To our knowledge, the relative efficacies of varying implant designs in terms of changing joint stability via prosthetic impingement-free ROM and posterior horizontal dislocation distance have not been evaluated.
Accordingly, we evaluated the effect of geometric differences of dual-mobility devices on the risk of dislocation in a computer-based 3-D simulation model. We asked the following questions: (1) Do variations in component geometry across dual-mobility designs (anatomic, modular, and subhemispheric) affect the posterior horizontal dislocation distances? (2) How do these compare with the measurements obtained with standard hemispheric fixed-bearing designs? (3) What is the effect of head size on posterior horizontal dislocation distances for dual-mobility and standard bearings? (4) What are the comparative differences in prosthetic impingement-free ROM between three modern dual-mobility components (subhemispheric, anatomic, and modular), and standard hemispheric fixed bearings?
Materials and Methods
We evaluated the posterior horizontal dislocation distance of various hip arthroplasty components using high-resolution, thin-slice CT scans of an adult pelvis. The images were extracted to Pro/ENGINEER 3-D computer-aided design (CAD) software (PTC® Inc, Needham, MA, USA) to generate a solid model (Fig. 1). The pelvis was used for orientation and observation purposes and does not affect the analysis. A progressive series of coronal, sagittal, and horizontal planes were defined to allow virtual placement of acetabular components in a predetermined orientation. The x- (pointing anteriorly), y- (pointing superiorly), and z- (pointing laterally) axes were determined with reference to the center of the femoral head. This solid pelvic model allowed the flexibility to change the inclination (forward or backward rotation) of the pelvis after placement of the acetabular component. For the purposes of this study, we performed all measurements with the acetabular component placed in 45° inclination and 20° anteversion in the socket and the pelvis rotated anteriorly to 26° forward tilt [19]. This amount of anterior tilt simulated a low-chair rise situation, which is considered a high-risk position for dislocation [19]. The 3-D jump distance or posterior horizontal dislocation distance was defined as the minimum translational distance in the coronal plane measured from the center of the acetabular component to the center of the femoral head placed at the 9 o’clock position (for the right hip) at the tangential edge of the acetabular component and was validated and described by Nevelos et al. (Fig. 2) [20]. The posterior horizontal dislocation distances were measured for four different acetabular cup sizes to represent different clinical scenarios (if the implant was available in that size): 48 mm (small primary), 54 mm (standard primary), 60 mm (standard revision), and 66 mm (large revision cups).
Fig. 1.
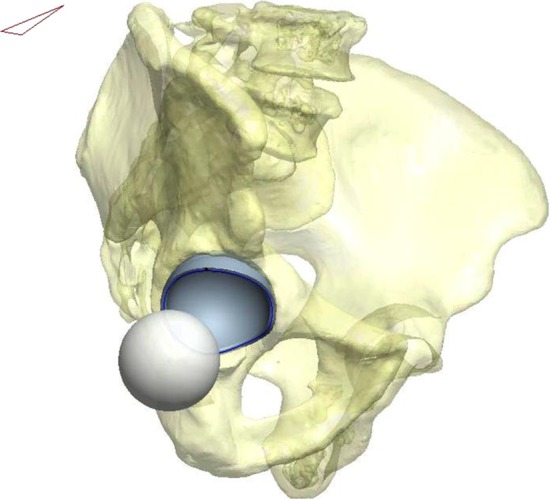
A three-dimensional pelvic model is generated after feeding the CT images of an adult pelvis into a software program. The acetabular cup was placed in 45° inclination and 20° anteversion, and the pelvis was tilted 26° to represent a low chair-sitting position. (Published with permission from Nevelos J, Johnson A, Heffernan C, Macintyre J, Markel DC, Mont MA. What factors affect posterior dislocation distance in THA? Clin Orthop Relat Res. 2013;471:519–526.)
Fig. 2.
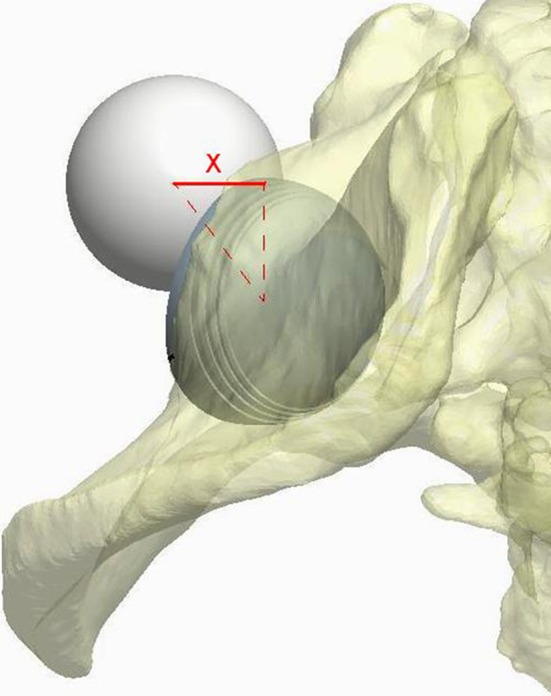
Measurement of the posterior horizontal dislocation distance using computerized software is shown. The posterior horizontal dislocation distance was defined as the minimum translational distance in the coronal plane measured from the center of the acetabular component to the center of the femoral head placed at the 9 o’clock position (for the right hip) at the tangential edge of the acetabular component and has been validated. (Published with permission from Nevelos J, Johnson A, Heffernan C, Macintyre J, Markel DC, Mont MA. What factors affect posterior dislocation distance in THA? Clin Orthop Relat Res. 2013;471:519–526.)
