Abstract
Background
The ideal local anesthetic regime for femoral nerve block that balances analgesia with mobility after total knee arthroplasty (TKA) remains undefined.
Questions/purposes
We compared two volumes and concentrations of a fixed dose of ropivacaine for continuous femoral nerve block after TKA to a single injection femoral nerve block with ropivacaine to determine (1) time to discharge readiness; (2) early pain scores and analgesic consumption; and (3) functional outcomes, including range of motion and WOMAC scores at the time of recovery.
Methods
Ninety-nine patients were allocated to one of three continuous femoral nerve block groups for this randomized, placebo-controlled, double-blind trial: a high concentration group (ropivacaine 0.2% infusion), a low concentration group (ropivacaine 0.1% infusion), or a placebo infusion group (saline 0.9% infusion). Infusions were discontinued on postoperative Day (POD) 2. The primary outcome was time to discharge readiness. Secondary outcomes included opioid consumption, pain, and functional outcomes. Ninety-three patients completed the study protocol; the study was halted early because of unanticipated changes to pain protocols at the host institution, by which time only 61% of the required number of patients had been enrolled.
Results
With the numbers available, the mean time to discharge readiness was not different between groups (high concentration group, 62 hours [95% confidence interval [CI], 51–72 hours]; low concentration group, 73 hours [95% CI, 63–83 hours]; placebo infusion group 65 hours [95% CI, 56–75 hours]; p = 0.27). Patients in the low concentration group consumed significantly less morphine during the period of infusion (POD 1, high concentration group, 56 mg [95% CI, 42–70 mg]; low concentration group, 35 mg [95% CI, 27–43 mg]; placebo infusion group, 48 mg [95% CI, 38–59 mg], p = 0.02; POD 2, high concentration group, 50 mg [95% CI, 41–60 mg]; low concentration group, 33 mg [95% CI, 24–42 mg]; placebo infusion group, 39 mg [95% CI, 30–48 mg], p = 0.04); however, there were no important differences in pain scores or opioid-related side effects with the numbers available. Likewise, there were no important differences in functional outcomes between groups.
Conclusions
Based on this study, which was terminated prematurely before the desired sample size could be achieved, we were unable to demonstrate that varying the concentration and volume of a fixed-dose ropivacaine infusion for continuous femoral nerve block influences time to discharge readiness when compared with a conventional single-injection femoral nerve block after TKA. A low concentration of ropivacaine infusion can reduce postoperative opioid consumption but without any important differences in pain scores, side effects, or functional outcomes. These pilot data may be used to inform the statistical power of future randomized trials.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Continuous femoral nerve blockade provides excellent postoperative analgesia for patients undergoing total knee arthroplasty (TKA) [20] with fewer side effects than intravenous opioids [6, 20] or epidural infusions [6, 24]. However, the ideal local anesthetic regime for femoral nerve block that balances pain relief with mobility after TKA, whether in the form of a continuous perineural infusion or a single bolus injection, remains undefined [17]. The modern goals of early active mobilization and short in-patient admissions demand a reappraisal of the way in which femoral nerve block is used. Previous studies comparing equal volumes of different concentrations of local anesthetic in continuous femoral nerve block lack robust mobility data but do indicate that adequate analgesia can likely be achieved with concentrations of ropivacaine between 0.1% and 0.2% [5, 18]. However, the effects of varying the volume and concentration of a fixed dose of local anesthetic on analgesia and function are unexamined in the context of continuous femoral nerve block. The impact of single-injection femoral nerve block compared with continuous infusion also merits thoughtful reconsideration in the modern-day context of early mobilization pathways and multimodal analgesia, particularly in terms of different infusate concentrations and their effect on mobilization and rehabilitation [19, 20]. We therefore aimed to answer the question of whether or not varying the concentration and volume of a fixed dose of ropivacaine for continuous femoral nerve block after TKA, compared with a single-injection femoral nerve block, can (1) decrease patients’ time to discharge readiness; (2) decrease early pain scores and analgesic consumption; and (3) improve functional outcomes, including range of motion (ROM), and WOMAC scores at the time of recovery.
Patients and Methods
Study Design
This randomized, placebo-controlled, double-blind study was designed to investigate clinically relevant analgesic and functional outcomes after three different local anesthetic regimes for femoral nerve block after TKA. To explore the role of local anesthetic concentration, the first regime consisted of a continuous femoral nerve block using an infusion of ropivacaine 0.2%, whereas the second regime included a continuous femoral nerve block using an infusion of ropivacaine 0.1%. Both of the continuous femoral nerve block infusion regimes deliberately contained the same mass of ropivacaine per unit of time (10 mg per hour infusion, 10 mg boluses allowable every 30 minutes). To evaluate the role of mode of administration, the third regime comprised a single-injection femoral nerve block using ropivacaine 0.375% followed by a continuous infusion of a normal saline placebo. Each of these three regimes is described in detail subsequently (see Study Intervention). The present report follows the CONSORT guidelines [22].
