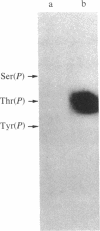
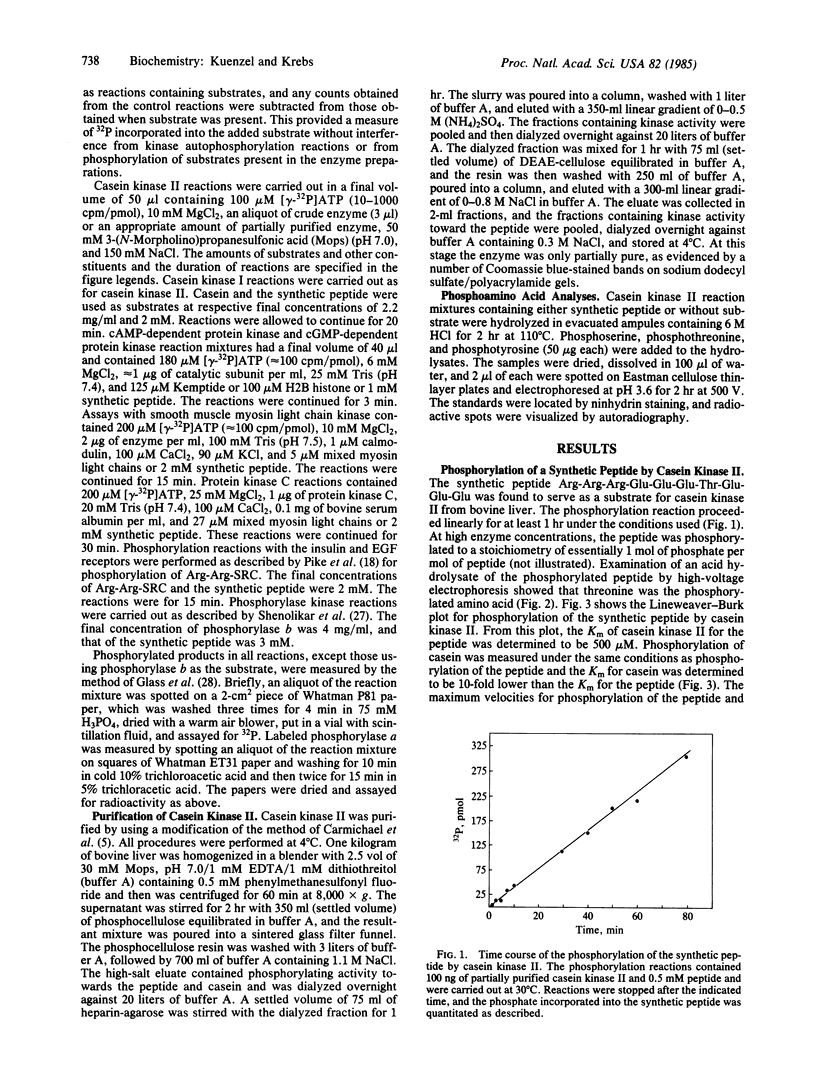
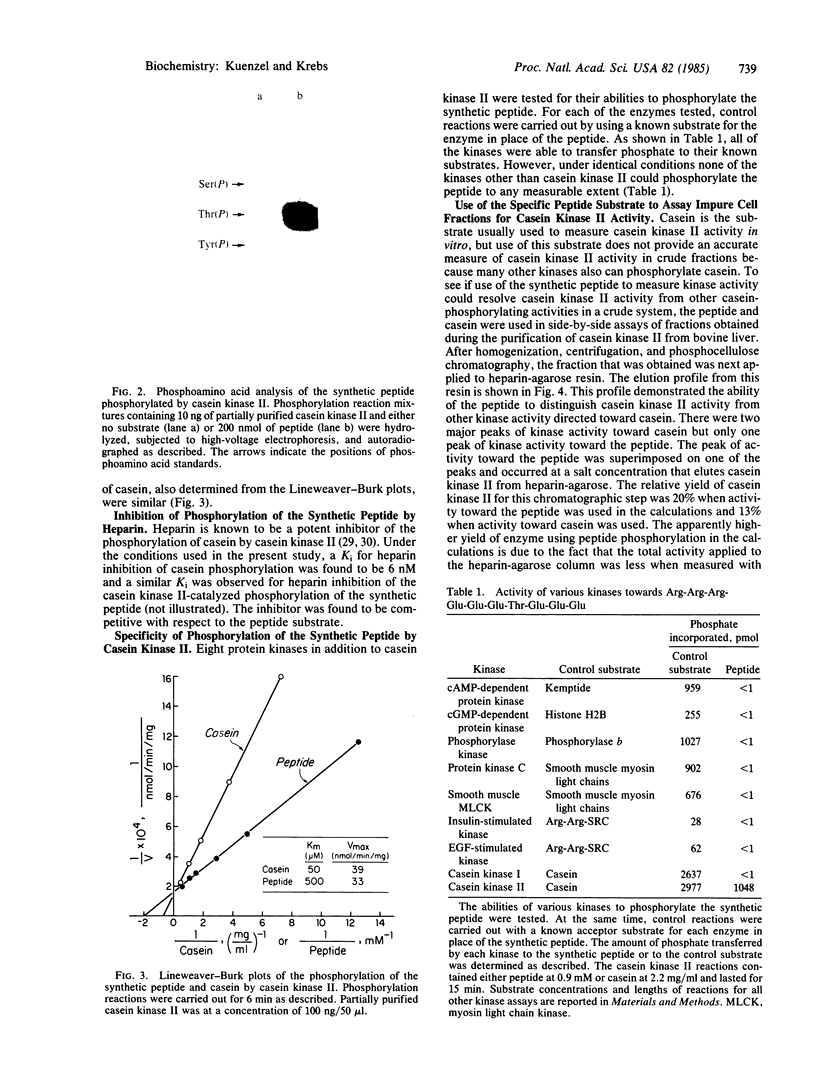
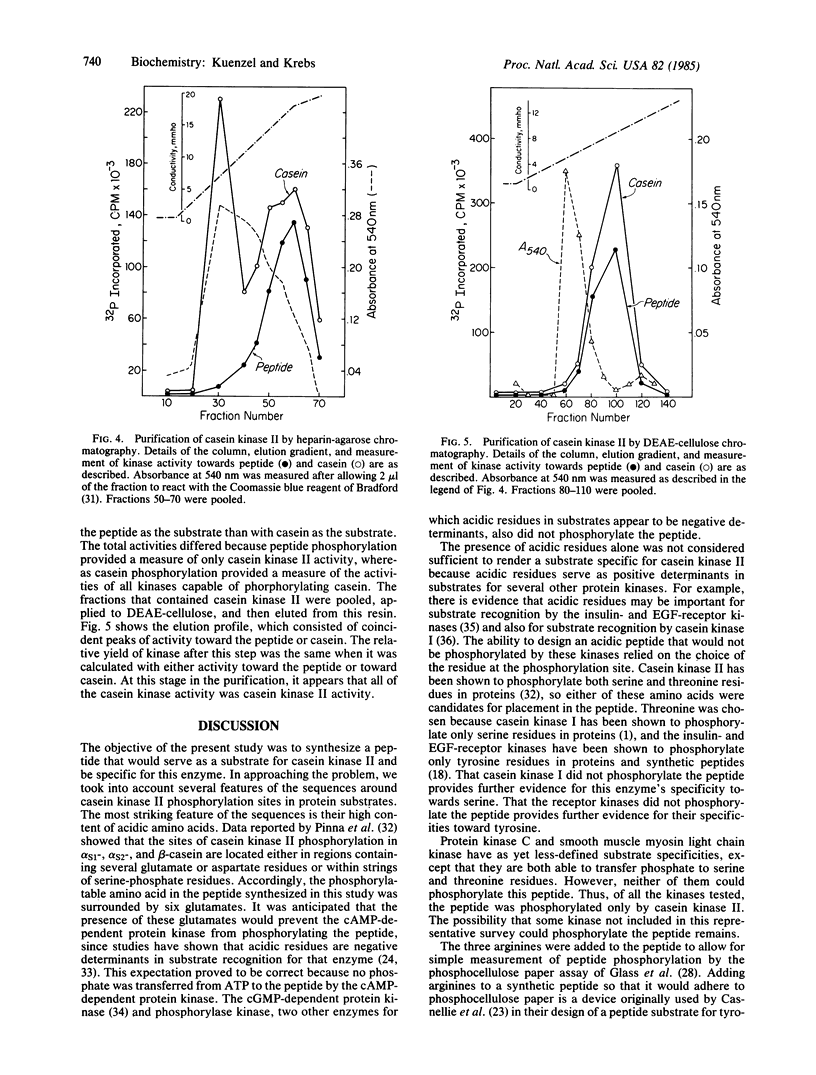
Abstract
A synthetic peptide having the sequence Arg-Arg-Arg-Glu-Glu-Thr-Glu-Glu-Glu was found to serve as a convenient substrate for the protein kinase generally referred to as casein kinase II. The enzyme exhibited an apparent Km of 500 microM for the peptide, as compared to an apparent Km of 50 microM for casein. The maximum velocities for phosphorylation of the peptide and of casein were similar. The peptide was not phosphorylated by any of eight other protein kinases, all of which were shown to be active toward their known substrates. The peptide was used to monitor activity during steps in the purification of casein kinase II from bovine liver. These experiments demonstrated that with this peptide it is now possible to obtain specific measurements of casein kinase II activity in crude enzyme preparations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camici M., Ahmad Z., DePaoli-Roach A. A., Roach P. J. Phosphorylation of rabbit liver glycogen synthase by multiple protein kinases. J Biol Chem. 1984 Feb 25;259(4):2466–2473. [PubMed] [Google Scholar]
- Carmichael D. F., Geahlen R. L., Allen S. M., Krebs E. G. Type II regulatory subunit of cAMP-dependent protein kinase. Phosphorylation by casein kinase II at a site that is also phosphorylated in vivo. J Biol Chem. 1982 Sep 10;257(17):10440–10445. [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Krebs E. G. The use of synthetic peptides for defining the specificity of tyrosine protein kinases. Adv Enzyme Regul. 1984;22:501–515. doi: 10.1016/0065-2571(84)90028-1. [DOI] [PubMed] [Google Scholar]