Three dual-mobility designs of different geometries were analyzed using the aforementioned cup sizes. These were compared with a generic hemispheric fixed-bearing type (with a head size typical for the cup outer diameter). For the dual-mobility bearings, the posterior horizontal dislocation distances were evaluated between the outer bearing and the acetabular component. Intraprosthetic dislocation risks were not evaluated in this study. The three dual-mobility bearings analyzed that are commonly used in the United States were the subhemispheric type (E1 Active®; Biomet, Inc, Warsaw, IN, USA) [4], a modular dual-mobility design with a 2.4-mm cylinder (MDM™; Stryker Orthopaedics, Mahwah, NJ, USA) (Fig. 3) [29], and a dual-mobility design with an anatomic rim (ADM™; Stryker Orthopaedics) (Fig. 4) [29]. A 3-D model of the subhemispheric dual-mobility design was constructed from the information obtained from the commercially available templates and the brochures. This design is hemispheric externally with a 3-mm lateralized internal bore to achieve approximate head coverage of 165°. The modular design has a cylindrospheric design with a constant 2.4-mm cylinder, while the anatomic dual-mobility cup has an anatomic-shaped rim to match the native acetabular socket. The anatomic design (shell range, 46–64 mm) has cutouts provided for the obturator foramen and for the psoas tendon to avoid psoas tendon-to-cup impingement. This anatomic design also makes the cup deeper than a hemisphere in certain regions, especially posteroinferior, where the rim extends beyond 180° to protect against dislocation. All components were implanted in the hip model based on the recommended surgical technique.
Fig. 3.

The Modular Dual Mobility (MDM™) cup design with a 2.4-mm cylinder is shown. (Photograph provided courtesy of Stryker Corp, Mahwah, NJ, USA).
Fig. 4.

Features of the Anatomic Dual Mobility (ADM™) cup with cut-outs at the rim of the acetabular component are shown. (Photograph provided courtesy of Stryker Corp, Mahwah, NJ, USA).
For prosthetic impingement-free ROM analysis, we used custom hip ROM simulator software for computation and animation of three isolated plane of hip movements based on the implant alignment, implant geometry, and patient activity. This software provided a 3-D model of the skeletal system containing the anatomic coordinate systems for the femur and the pelvis for the user. The anterior superior iliac spine (ASIS) and the pubic symphysis provided the landmarks for the pelvic coordinates. For the purposes of this study, we defined pelvic tilt as the angle between the plane containing the ASIS and the pubic symphysis with an imaginary vertical plane. The orientation of these coordinates could be changed based on the amount of pelvic tilt desired. Only bearing designs commercially available in a 54-mm shell (standard primary) were analyzed.
CAD models of hip implants were imported into the model. The software then was used to measure interprosthetic (ie, component-to-component) impingement-free ROM in three different planes of motion (flexion-extension, abduction-adduction, and internal and external rotation in neutral flexion). For all dual-mobility bearings, motion was considered between the outer bearing and the metal acetabular component. Both articulations were moved simultaneously in the simulation model, however, the frictional coefficients between the articulating surfaces of the dual-mobility designs were not evaluated. Intraprosthetic mobility between the inner and the outer bearings was not measured in the study. The implants used for prosthetic impingement-free ROM analysis included a standard stem with a 132° neck-shaft angle, which was used for all designs. The 28-mm and 36-mm head sizes were used for assessment of prosthetic impingement-free ROM as anecdotally these were the two most common head diameters we used.
All data generated from the 3-D modeling measurements initially were tabulated in an Excel® spreadsheet (Microsoft Corp, Redmond, WA, USA). These then were compared among various component designs and across all head sizes and cup diameters using Excel, Minitab® (Minitab Inc, State College, PA, USA), and GraphPad (GraphPad Software, Inc, La Jolla, CA, USA). Single-factor ANOVA and t-test statistics were used to compare the association of head size and posterior horizontal dislocation distances. Nested ANOVA and regression analyses were used to determine the relationship between head size, shell size, and design type with posterior horizontal dislocation distance values. Paired t-test statistics were used to compare the prosthetic impingement-free ROM between various designs. A p value less than 0.05 was considered statistically significant.