Patient Demographics
Ethical approval was granted by the University Health Network Research Ethics Board (REB number 08-0895-B), and the trial was prospectively registered on Clinicaltrials.gov (NCT00803348). Between April 22, 2009, and October 17, 2011, all patients of American Society of Anesthesiologists physical status I to III, aged between 18 and 85 years scheduled to undergo primary unilateral TKA at Toronto Western Hospital, who were able to walk 30 m without stopping before surgery, were actively recruited to participate in this study. Informed written consent was obtained. Exclusion criteria included intended discharge to an in-patient rehabilitation facility; patient refusal of femoral nerve block, spinal anesthetic, or sciatic nerve block; contraindications to regional anesthesia (eg, allergy to local anesthetics, coagulopathy); significant peripheral neuropathy or neurological disorder affecting the lower extremity; contraindication to a component of multimodal analgesia; pregnancy; opioid tolerance (chronic use of over 30 mg oxycodone or equivalent per day); cognitive or psychiatric condition that might affect patient assessment; and/or ability to provide informed consent.
Study Intervention
All nerve blocks were performed by a staff regional anesthesiologist or a regional anesthesia fellow under supervision of a staff regional anesthesiologist. The patients were positioned supine and standard monitors were applied. Midazolam at 1 to 4 mg intravenously was administered for anxiolysis as needed. The inguinal crease was sterilized with chlorhexidine 2% solution and the skin was infiltrated with 3 mL lidocaine 1%. Using a high-frequency linear array transducer (6–13 MHz, SonoSite M-Turbo; SonoSite, Inc, Bothell, WA, USA), a 17-gauge 90-mm insulated Tuohy needle was inserted out of plane with the ultrasound beam and advanced until the tip was deep to the fascia iliaca and superficial to the midpoint of the femoral nerve in the short-axis view. A catheter (StimuCath®; Arrow International, Inc, Reading, PA, USA) was then advanced through the needle along the longitudinal axis of the femoral nerve until the catheter tip was 2 to 4 cm beyond the needle tip. The catheter was secured using liquid skin adhesive. Immediately after catheter placement, 10 mL mepivacaine 2% was injected through the catheter. Ten minutes later, sensation to pinprick was evaluated in distribution of the femoral nerve. Only patients with evidence of loss of sensation to pinprick continued as study patients. All patients then received an ultrasound-guided sciatic nerve block and a spinal anesthetic in line with institutional practice for TKA. Sciatic nerve block was performed in the lateral decubitus position at the subgluteal level as previously reported [7] using 30 mL ropivacaine 0.2%. Spinal anesthesia was performed in the sitting position with 2.5 to 3.0 mL isobaric bupivacaine 0.5% and 0.1 mg intrathecal morphine.
In the postanesthesia care unit (PACU), patients were randomized to receive one of three local anesthetic infusion regimes. Patients in the first experimental group (high concentration group) received a bolus of 20 mL ropivacaine 0.2% with epinephrine 1:400,000 into the femoral catheter followed by ropivacaine 0.2% at a rate of 5 mL/h with patient-controlled boluses of 5 mL available every 30 minutes. Patients in the second experimental group (low concentration group) received the same bolus of 20 mL ropivacaine 0.2% with epinephrine 1:400,000 into the femoral catheter followed by ropivacaine 0.1% at a rate of 10 mL/h with patient-controlled boluses of 10 mL available every 30 minutes. Patients in the control group (placebo infusion group) received a bolus of 30 mL ropivacaine 0.375% with epinephrine 1:400,000 into the femoral catheter [21] followed by normal saline at a rate of 1 mL/h with patient-controlled boluses of 1 mL available every 30 minutes.
Randomization
Because of the possibility of preoperative quadriceps muscle strength and function impacting on postoperative strength and functional recovery, patients were stratified according to these two parameters before assignment to a treatment group. Quadriceps strength in the operative extremity was measured using an isometric force dynamometer (microFET2™; HOGGAN Health Industries, Inc, Salt Lake City, UT, USA) and a functional baseline was obtained using the WOMAC score [3]. The threshold levels for stratification were: WOMAC = 54.5 and quadriceps strength = 16.34 lbs. If the WOMAC score was above 54.5, the patient was designated to be in the high WOMAC group; if equal or less, then the patient was designated to be in the low WOMAC group. Also, if the quadriceps strength was above 16.34, the patient was designated to be in the high quadriceps strength group; if equal or less, then the patient was designated to be in the low quadriceps strength group. Therefore, there were four possible stratification groups: high WOMAC/high quadriceps strength; high WOMAC/low quadriceps strength; low WOMAC/high quadriceps strength; and low WOMAC/low quadriceps strength. Patients were randomly allocated into one of the three study groups according to a computer-generated list of random numbers with randomization taking this stratification into account so that there were approximately equal numbers of patients with high or low muscle strength and high or low WOMAC scores in each study group. The pharmacy department established the randomization schedule.
Characteristics of the 93 patients were similar between groups (Table 1).
Table 1.