- Cohen P., Yellowlees D., Aitken A., Donella-Deana A., Hemmings B. A., Parker P. J. Separation and characterisation of glycogen synthase kinase 3, glycogen synthase kinase 4 and glycogen synthase kinase 5 from rabbit skeletal muscle. Eur J Biochem. 1982 May;124(1):21–35. doi: 10.1111/j.1432-1033.1982.tb05902.x. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E. Phosphorylation of eukaryotic DNA-dependent RNA polymerase. Identification of calf thymus RNA polymerase subunits phosphorylated by two purified protein kinases, correlation with in vivo sites of phosphorylation in HeLa cell RNA polymerase II. J Biol Chem. 1981 Apr 10;256(7):3332–3339. [PubMed] [Google Scholar]
- Dahmus M. E. Purification and properties of calf thymus casein kinases I and II. J Biol Chem. 1981 Apr 10;256(7):3319–3325. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Roach P. J. Characterization of a rabbit skeletal muscle protein kinase (PC0.7) able to phosphorylate glycogen synthase and phosvitin. J Biol Chem. 1981 Sep 10;256(17):8955–8962. [PubMed] [Google Scholar]
- Erdmann H., Böcher M., Wagner K. G. Two protein kinases from nuclei of cultured tobacco cells with properties similar to the cyclic nucleotide-independent enzymes (NI and NII) from animal tissue. FEBS Lett. 1982 Jan 25;137(2):245–248. doi: 10.1016/0014-5793(82)80359-1. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Feramisco J. R., Krebs E. G. Inhibition of cyclic AMP-dependent protein kinase by analogues of a synthetic peptide substrate. J Biol Chem. 1978 Dec 25;253(24):8968–8971. [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Comparison of the substrate specificity of adenosine 3':5'-monophosphate- and guanosine 3':5'-monophosphate-dependent protein kinases. Kinetic studies using synthetic peptides corresponding to phosphorylation sites in histone H2B. J Biol Chem. 1979 Oct 10;254(19):9728–9738. [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Comparison of the substrate specificity of adenosine 3':5'-monophosphate- and