Results
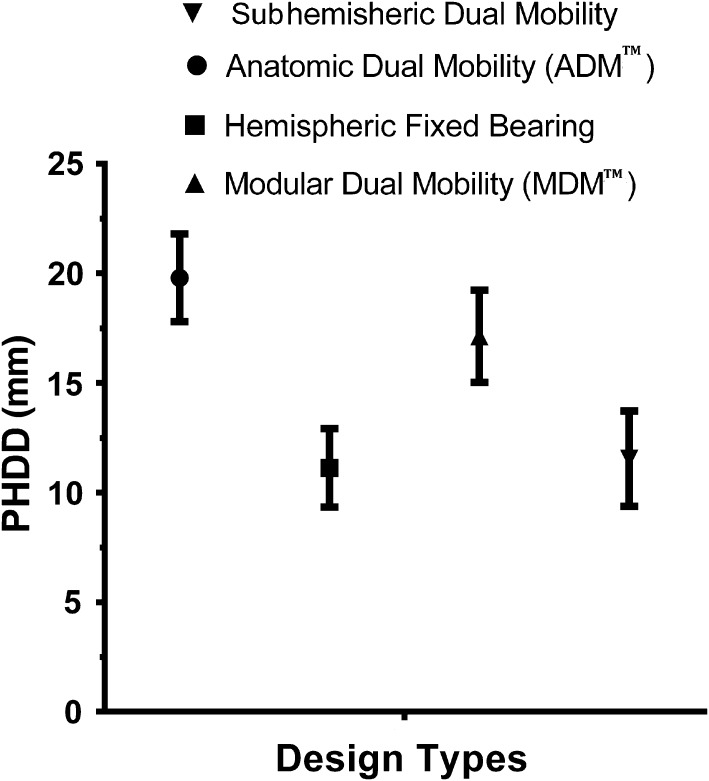
Component design type was found to be the most important factor affecting posterior horizontal dislocation distance variance (shell size, p = 0.29, R2 = 0.12, 95% CI, ± 2.21; head size, p = 0.06, R2 = 0.42, 95% CI, ± 2.42; design type, p < 0.001, R2 = 0.81, 95% CI, ± 1.026). Although shell size did not contribute to the variance of posterior horizontal dislocation distance variance values, head size was found to contribute to 21% (SD, ± 2%) and design type 79% (SD, ± 4%) to the variance seen in posterior horizontal dislocation distance variance values, showing that while larger heads increase posterior horizontal dislocation distance variance, design type had a more considerable effect on posterior horizontal dislocation distance variance. For all components analyzed, the anatomic dual-mobility design provided the greatest posterior horizontal dislocation distance variances compared with the modular dual-mobility, subhemispheric dual-mobility, and standard fixed-bearing designs in cup ranges between 48 mm and 60 mm (p < 0.01 for all comparisons; Table 1). The modular design provided the second greatest posterior horizontal dislocation distance variance in the 48- to 60-mm cup range and the greatest in the 66-mm shell size (p < 0.01 for all comparisons; Table 2). The anatomic and modular dual-mobility designs had greater posterior horizontal dislocation distances compared with the subhemispheric dual-mobility designs (p < 0.001, R2 = 0.99, 95% CI, −10.31 to −7.96; and p < 0.001, R2 = 0.99, 95% CI, 5.12–6.03, respectively) and hemispheric fixed-bearing components (p < 0.001, R2 = 0.99, 95% CI, 8.20–9.99; and p < 0.001, R2 = 0.98, 95% CI, 4.65–7.35, respectively). In addition, the anatomic dual-mobility design was found to have greater posterior horizontal dislocation distances compared with modular dual-mobility designs (p = 0.005; R2 = 0.98; 95% CI, 2.42–4.50) (Fig. 5). The difference in posterior horizontal dislocation distance between subhemispheric dual-mobility and hemispheric fixed-bearing designs was not significant (p = 0.47; R2 = 0.18; 95% CI, −1.23 to 2.08), despite larger head diameters used with the subhemispheric dual-mobility design.
Table 1.
Comparison of four component designs
| Design | Head diameter (millimeters) | Shell outer diameter (millimeters) | Posterior horizontal dislocation distances (millimeters) | Correlation of head size to posterior horizontal dislocation distance for each design type |
|---|---|---|---|---|
| Hemispheric fixed bearing | 28 | 48 | 8.6 | |
| 36 | 54 | 11.1 | ||
| 40 | 60 | 12.4 | ||
| 40 | 66 | 12.4 | ||
| Average (SD) | 11.1 (1.8) | p < 0.001; R2 = 0.98; 95% CI, 0.99–1.00 | ||
| Subhemispheric dual mobility | 42 | 48 | 9.2 | |
| 48 | 54 | 10.5 | ||
| 54 | 60 | 12.3 | ||
| 60 | 66 | 14.2 | ||
| Average (SD) | 11.6 (2.2) | p = 0.004; R2 = 0.98; 95% CI, 0.83–0.99 | ||
| Modular Dual Mobility (MDM™) | 36 | 48 | 14.6 | |
| 42 | 54 | 16.5 | ||
| 46 | 60 | 17.8 | ||
| 52 | 66 | 19.6 | ||
| Average (SD) | 17.1 (2.1) | p < 0.001; R2 = 0.99; 95% CI, 0.99–1.00 | ||
| Anatomic Dual Mobility (ADM™) | 42 | 48 | 17.8 | |
| 48 | 54 | 19.8 | ||
| 54 | 60 | 21.8 | ||
| Average (SD) | 19.8 (2.0) | p = 0.0000; R2 = 1 |
MDM™ and ADM™, Stryker Orthopaedics, Mahwah, NJ, USA.