Patient characteristics
| High concentration group | Low concentration group | Placebo infusion group | p value | |
|---|---|---|---|---|
| Number | 28 | 32 | 33 | |
| Age (years) | 61 (57–64) | 63 (60–67) | 63 (60–66) | 0.58 |
| Sex (female/male) | 13/15 | 14/18 | 17/16 | 0.82 |
| Height (cm) | 169 (165–172) | 169 (167–172) | 170 (167–174) | 0.76 |
| Weight (kg) | 91 (84–98) | 92 (86–99) | 90 (83–96) | 0.83 |
| Body mass index (kg/m2) | 32 (30–34) | 32 (30–34) | 31 (29–33) | 0.63 |
| ASA status I/II/III | 2/12/14 | 0/13/19 | 2/21/10 | 0.12 |
| Surgery duration (minutes) | 100 (94–106) | 98 (92–103) | 97 (92–102) | 0.84 |
Data are presented as mean (95% confidence interval) or absolute number as appropriate. High concentration group, infusion of ropivacaine 0.2%; low concentration group, infusion of ropivacaine 0.1%; placebo infusion group, infusion of normal saline 0.9%; ASA = American Society of Anesthesiologists.
Blinding
An attending anesthesiologist, who was unrelated to the study and blinded to group allocation, administered the initial bolus dose through the catheter in the PACU. All local anesthetic solutions administered postoperatively as part of the study intervention were prepared by the hospital pharmacy in identical size syringes and infusate bags and concealed to maintain double-blinding. A nurse unrelated to the study programmed the infusion pumps according to group allocation and covered the pump with an opaque bag. Physiotherapists, surgeons, research assistants collecting data, and members of the Acute Pain Service were kept blinded to group allocation. When necessary, infusion bags were replaced by nurses who had been instructed not to communicate group allocation information to patients or other healthcare providers.
Postoperative Treatment
All patients received our institutional standard multimodal analgesia of 1 g acetaminophen every 6 hours, 200 mg celecoxib every 12 hours (100 mg if age older than 65 years or weight less than 70 kg), 200 mg gabapentin every 8 hours (100 mg if age older than 65 years), 10 mg oxycodone controlled release every 8 hours (5 mg if age older than 65 years), and 10 mg oxycodone immediate release every 2 hours as required (5 mg if age older than 65 years). In case of sulpha allergy, 15 mg meloxicam daily (7.5 mg if age older than 65 years) was substituted instead of celecoxib.
Follow-up Routine
Discharge readiness criteria were reviewed after morning and afternoon physical therapy sessions. Early functional and analgesic outcomes were measured twice daily up to and including postoperative Day (POD) 3 during and after physical therapy sessions. Later functional outcomes were measured at 6 weeks, 3 months, and 12 months postoperatively.
The Acute Pain Service visited patients twice daily. If deemed necessary, the oral opioid dose was increased to achieve a visual analog scale (VAS) score for pain of 4 or less. In the event of inadequate pain control such that the VAS pain score was greater than 7, intravenous morphine patient-controlled analgesia was started. If patients had severe pain despite these measures, their group allocation was unblinded, and the continuous femoral nerve block was dosed with ropivacaine as judged clinically necessary by the Acute Pain Service in line with institutional practice. These patients were analyzed on an intention-to-treat basis according to the group to which they were assigned. In the event that pain was well controlled, but a patient had a significant motor block or failed to walk at their first or subsequent physiotherapy sessions, it was assumed that the continuous femoral nerve block was contributory, and both the continuous infusion and bolus volumes were halved. This provided a standardized clinical strategy to reduce the dose in the continuous perineural catheter-based infusions. The continuous femoral nerve block infusion was discontinued at 6:00 am on POD 2.
Outcome Measures
The primary outcome was the time to discharge readiness, defined as the duration of time (in hours) from patient arrival in the PACU until the achievement of all of the following criteria [14]: (1) walk 30 m (wheeled walker allowed); (2) no parenteral opioids for at least 12 hours; and (3) resting VAS pain score less than or equal to 4. In the event that a patient was discharged from the hospital before meeting these criteria, the actual discharge time from the hospital was considered as the time to discharge readiness. Secondary outcomes were divided into analgesic and functional outcomes. Analgesic outcomes included opioid consumption on POD 1, 2, and 3 (mg, converted to equianalgesic intravenous morphine [25]), pain (VAS) at rest and during physiotherapy, incidence of opioid-related side effects (occurrence of nausea and vomiting, pruritus, and drowsiness), and satisfaction with analgesia (VAS). Early functional outcomes included distance walked (meters) during physiotherapy, active and passive knee flexion (degrees, measured by goniometer), maximum quadriceps strength (pounds, measured by isometric force dynamometer), and the occurrence of falls.
Later functional outcomes were measured using the WOMAC [3] and SF-36 health survey [4].