guanosine 3':5'-monophosphate-dependent protein kinases. Kinetic studies using synthetic peptides corresponding to phosphorylation sites in histone H2B. J Biol Chem. 1979 Oct 10;254(19):9728–9738. [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Glover C. V., Shelton E. R., Brutlag D. L. Purification and characterization of a type II casein kinase from Drosophila melanogaster. J Biol Chem. 1983 Mar 10;258(5):3258–3265. [PubMed] [Google Scholar]
- Hathaway G. M., Lubben T. H., Traugh J. A. Inhibition of casein kinase II by heparin. J Biol Chem. 1980 Sep 10;255(17):8038–8041. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Itarte E., Singh T. J., Akatsuka A. Phosphorylation of glycogen synthase by cyclic AMP-independent casein kinase-2 from rabbit skeletal muscle. J Biol Chem. 1982 Mar 25;257(6):3236–3242. [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Kudlicki W., Grankowski N., Gasior E. Isolation and properties of two protein kinases from yeast which phosphorylate casein and some ribosomal proteins. Eur J Biochem. 1978 Mar 15;84(2):493–498. doi: 10.1111/j.1432-1033.1978.tb12191.x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Donella-Deana A., Pinna L. A. Studies on the structural requirements of a microsomal cAMP-independent protein kinase. FEBS Lett. 1979 Oct 1;106(1):76–80. doi: 10.1016/0014-5793(79)80698-5. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H. Effects of polyamines and polyanions on a cyclic nucleotide-independent and a cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1977 Jul 21;498(1):294–305. doi: 10.1016/0304-4165(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Pinna L. A., Donella-Deana A., Meggio F. Structural features determining the site specificity of a rat liver cAMP-independent protein kinase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):114–120. doi: 10.1016/0006-291x(79)91654-1. [DOI] [PubMed] [Google Scholar]