Table 2.
Statistical comparison of the posterior horizontal dislocation distances*
| Design type | Hemispheric fixed bearing | Subhemispheric dual mobility | Modular dual mobility |
|---|---|---|---|
| Subhemispheric dual mobility | p = 0.474; R2 = 0.182; 95% CI, −1.231 to 2.081 | N/A | |
| Modular Dual Mobility (MDM™) | p < 0.001; R2 = 0.985; 95% CI, 4.65–7.35 | p < 0.001; R2 = 0.998; 95% CI, 5.118–6.032 | N/A |
| Anatomic Dual Mobility (ADM™) | p < 0.001; R2 = 0.999; 95% CI, 8.204–9.996 | p< 0.001; R2 = 0.998; 95% CI, −10.31 to −7.959 | p = 0.005; R2 = 0.98; 95% CI, 2.417–4.5 |
* Paired 2-tail t-test; N/A = not applicable; MDM™ and ADM™, Stryker Orthopaedics, Mahwah, NJ, USA.
Fig. 5.
The Anatomic Dual Mobility (ADM™, Stryker Orthopaedics, Mahwah, NJ, USA) design provided significantly greater posterior horizontal dislocation distances compared with the Modular Dual Mobility (MDM™, Stryker Orthopaedics), subhemispheric dual-mobility, and standard fixed-bearing designs in cup ranges between 48 and 60 mm. PHDD = posterior horizontal dislocation distance.
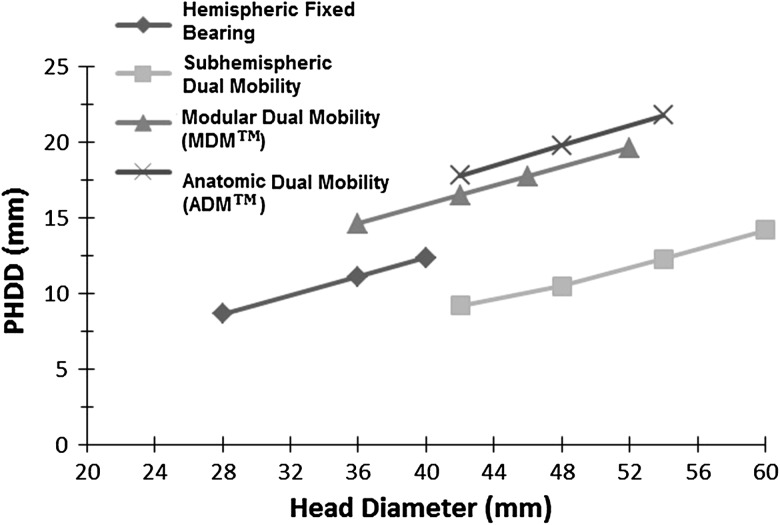
We also found that regardless of design type, increasing head size had a similar effect on posterior horizontal dislocation distance values, with increase in posterior horizontal dislocation distances for a given increase in head size (Fig. 6). A strong positive correlation was found between increased head sizes and posterior horizontal dislocation distance for standard hemispheric fixed-bearing (p < 0.001; R2 = 0.98; 95% CI, 0.99–1.00), subhemispheric dual-mobility (p = 0.004; R2 = 0.98; 95% CI, 0.83–0.99), modular (p < 0.001; R2 = 0.99; 95% CI, 0.99–1.00), and anatomic dual-mobility (p = 0.000; R2 = 1) designs. For standard hemispheric fixed-bearing components, only a minor increase in posterior horizontal dislocation distances (11.4 versus 12.4 mm) was observed by increasing head diameters from 36 to 40 mm. With the dual-mobility bearings, steady increases in posterior horizontal dislocation distances were found across their respective head diameters from 36 to 60 mm. At equivalent head diameters, the anatomic and the modular designs provided greater posterior horizontal dislocation distances compared with the subhemispheric dual-mobility and standard fixed bearings. However, the greatest percentage improvement in posterior horizontal dislocation distances with increasing head sizes was found with the subhemispheric dual-mobility designs (54%; 5 mm; head diameter, 42–60 mm) compared with the hemispheric fixed-bearing (44%; 3.8 mm; head diameter, 28–40 mm), modular dual-mobility (34%; 5 mm; head diameter, 36–52 mm), and the anatomic dual-mobility bearings (22%; 4 mm; head diameter, 42–54 mm).
Fig. 6.
Increasing the head size increased the posterior horizontal dislocation distance for all designs, however design features had a stronger effect on posterior horizontal dislocation distance. PHDD = posterior horizontal dislocation distance. (ADM™ and MDM™, Stryker Orthopaedics, Mahwah, NJ, USA).