Statistical Analysis
Primary outcome and other continuous data were compared across groups using an analysis of variance. Noncontinuous data were compared using a Pearson’s test. Data are presented as mean along with the 95% confidence intervals (95% CI), absolute numbers, or percentages as appropriate. Kaplan-Meier life table analysis (survival analysis) was performed for effect of mode of administration on time to discharge readiness and a log-rank test was applied. Significance was considered at p < 0.05. Statistical analyses were performed using the JMP 9 statistical package (SAS Institute, Cary, NC, USA).
Power Analysis
Based on our institutional database, patients who had undergone TKA at Toronto Western Hospital in 2008 were discharged from the hospital to home after a mean (SD) of 100 (36) hours. This value excluded patients for whom discharge to a rehabilitation facility was planned. We considered a clinically significant difference in time to discharge readiness to be 20%. A total of 153 patients, 51 patients in each group, was required to detect an effect size of 0.5 SDs in the time to discharge readiness between the two experimental groups with a power of 80% using a two-sided p = 0.05 level test. Allowing for approximately a 10% incomplete followup or patient dropout, we planned to enroll 56 patients per group for a total of 168 patients.
Premature Study Termination
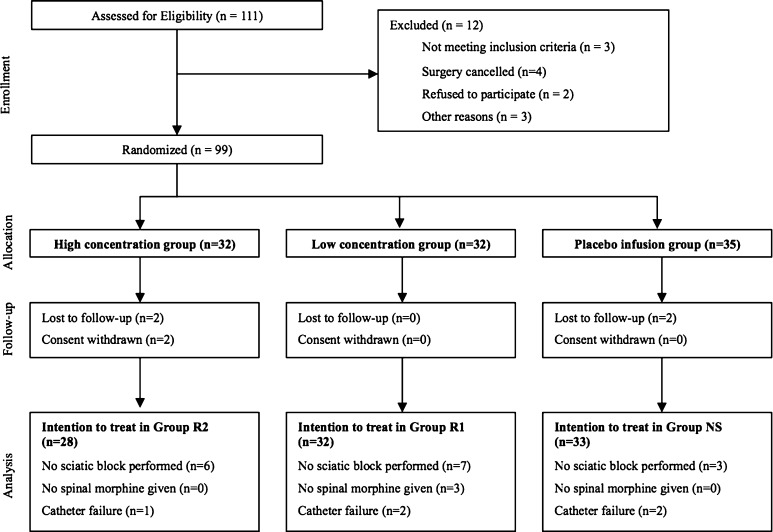
Recruitment was halted after the enrollment of 99 patients (59% of the desired 168) as a result of an unanticipated change in standard practice at our home institution such that single-injection sciatic nerve blocks were no longer routinely administered for postoperative analgesia after TKA. Six patients were lost to followup or withdrew consent (Fig. 1) without a significant difference in loss between groups (p = 0.11). Sixteen patients had no sciatic nerve block because of protocol violations related to time constraints in the operating room. Five patients had primary catheter failure after the mepivacaine bolus. Spinal morphine was withheld from three patients on suspicion of obstructive sleep apnea by the provider anesthesiologist. The group allocations of one patient in the high concentration group and one patient in the placebo infusion group were unblinded because of severe pain on POD 0 and POD 1, respectively. Both of these patients received a rescue bolus of 10 mL ropivacaine 0.2% followed by an infusion of 0.2% ropivacaine at a rate of 5 mL/h with patient-controlled boluses of 5 mL available every 30 minutes. The data collected from all these patients were analyzed on an intention-to-treat basis.
Fig. 1.
This diagram presents a patient flow chart.
With the numbers available (61% of the required 153, according to our a priori sample size calculation), we had 39% power to detect a difference of 11 hours between groups in terms of time to discharge readiness.
Results
Time to Discharge Readiness
With the numbers available, we detected no differences in the time to discharge readiness among the three groups (high concentration group, 62 hours [95% CI, 51–72 hours]; low concentration group, 73 hours [95% CI, 63–83 hours]; placebo infusion group, 65 hours [95% CI, 56–75 hours]; p = 0.27).
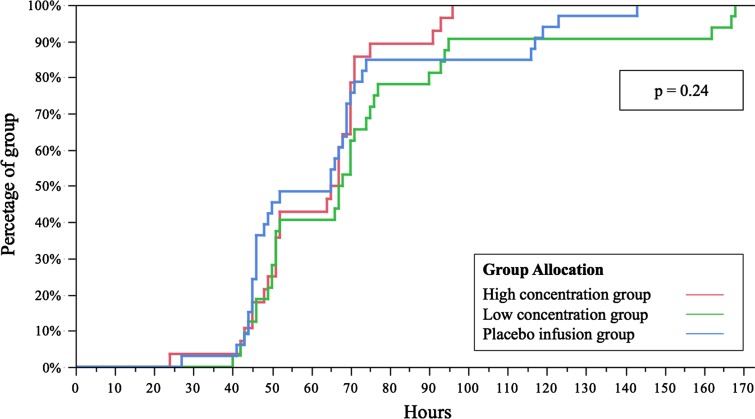
The proportion of patients in each group who met discharge readiness criteria did not differ between groups at any time postoperatively (Fig. 2; p = 0.24).
