Abstract
Background
Total joint arthroplasty (TJA) is one of the most widely performed elective procedures; however, there are wide variations in cost and quality among facilities where the procedure is performed.
Questions/purposes
The purposes of this study were to (1) develop a generalizable clinical care pathway for primary TJA using inputs from clinical, academic, and patient stakeholders; and (2) identify system- and patient-level processes to provide safe, effective, efficient, and patient-centered care for patients undergoing TJA.
Methods
We used a combination of quantitative and qualitative methods to design a care pathway that spans 14 months beginning with the presurgical office visit and concluding 12 months after discharge. We derived care suggestions from interviews with 16 hospitals selected based on readmission rates, cost, and quality (n = 10) and author opinion (n = 6). A 32-member multistakeholder panel refined the pathway during a 1-day workshop. Participants were selected based on leadership in orthopaedic (n = 4) and anesthesia (n = 1) specialty societies; involvement in organizations specializing in safety and high reliability care (n = 3), lean production/consumption of care (n = 3), and patient experience of care (n = 3); membership in an interdisciplinary care team of a hospital selected for interviewing (n = 8); recent receipt of a TJA (n = 1); and participation in the pathway development team (n = 9).
Results
The care pathway includes 40 suggested processes to improve care, 37 techniques to reduce waste, and 55 techniques to improve communication. Central themes include standardization and process improvement, interdisciplinary communication and collaboration, and patient/family engagement and education. Selected recommendations include standardizing care protocols and staff roles; aligning information flow with patient and process flow; identifying a role accountable for care delivery and communication; managing patient expectations; and stratifying patients into the most appropriate care level.
Conclusions
We developed a multidisciplinary clinical care pathway for patients undergoing TJA based on principles of high-value care. The pathway is ready for clinical testing and context-specific adaptation.
Level of Evidence
Level V, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
There is growing national interest in improving the value of total joint arthroplasties (TJA) of the hip and knee [6, 28]. Nearly one million TJAs are performed annually [21], and the frequency of these procedures increases by 10% to 15% per year [12, 20]. Despite the high volume of TJAs, there is substantial variation across facilities in adherence to evidence-based care processes, operative times, length of stay, discharge disposition, complication rates, patient-reported outcomes, and episode costs [9, 15, 19, 26, 30, 31].
Several tools have been developed to standardize care delivery and support modeling of care for different patient groups. For instance, evidence-based clinical practice guidelines produced by the American Academy of Orthopaedic Surgeons focus on improving TJA safety, including venous thromboembolism (VTE) prophylaxis and surgical site infection prevention [1–3, 17]. TJA clinical practice guidelines produced in Canada and the United Kingdom focus on care from initial assessment through outpatient rehabilitation and followup [5, 23]. A set of quality indicators has been developed to guide surgical practice [29], numerous care pathways target inpatient care [4], and some institution-specific pathways target the care continuum. For instance, the Dartmouth “GreenCare” model [11] implemented in 2011 addresses care from initial surgeon referral through 1 year after surgery, with attention to role-task alignment of providers involved in delivering care, measurement of compliance with clinical evidence for each patient, incorporation of patient-reported outcomes into clinical decisions, use of formal shared decision-making, and per appointment and per case cost reduction.
Despite development of best practice guidelines, there has been limited attention to developing guidelines that consider patient-centered care processes [27]; lean consumption (eg, redesigning care processes to meet consumers’ demands without wasting time, effort, or resources) [35]; or processes to improve communication across settings [34]. As value-based payment reforms emphasize the shift from volume and intensity of services toward high-value, patient-centered care [6], care pathways that can consistently guide reliable delivery of safe, effective, efficient, and patient-centered care are required.
Recognizing this need, our study expands on prior pathway development efforts (including Dartmouth’s development of GreenCare) with the purposes of (1) developing a generalizable clinical care pathway for primary TJA using inputs from clinical, academic, and patient stakeholders; and (2) identifying system- and patient-level processes that may provide safe, effective, efficient, and patient-centered care for patients undergoing TJA.