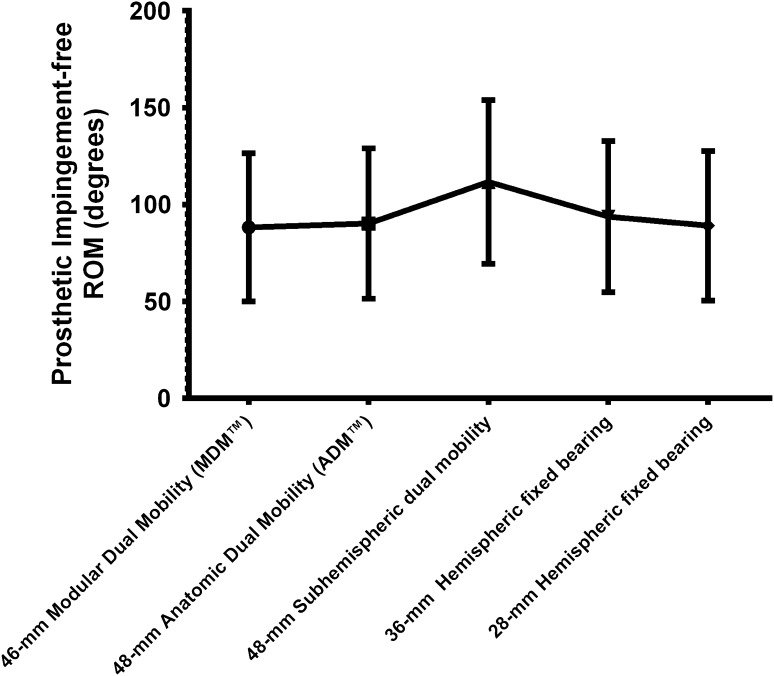
For the prosthetic impingement-free ROM analysis, among the bearings available for use with a 54-mm shell, the 48-mm subhemispheric implant had the greatest overall motion in all three planes, across all component designs tested. The subhemispheric dual-mobility design had greater prosthetic impingement-free ROM in all axes than all other designs (p < 0.001 for all designs but the Anatomic Dual Mobility [ADM™, Stryker Orthopaedics; p = 0.001) (Table 3). No differences in prosthetic impingement-free ROM for all axes of movement were found between the anatomic and modular dual-mobility designs (p = 0.55; R2 = 0.1; 95% CI, −5.9 to 9.9) (Fig. 7). Moreover, the prosthetic impingement-free ROM for the anatomic and modular dual-mobility designs were not different from the 28-mm hemispheric fixed-bearing design (p = 0.708, R2 = 0.03, 95% CI, −8.7 to 6.3; p = 0.22, R2 = 0.3, 95% CI, −0.7 to 2.3). Similarly, there were no significant differences between 36-mm hemispheric fixed-bearing design and the anatomic dual-mobility design (p = 0.3; R2 = 0.24; 95% CI, −3.6 to 10.6) (Table 4). However, the 36-mm hemispheric fixed-bearing design had significantly greater prosthetic impingement-free ROM compared with the modular dual-mobility design (p < 0.001; R2 = 0.91; 95% CI, 3.54–7.46).
Table 3.
Prosthetic impingement-free ROM across various component designs for 54-mm shell
| Component | Internal rotation (degrees) | External rotation (degrees) | Adduction (degrees) | Abduction (degrees) | Flexion (degrees) | Extension (degrees) |
|---|---|---|---|---|---|---|
| 28-mm hemispheric fixed bearing | 145 | 69 | 64 | 63 | 132 | 61 |
| 36-mm hemispheric fixed bearing | 151 | 75 | 67 | 66 | 136 | 67 |
| 48-mm subhemispheric dual mobility | 176 | 98 | 78 | 77 | 153 | 88 |
| 48-mm Anatomic Dual Mobility (ADM™) | 146 | 81 | 70 | 54 | 131 | 59 |
| 46-mm Modular Dual Mobility (MDM™) | 145 | 67 | 64 | 62 | 129 | 62 |
ADM™ and MDM™, Stryker Orthopaedics, Mahwah, NJ, USA.
Fig. 7.
The subhemispheric implant had the highest overall motion in all three planes across all component designs tested. (ADM™ and MDM™, Stryker Orthopaedics, Mahwah, NJ, USA).
Table 4.
Statistical comparison of the prosthetic impingement-free ROM between various component designs*
| Component | 28-mm hemispheric fixed bearing | 36-mm hemispheric fixed bearing | 48-mm subhemispheric dual mobility | 48-mm anatomic dual mobility | 46-mm modular dual mobility |
|---|---|---|---|---|---|
| 28-mm hemispheric fixed bearing | N/A | ||||
| 36-mm hemispheric fixed bearing | p < 0.001; R2 = 0.9295% CI, −6.2 to 3.1 | N/A | |||
| 48-mm subhemispheric dual mobility | p < 0.001; R2 = 0.91; 95% CI, −30.5 to −14.8 | p < 0.001; R2 = 0.91; 95% CI, −24.3 to −11.7 | N/A | ||
| 48-mm Anatomic Dual Mobility (ADM™) | p = 0.71; R2 = 0.03; 95% CI, −8.7 to 6.3 | p = 0.3; R2 = 0.24; 95% CI, −3.6 to 10.6 | p = 0.001; R2 = 0.89; 95% CI, 12.9–30.1 | N/A | |
| 46-mm modular dual mobility (MDM™) | p = 0.22; R2 = 0.3; 95% CI, −0.7 to 2.3 | p < 0.001; R2 = 0.91; 95% CI, 3.54–7.46 | p < 0.001; R2 = 0.92; 95% CI, 15.6–31.3 | p = 0.55; R2 = 0.1; 95% CI, −5.9 to 9.9 | N/A |
* Paired 2-tailed t-test; N/A = not available; ADM™ and MDM™, Stryker Orthopaedics, Mahwah, NJ, USA.