Fig. 2.
This survival analysis curve describes the effect of mode of administration on time to discharge readiness. High concentration group, infusion of ropivacaine 0.2%; low concentration group, infusion of ropivacaine 0.1%; and placebo infusion group, infusion of normal saline 0.9%.
Analgesic Outcomes
Patients in the low concentration group consumed significantly less morphine during the period of infusion (POD 1, high concentration group, 56 mg [95% CI, 42–70 mg]; low concentration group, 35 mg [95% CI, 27–43 mg]; placebo infusion group, 48 mg [95% CI, 38–59 mg], p = 0.02; POD 2, high concentration group, 50 mg [95% CI, 41–60 mg]; low concentration group, 33 mg [95% CI, 24–42 mg]; placebo infusion group, 39 mg [95% CI, 30–48 mg], p = 0.04); however, there were no important differences in pain scores or opioid-related side effects with the numbers available (Table 2).
Table 2.
Analgesic outcomes on postoperative Day (POD) 1, 2, and 3
| Analgesic outcome | High concentration group | Low concentration group | Placebo infusion group | p value |
|---|---|---|---|---|
| Opioid consumption (intravenous morphine equivalent, mg) | ||||
| POD 1 | 56 (42–70) | 35 (27–43) | 48 (38–59) | 0.02* |
| POD 2 | 50 (41–60) | 33 (24–42) | 39 (30–48) | 0.04* |
| POD 3 | 27 (20–35) | 18 (12–25) | 21 (14–28) | 0.18 |
| Pain at rest (VAS) | ||||
| POD 1 am | 5 (4–6) | 4 (3–5) | 5 (4–6) | 0.17 |
| POD 1 pm | 5 (4–6) | 4 (3–5) | 5 (4–6) | 0.37 |
| POD 2 am | 4 (3–5) | 3 (2–4) | 3 (2–4) | 0.60 |
| POD 2 pm | 4 (3–5) | 2 (1–3) | 3 (2–4) | 0.03* |
| POD 3 am | 2 (2–3) | 2 (1–3) | 2 (1–3) | 0.76 |
| POD 3 pm | 2 (2–3) | 3 (2–4) | 2 (1–4) | 0.93 |
| Pain during physiotherapy (VAS) | ||||
| POD 1 am | 7 (6–8) | 7 (6–7) | 7 (6–8) | 0.65 |
| POD 1 pm | 7 (5–8) | 6 (5–7) | 6 (6–7) | 0.56 |
| POD 2 am | 7 (6–8) | 6 (5–7) | 7 (6–7) | 0.67 |
| POD 2 pm | 6 (5–7) | 5 (4–6) | 6 (5–7) | 0.50 |
| POD 3 am | 6 (5–7) | 5 (4–6) | 5 (4–6) | 0.54 |
| POD 3 pm | 6 (5–7) | 5 (4–7) | 5 (4–7) | 0.86 |
| Nausea and vomiting (incidence) | ||||
| POD 1 am | 40% | 33% | 50% | 0.76 |
| POD 1 pm | 12% | 7% | 14% | 0.73 |
| POD 2 am | 17% | 3% | 18% | 0.23 |
| POD 2 pm | 33% | 10% | 32% | 0.21 |
| POD 3 am | 22% | 7% | 19% | 0.36 |
| POD 3 pm | 12% | 3% | 6% | 0.49 |
| Pruritus (incidence) | ||||
| POD 1 am | 27% | 14% | 27% | 0.58 |
| POD 1 pm | 33% | 11% | 27% | 0.28 |
| POD 2 am | 27% | 7% | 14% | 0.21 |
| POD 2 pm | 17% | 3% | 10% | 0.30 |
| POD 3 am | 12% | 7% | 14% | 0.69 |
| POD 3 pm | 17% | 0% | 10% | 0.10 |
| Drowsiness (incidence) | ||||
| POD 1 am | 40% | 19% | 32% | 0.47 |
| POD 1 pm | 17% | 29% | 32% | 0.60 |
| POD 2 am | 12% | 23% | 14% | 0.62 |
| POD 2 pm | 40% | 10% | 14% | 0.10 |
| POD 3 am | 12% | 3% | 10% | 0.49 |
| POD 3 pm | 12% | 0% | 14% | 0.13 |
| Satisfaction (VAS) | ||||
| POD 1 am | 6 (5–7) | 8 (7–8) | 6 (5–7) | 0.12 |
| POD 1 pm | 6 (5–7) | 7 (6–8) | 7 (7–8) | 0.08 |
| POD 2 am | 6 (5–7) | 7 (5–8) | 8 (7–9) | 0.06 |
| POD 2 pm | 6 (5–7) | 8 (7–9) | 8 (7–9) | 0.08 |
| POD 3 am | 7 (6–8) | 8 (7–9) | 9 (8–9) | 0.11 |
| POD 3 pm | 8 (7–9) | 8 (7–9) | 9 (8–9) | 0.18 |
Data are presented as mean (95% confidence interval) or percentage. Significant results are underscored by an asterisk. High concentration group, infusion of ropivacaine 0.2%; low concentration group, infusion of ropivacaine 0.1%; placebo infusion group, infusion of normal saline 0.9%; VAS = visual analog scale.