- Pinna L. A., Meggio F., Marchiori F., Borin G. Opposite and mutually incompatible structural requirements of type-2 casein kinase and cAMP-dependent protein kinase as visualized with synthetic peptide substrates. FEBS Lett. 1984 Jun 11;171(2):211–214. doi: 10.1016/0014-5793(84)80490-1. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Titani K., Ericsson L. H., Wade R. D., Fischer E. H., Walsh K. A. Homology of the gamma subunit of phosphorylase b kinase with cAMP-dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4185–4192. doi: 10.1021/bi00313a027. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Cohen P. T., Cohen P., Nairn A. C., Perry S. V. The role of calmodulin in the structure and regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1979 Oct 15;100(2):329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Cohen P. The substrate specificity of cyclic AMP-dependent protein kinase: amino acid sequences at the phosphorylation sites of herring protamine (clupeine). FEBS Lett. 1978 Feb 1;86(1):92–98. doi: 10.1016/0014-5793(78)80106-9. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A., Sharp S. B., Lundak T. S., Safer B., Merrick W. C. Effect of hemin on site-specific phosphorylation of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):789–793. doi: 10.1073/pnas.75.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper J. P., Bacon G. W., Witters L. A. Phosphorylation of acetyl-coenzyme A carboxylase by casein kinase I and casein kinase II. Arch Biochem Biophys. 1983 Dec;227(2):386–396. doi: 10.1016/0003-9861(83)90468-x. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Tipper J. P., Bacon G. W. Stimulation of site-specific phosphorylation of acetyl coenzyme A carboxylase by insulin and epinephrine. J Biol Chem. 1983 May 10;258(9):5643–5648. [PubMed] [Google Scholar]