Discussion
Technologic improvements in THA component designs have led to the development of new dual-mobility designs during the past few years. Various dual-mobility designs have been marketed with purported improvements in stability, without compromising ROM compared with conventional 28-mm THA bearings. Although conceptually all dual-mobility designs should reduce the risk of dislocation owing to the improvement in their effective head diameters and thus the jump distances, these changes may not translate into a reduction in dislocation in vivo. This potentially is related to the effect of multiple factors on dislocation risks other than head size or the stable arc of motion of an implant’s bearing couple. In addition, the efficacy of some of these newer designs with varying geometries has not been conclusively proven in terms of increasing jump distances. Previous studies showed that the posterior horizontal dislocation distances and prosthetic impingement-free ROM may vary for different component designs [12, 20]. Therefore, in this study, we aimed to compare the posterior horizontal dislocation distances and the prosthetic impingement-free ROMs of three new dual-mobility designs with those of standard components to determine whether differences in component geometries alter these measurements, which thereby may affect their resistance to dislocation. We found cup design had a much greater effect on posterior horizontal dislocation distance than pure head size (p < 0.001). Moreover, using a subhemispheric dual-mobility design was found to have no significant benefit in increasing the posterior horizontal dislocation distance compared with a hemispheric fixed-bearing design (p = 0.474). Of all tested components, the anatomic and the modular dual mobility provided significantly greater posterior horizontal dislocation distances than all other designs.
There were several limitations of this study. In the hip model we used, measurements of prosthetic impingement-free ROM did not take into consideration the static or dynamic effects of bony or soft tissue impingement on ROM and dislocation. The 3-D simulation model considers a simple translational mechanism for evaluating potential risks for pure posterior dislocation among the various component designs using a predetermined computer algorithm. Although dislocations can occur in directions other than posterior in various clinical scenarios, we did not evaluate them in this study. In addition, no evaluation was made of effect of elevated posterior liners that might prevent posterior dislocations but may cause impingement on extremes of motion with the stem. Moreover, the study does not take into consideration the role of femoral anteversion, femoral neck geometry, soft tissue tension, horizontal offset, soft tissue stabilizers, and abductor muscle function in preventing dislocations and the relative contribution of the weight of posterior horizontal dislocation distance and prosthetic impingement-free ROM in estimating the risks of instability. It is also difficult to speculate from the findings of the study whether posterior horizontal dislocation distance and prosthetic impingement-free ROM in flexion-extension, abduction-adduction, and internal and external rotation in neutral flexion-only correlates with dislocations in clinical scenarios. The model assumes that dislocations occur in a purely posterior direction rather than in a posterosuperior or a more generalized posterior direction. Joint laxity was not assessed in our study, and pelvic tilt, which is known to vary widely among individuals and with changes in posture, was held constant in this model. Furthermore, the posterior horizontal dislocation distances and prosthetic impingement-free ROMs in less than optimal cup placements (which were not evaluated in this study) among the various dual-mobility designs may differ. However, the results of our study are valuable, because to our knowledge, this is the first study that has compared the posterior horizontal dislocation distance across the different dual-mobility designs, using CT-based 3-D modeling to predict the posterior horizontal dislocation distance, prosthetic impingement-free ROM, and consequently the risk of dislocation.
Previous studies showed that 3-D modeling can provide an accurate estimate of the risks of dislocation across various component designs from measurements of posterior horizontal dislocation distance (3-D jump distance) and prosthetic impingement-free ROM [20, 21, 27]. However, to our knowledge, no previous clinical or biomechanical studies have compared the risk of dislocation among various dual-mobility designs. Nevelos et al. [20], in a hip modeling study, compared the 3-D jump distances among standard, resurfacing, and anatomic dual-mobility bearings. They found that an anatomic design provided the greatest posterior horizontal dislocation distance across all positions and activities. They also found that the posterior horizontal dislocation distance increased with increasing head sizes and inclination angles between 30° and 60°. They further reported that minimal differences in posterior horizontal dislocation distances were found between 28- and 36-mm bearings. In the current study, we found that the 3-D jump distances (posterior horizontal dislocation distance) can differ across various dual-mobility and conventional hemispheric fixed-bearing designs. Regardless of head sizes, the anatomic dual-mobility design provided the greatest posterior horizontal dislocation distance in comparison to the modular, subhemispheric dual-mobility and standard hemispheric fixed-bearing designs in cup sizes ranging between 48 to 60 mm. The lower posterior horizontal dislocation distances observed with the subhemispheric dual-mobility bearings may be attributable to the positive offset of the head center in relation to the opening plane of the cup with this 165° subspheric design. Improved head coverage with the modular and anatomic designs (> 180° in certain regions) results in a 2-mm or neutral head offset, respectively, which improves the posterior horizontal dislocation distances and potentially decreases the risk of dislocation.