Functional Outcomes
With the numbers available, there were no important differences in any early or later functional outcome measures detected between groups (Table 3). One patient in the placebo infusion group fell on POD 1.
Table 3.
Early and later functional outcomes on postoperative Day (POD) 1, 2, 3, at 6 weeks, 3 months, and 12 months
| Functional outcome | High concentration group | Low concentration group | Placebo infusion group | p value |
|---|---|---|---|---|
| Distance walked (m) | ||||
| POD 1 am | 3 (0–6) | 4 (0–10) | 6 (0–11) | 0.71 |
| POD 1 pm | 8 (1–15) | 12 (3–21) | 14 (6–23) | 0.57 |
| POD 2 am | 39 (28–51) | 40 (28–51) | 44 (32–56) | 0.83 |
| POD 2 pm | 48 (37–57) | 55 (37–73) | 52 (41–63) | 0.72 |
| POD 3 am | 65 (55–75) | 66 (51–81) | 57 (45–69) | 0.48 |
| POD 3 pm | 54 (41–67) | 51 (33–68) | 47 (33–62) | 0.78 |
| Active flexion (degrees) | ||||
| POD 1 am | 61 (53–69) | 55 (47–63) | 56 (49–62) | 0.41 |
| POD 1 pm | 72 (63–80) | 66 (57–75) | 60 (53–67) | 0.09 |
| POD 2 am | 71 (63–79) | 61 (54–69) | 66 (58–72) | 0.18 |
| POD 2 pm | 75 (68–82) | 68 (62–75) | 64 (56–71) | 0.08 |
| POD 3 am | 79 (73–86) | 78 (71–85) | 71 (64–78) | 0.15 |
| POD 3 pm | 78 (67–89) | 78 (69–87) | 71 (63–78) | 0.39 |
| Passive flexion (degrees) | ||||
| POD 1 am | 86 (80–91) | 84 (79–89) | 76 (69–83) | 0.03* |
| POD 1 pm | 91 (85–96) | 92 (87–98) | 76 (70–82) | < 0.001* |
| POD 2 am | 86 (81–93) | 81 (76–86) | 81 (76–87) | 0.23 |
| POD 2 pm | 88 (83–93) | 86 (81–91) | 81 (73–88) | 0.18 |
| POD 3 am | 91 (85–96) | 87 (82–92) | 84 (79–89) | 0.15 |
| POD 3 pm | 88 (79–97) | 85 (81–88) | 81 (76–86) | 0.23 |
| Quadriceps strength (pounds) | ||||
| Preoperative | 18 (15–20) | 17 (15–20) | 17 (15–19) | 0.98 |
| POD 1 am | 4 (2–6) | 6 (4–8) | 5 (3–7) | 0.47 |
| POD 1 pm | 5 (3–7) | 6 (4–8) | 6 (4–7) | 0.67 |
| POD 2 am | 5 (4–7) | 6 (4–7) | 5 (4–7) | 0.78 |
| POD 2 pm | 6 (5–8) | 7 (5–9) | 6 (5–8) | 0.60 |
| POD 3 am | 8 (6–9) | 8 (6–11) | 7 (5–8) | 0.41 |
| POD 3 pm | 8 (6–10) | 11 (8–13) | 9 (7–11) | 0.14 |
| WOMAC score | ||||
| Preoperative | 44 (40–48) | 46 (41–51) | 43 (37–49) | 0.70 |
| 6 weeks | 30 (23–38) | 31 (25–36) | 33 (28–38) | 0.74 |
| 3 months | 22 (15–30) | 21 (16–25) | 24 (17–30) | 0.81 |
| 12 months | 17 (7–27) | 22 (14–30) | 18 (9–27) | 0.68 |
| SF-36 score | ||||
| Preoperative | 91 (86–97) | 91 (86–96) | 90 (84–97) | 0.96 |
| 6 weeks | 70 (58–81) | 69 (57–81) | 73 (63–83) | 0.89 |
| 3 months | 80 (68–91) | 75 (64–86) | 88 (78–98) | 0.17 |
| 12 months | 97 (90–104) | 102 (100–104) | 103 (100–106) | 0.07 |
Data are presented as mean (95% confidence interval). Significant results are underscored by an asterisk. High concentration group, infusion of ropivacaine 0.2%; low concentration group, infusion of ropivacaine 0.1%; placebo infusion group, infusion of normal saline 0.9%.
Discussion
TKA is associated with severe postoperative pain [9, 24]. Pain relief is essential for patient comfort, and neither rehabilitation nor discharge from the hospital is possible without adequate analgesia. However, modern clinical practice demands analgesia with function. Although the analgesic properties of continuous femoral nerve block after TKA are well recognized, continuous femoral nerve block has been associated with quadriceps weakness [2, 14] and may increase the risk of falls [13, 27]. Earlier trials supporting the analgesic efficacy of continuous femoral nerve block for TKA were based on a now outdated intention to cause motor block to reduce muscle spasm and facilitate passive motion rehabilitation techniques during prolonged in-patient admissions [6, 24]. Furthermore, previous studies comparing different formulations of femoral nerve block are variously retrospective [8], do not use complete multimodal analgesia [5, 8, 12, 21], or primarily address analgesic outcomes with little or no consideration of mobility or function [8, 12, 18, 26]. The multiplicity of differences in local anesthetic volumes and concentrations used for the femoral nerve block as well as differences in modes of administration and outcome measures make comparisons between studies and meaningful interpretation problematic. In the present study, we investigated whether altering the concentration and volume of a fixed dose of ropivacaine in a continuous femoral nerve block would affect time to discharge readiness after TKA compared with a conventional single-injection femoral nerve block. Our secondary questions were whether the concentration and volume of ropivacaine in continuous femoral nerve blocks would affect the quality of analgesia compared with a single-injection femoral nerve block, and finally, whether functional outcomes would be affected.
The present study has a number of limitations. First, and most importantly, our data must be interpreted with caution because this trial was terminated early, resulting in a much smaller sample size than intended and an increased likelihood that we failed to detect differences between treatment groups that might have been present. The premature termination resulted in only 61% of the planned sample size being recruited, resulting in a 39% power to detect a clinically relevant difference on our primary study end point of time to discharge readiness. The reason for early termination was a change in practice at our home institution such that sciatic nerve block was discontinued as a component of standard postoperative analgesia after TKA. This change was made after our review of the evidence found that the analgesic benefit of adding a sciatic nerve block to a femoral nerve block for TKA is questionable [1]. As such, recruitment for the present study was terminated, because a change in the standard postoperative analgesic regimen would introduce considerable bias and render the interpretation of our results problematic. Our sample size was further restricted by loss of four patients to followup and withdrawal of consent by two patients. Second, the multimodal analgesia treatment prescribed in our institution, including intrathecal morphine, opioids, gabapentin, NSAIDs, and acetaminophen, may mean that our data are not generalizable to other settings that do not use a similar approach. Finally, continuous infusion and bolus volumes were not routinely increased as part of the study because there was insufficient evidence in the literature for increasing the local anesthetic dose beyond what was provided [5, 14, 18]. Nonetheless, we believe our results are worthy of dissemination and thoughtful consideration as the combined analgesic and functional impact of different formulations of femoral nerve block has been very little studied up to now. Finally, despite the insufficient sample size, our results may serve as useful pilot data for future studies.
There is very little published evidence regarding the impact of different femoral nerve block formulations on time to discharge readiness. The present study replicated the discharge criteria used by Ilfeld and colleagues [14, 15] in two previous studies examining the effect of the duration of continuous femoral nerve block on time to discharge readiness after TKA. Patients with a continuous femoral nerve block were randomly allocated to a ropivacaine 0.2% infusion for 4 days or an identical infusion stopped on the morning of the first postoperative day. Ilfeld found that the time to discharge readiness in the 4-day infusion groups was significantly shorter than the comparison groups, a finding that appears to be at odds with the present study wherein we found similar times to discharge readiness across all three groups, including the placebo infusion group. It is noteworthy that, unlike our study, the patients in these two previous trials were all American Society of Anesthesiologists physical status II and were subject to strict exclusion criteria around preoperative analgesic use. For these reasons, we believe our results more closely represent the TKA population at large. Finally, unlike several other comparative studies of continuous and single-injection femoral nerve block after TKA [8, 21, 26], we purposefully avoided using length of hospital stay as an outcome measure, because this is notoriously influenced by multiple factors [16].
Another recurring problem that undermines comparisons of analgesic efficacy between previous studies and the present one is the inconsistency in the use of multimodal analgesia across study groups [5, 8, 12, 21]. Multimodal analgesia has been shown to improve analgesic and functional outcomes after total joint arthroplasty [11] and has been supported by a procedure-specific systematic review [9]. The multimodal analgesic regimen used here includes intrathecal morphine, peripheral nerve blockade, and three nonopioid oral analgesics and reflects the standard, evidence-based practice at our institution.
The effects of different formulations of femoral nerve block on functional end points other than time to discharge readiness or length of hospital stay have been studied very little, and many of the studies of femoral nerve block after TKA do not report any detail on postoperative mobility, muscle strength, or long-term functional outcomes [8, 12, 21, 23, 26]. Different measures of function also make comparisons between studies problematic. Consistency in functional measures, whether ROM, quadriceps strength, or questionnaire-based scales such as SF-36, is required for clinically meaningful, patient-centered results [10].
Based on this study, which was terminated prematurely before the desired sample size could be achieved, we were unable to demonstrate that varying the concentration and volume of a fixed-dose ropivacaine infusion for continuous femoral nerve block influences time to discharge readiness when compared with a conventional single-injection femoral nerve block after TKA. A low concentration of ropivacaine infusion can reduce postoperative opioid consumption but without any important differences in pain scores, side effects, or functional outcomes. These pilot data may be used to inform the statistical power of future randomized trials.