Although it generally is believed that large head diameters decrease the risk of dislocation in THA through improvement in translational distances needed to dislodge the femoral head from the socket, few authors have quantified the improvements in jump distances achieved with incremental head diameters [20, 27]. Nevelos et al. [20], in a 3-D CT modeling study, found that the posterior horizontal dislocation distances increased with increasing head sizes with standard THA designs. However, they found that the beneficial effects of increased head diameters were more obvious at greater acetabular inclination angles (60°) compared with lower cup abduction angles (30°). Sariali et al. [27], in a two-dimensional mathematical model, evaluated the effect of increasing head diameters on jump distance with standard bearings. They also reported improvement in jump distances with increasing head diameters from 22 to 36 mm. The jumping distance was found to increase by 0.4 mm for every 1-mm increase in head size at cup inclination angles of 45°. However, they found only marginal improvement in jump distances between a 36- and a 48-mm head size (14.1 versus 15.8 mm; 12% increase). We also found that there was a positive correlation between increase in head sizes and posterior horizontal dislocation distances, affirming larger heads result in increasingly stable joints, with only minor improvement in the 3-D jump distances between 36- and 40-mm head sizes with standard bearings.
Prosthetic impingement has been reported in previous computerized modeling studies as an important cause for wear and dislocations after THAs [1, 12, 18, 21]. Padgett et al. [21], in a computerized 3-D modeling study, reported that the prosthetic impingement-free ROM improved with increasing head sizes (from 22 to 28 mm; p = 0.03) and increased femoral anteversion. Similarly, we also found marked improvement in the posterior horizontal dislocation distance with increasing head sizes across all dual-mobility and standard acetabular components (r ≥ 0.99). We also found that the prosthetic impingement-free ROMs of the anatomic and modular dual-mobility designs were comparable to those of standard 28- and 36-mm bearings with no considerable difference between these two dual-mobility designs. Klingenstein et al. [12], in a computerized 3-D model of motion analysis generated after obtaining segmentation CT data from five cadaveric femurs and acetabula, compared the ROM analysis of standard, resurfacing, and anatomic dual-mobility designs. Consistent with our findings on prosthetic impingement-free ROM, they reported no differences in the ROM between the anatomic dual-mobility and the standard 28- and 36-mm bearings.
We found that the posterior horizontal dislocation distances differed substantially among dual-mobility designs regardless of head size. The subhemispheric dual-mobility designs offered little improvement in the 3-D jump distances over standard hemispheric fixed-bearing designs (p = 0.474). Not surprisingly, an increase in head size did provide an increase in posterior horizontal dislocation distance for dual-mobility and standard bearings. The anatomic dual-mobility design provided the highest posterior horizontal dislocation distances compared with the standard hemispheric fixed-bearing (p < 0.001), subhemispheric dual-mobility (p < 0.001), and modular dual-mobility designs (p = 0.005). Moreover, this beneficial effect occurred without a marked decrease in the prosthetic impingement-free ROM. Further clinical studies are necessary to confirm if the improvement in posterior horizontal dislocation distances found with anatomic and dual-mobility designs in 3-D simulation studies translate to a reduction in dislocation rates in patients undergoing primary and revision THAs who have increased risk for dislocations. Further studies on systematic computerized simulation techniques incorporating mechanical and kinematic assessments evaluating various pelvic positions and the dynamic effect of joint loading may provide better insight in understanding the risk of dislocation with different component designs.
Footnotes
One of the authors certifies that he (MAM) or his institution has received or may receive payments or benefits, during the study period, an amount of USD (not less than USD 10,000). Three authors (CH, JM, and JN) are employees of Stryker Inc, Mahwah, NJ, USA.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at Stryker Orthopaedics Inc, Mahwah, NJ, USA.
References
- 1.Bader R, Willmann G. [Ceramic cups for hip endoprostheses. 6: Cup design, inclination and antetorsion angle modify range of motion and impingement][in German]. Biomed Tech (Berl). 1999;44:212–219. [DOI] [PubMed]
- 2.Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11:89–99. doi: 10.5435/00124635-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bauchu P, Bonnard O, Cypres A, Fiquet A, Girardin P, Noyer D. The dual-mobility POLARCUP: first results from a multicenter study. Orthopedics. 2008;31(12 suppl 2). pii: orthosupersite.com/view.asp?rID=37180. [PubMed]
- 4.Biomet. Active ArticulationTM Dual Mobility Hip System. Available at: http://www.biomet.com/orthopedics/productDetail.cfm?category=1&product=260. Accessed June 24, 2013.
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 6.Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don’t do. Clin Orthop Relat Res. 2004;429:102–107. doi: 10.1097/01.blo.0000150117.42360.f9. [DOI] [PubMed] [Google Scholar]
- 7.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility: a twelve-year follow-up study. Int Orthop. 1998;22:219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garbuz DS, Masri BA, Duncan CP, Greidanus NV, Bohm ER, Petrak MJ, Della Valle CJ, Gross AE. The Frank Stinchfield Award: Dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop Relat Res. 2012;470:351–356. doi: 10.1007/s11999-011-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamadouche M, Biau DJ, Huten D, Musset T, Gaucher F. The use of a cemented dual mobility socket to treat recurrent dislocation. Clin Orthop Relat Res. 2010;468:3248–3254. doi: 10.1007/s11999-010-1404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton WG, McAuley JP. Evaluation of the unstable total hip arthroplasty. Instr Course Lect. 2004;53:87–92. [PubMed] [Google Scholar]
- 11.Jameson SS, Lees D, James P, Serrano-Pedraza I, Partington PF, Muller SD, Meek RM, Reed MR. Lower rates of dislocation with increased femoral head size after primary total hip replacement: a five-year analysis of NHS patients in England. J Bone Joint Surg Br. 2011;93:876–880. doi: 10.1302/0301-620X.93B7.26657. [DOI] [PubMed] [Google Scholar]
- 12.Klingenstein GG, Yeager AM, Lipman JD, Westrich GH. Computerized range of motion analysis following dual mobility total hip arthroplasty, traditional total hip arthroplasty, and hip resurfacing. J Arthroplasty. 2013;28:1173–1176. doi: 10.1016/j.arth.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Kostensalo I, Junnila M, Virolainen P, Remes V, Matilainen M, Vahlberg T, Pulkkinen P, Eskelinen A, Makela KT. Effect of femoral head size on risk of revision for dislocation after total hip arthroplasty: a population-based analysis of 42,379 primary procedures from the Finnish Arthroplasty Register. Acta Orthop. 2013;84:342–347. doi: 10.3109/17453674.2013.810518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 15.Loving L, Lee RK, Herrera L, Essner AP, Nevelos JE. Wear performance evaluation of a contemporary dual mobility hip bearing using multiple hip simulator testing conditions. J Arthroplasty. 2013;28:1041–1046. doi: 10.1016/j.arth.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Lyons MC, MacDonald SJ. Dual poly liner mobility optimizes wear and stability in THA: opposes. Orthopedics. 2011;34:e449–451. doi: 10.3928/01477447-20110714-24. [DOI] [PubMed] [Google Scholar]
- 17.Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21–25. doi: 10.1016/j.arth.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech (Bristol, Avon). 2013;28:182–186. [DOI] [PubMed]
- 19.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. doi: 10.1016/S0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 20.Nevelos J, Johnson A, Heffernan C, Macintyre J, Markel DC, Mont MA. What factors affect posterior dislocation distance in THA? Clin Orthop Relat Res. 2013;471:519–526. doi: 10.1007/s11999-012-2559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padgett DE, Lipman J, Robie B, Nestor BJ. Influence of total hip design on dislocation: a computer model and clinical analysis. Clin Orthop Relat Res. 2006;447:48–52. doi: 10.1097/01.blo.0000218748.30236.40. [DOI] [PubMed] [Google Scholar]
- 22.Padgett DE, Warashina H. The unstable total hip replacement. Clin Orthop Relat Res. 2004;420:72–79. doi: 10.1097/00003086-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Kim KI, Goldberg G, Mallo G, Hozack WJ. Recurrent instability after total hip arthroplasty: beware of subtle component malpositioning. Clin Orthop Relat Res. 2006;447:60–65. doi: 10.1097/01.blo.0000218749.37860.7c. [DOI] [PubMed] [Google Scholar]
- 24.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot F, Curvale G, Farizon F. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res. 2009;95:407–413. doi: 10.1016/j.otsr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. Survival of cementless dual mobility socket with a mean 17 years follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:e23–27. doi: 10.1016/j.rco.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Robinson M, Bornstein L, Mennear B, Bostrom M, Nestor B, Padgett D, Westrich G. Effect of restoration of combined offset on stability of large head THA. Hip Int. 2012;22:248–253. doi: 10.5301/HIP.2012.9283. [DOI] [PubMed] [Google Scholar]
- 27.Sariali E, Lazennec JY, Khiami F, Catonne Y. Mathematical evaluation of jumping distance in total hip arthroplasty: influence of abduction angle, femoral head offset, and head diameter. Acta Orthop. 2009;80:277–282. doi: 10.3109/17453670902988378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroh DA, Issa K, Johnson AJ, Delanois RE, Mont MA. Reduced dislocation rates and excellent functional outcomes with large-diameter femoral heads. J Arthroplasty. 2013;28:1415–1420. doi: 10.1016/j.arth.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Stryker. Stryker Mobile Bearing Hip™ System. Available at: http://www.stryker.com/en-us/products/Orthopaedics/HipReplacement/Acetabular/mobilebearing/139617. Accessed June 24, 2013.
- 30.Vielpeau C, Lebel B, Ardouin L, Burdin G, Lautridou C. The dual mobility socket concept: experience with 668 cases. Int Orthop. 2011;35:225–230. doi: 10.1007/s00264-010-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]