References
- 1.Abdallah FW, Brull R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A systematic review. Reg Anesth Pain Med. 2011;36:493–498. doi: 10.1097/AAP.0b013e318228d5d4. [DOI] [PubMed] [Google Scholar]
- 2.Barrington MJ, Olive D, Low K, Scott DA, Brittain J, Choong P. Continuous femoral nerve blockade or epidural analgesia after total knee replacement: a prospective randomized controlled trial. Anesth Analg. 2005;101:1824–1829. doi: 10.1213/01.ANE.0000184113.57416.DD. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 4.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodner G, Buerkle H, Van Aken H, Lambert R, Schweppe-Hartenauer ML, Wempe C, Gogarten W. Postoperative analgesia after knee surgery: a comparison of three different concentrations of ropivacaine for continuous femoral nerve blockade. Anesth Analg. 2007;105:256–262. doi: 10.1213/01.ane.0000265552.43299.2b. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Chan VW, Nova H, Abbas S, McCartney CJ, Perlas A, Xu DQ. Ultrasound examination and localization of the sciatic nerve: a volunteer study. Anesthesiology. 2006;104:309–314. doi: 10.1097/00000542-200602000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Duarte VM, Fallis WM, Slonowsky D, Kwarteng K, Yeung CK. Effectiveness of femoral nerve blockade for pain control after total knee arthroplasty. J Perianesth Nurs. 2006;21:311–316. doi: 10.1016/j.jopan.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Fischer HB, Simanski CJ, Sharp C, Bonnet F, Camu F, Neugebauer EA, Rawal N, Joshi GP, Schug SA, Kehlet H. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105–1123. doi: 10.1111/j.1365-2044.2008.05565.x. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi R, Liu SS, Brull R. What matters most. Reg Anesth Pain Med. 2013;38:257–258. doi: 10.1097/AAP.0b013e318298692e. [DOI] [PubMed] [Google Scholar]
- 11.Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, Hanssen AD, Horlocker TT. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. [PubMed] [Google Scholar]
- 12.Hirst GC, Lang SA, Dust WN, Cassidy JD, Yip RW. Femoral nerve block. Single injection versus continuous infusion for total knee arthroplasty. Reg Anesth. 1996;21:292–297. [PubMed] [Google Scholar]
- 13.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–713. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–484. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehlet H. Postoperative analgesia and hospital stay: a call for better study design. Anesth Analg. 2007;104:212. doi: 10.1213/01.ane.0000247702.97788.57. [DOI] [PubMed] [Google Scholar]
- 17.Morfey DH, Chan VW, Brull R. Tripping over perineural catheters. Anesth Analg. 2011;113:689–691. doi: 10.1213/ANE.0b013e31822dad0a. [DOI] [PubMed] [Google Scholar]
- 18.Paauwe JJ, Thomassen BJ, Weterings J, van Rossum E, Ausems ME. Femoral nerve block using ropivacaine 0.025%, 0.05% and 0.1%: effects on the rehabilitation programme following total knee arthroplasty: a pilot study. Anaesthesia. 2008;63:948–953. doi: 10.1111/j.1365-2044.2008.05538.x. [DOI] [PubMed] [Google Scholar]
- 19.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, Murthy Y. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–1162. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 20.Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, Cohen SR, Wu CL. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248–257. doi: 10.1213/01.ANE.0000181289.09675.7D. [DOI] [PubMed] [Google Scholar]
- 21.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seet E, Leong WL, Yeo AS, Fook-Chong S. Effectiveness of 3-in-1 continuous femoral block of differing concentrations compared to patient controlled intravenous morphine for post total knee arthroplasty analgesia and knee rehabilitation. Anaesth Intensive Care. 2006;34:25–30. doi: 10.1177/0310057X0603400110. [DOI] [PubMed] [Google Scholar]
- 24.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Skaer TL. Practice guidelines for transdermal opioids in malignant pain. Drugs. 2004;64:2629–2638. doi: 10.2165/00003495-200464230-00002. [DOI] [PubMed] [Google Scholar]
- 26.Soto Mesa D, Del Valle Ruiz V, Fayad Fayad M, Cosio Carreno F, Blanco Rodriguez I, Gonzalez Castano R, Bermejo Alvarez MA. [Control of postoperative pain in knee arthroplasty: single dose femoral nerve block versus continuous femoral block] [in Spanish]. Rev Esp Anestesiol Reanim. 2012;59:204–209. [DOI] [PubMed]
- 27.Wasserstein D, Farlinger C, Brull R, Mahomed N, Gandhi R. Advanced age, obesity and continuous femoral nerve blockade are independent risk factors for inpatient falls after primary total knee arthroplasty. J Arthroplasty. 2012 Dec 19 [Epub ahead of print]. [DOI] [PubMed]