Materials and Methods
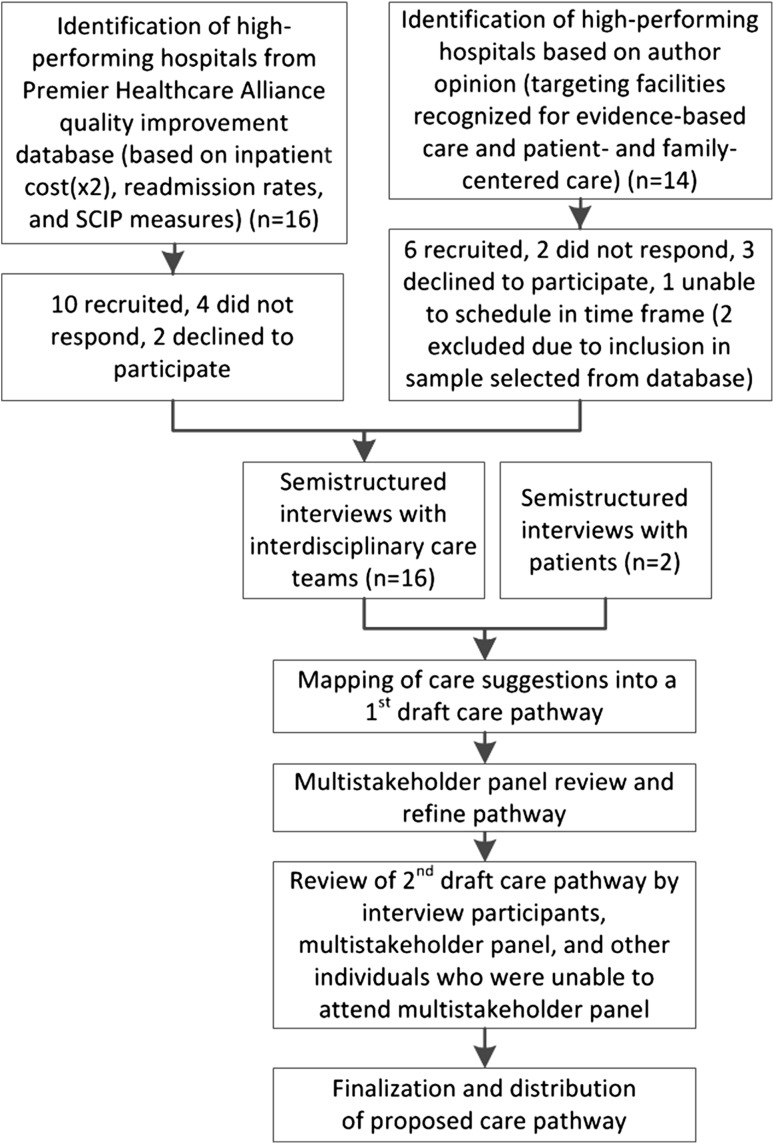
We used a combination of quantitative and qualitative methods [10] to develop a care pathway for elective TJA that begins at the presurgical office visit and continues through the first year of postoperative followup. This approach included (1) identification of high-performing hospitals using available data and author opinion; (2) semistructured interviews with interdisciplinary care teams from these hospitals and independently selected patients undergoing TJA; (3) drafting the care pathway; and (4) consolidation by a multistakeholder panel (Fig. 1).
Fig. 1.
The process used to develop the care pathway is shown. SCIP = Surgical Care Improvement Project.
Population Studied
We used the Premier Healthcare Alliance quality improvement database (n = 234 hospital members) to identify 16 high-performing hospitals with at least 150 primary THA discharges and 300 primary TKA discharges during a 2-year period. We recruited 10 of 16 selected hospitals, including six of eight teaching hospitals and four of eight nonteaching hospitals. We were unable to contact four hospitals and two declined, stating lack of interest. To identify high-performing hospitals, we calculated standardized z-scores for (1) 30-day readmission rates and (2) inpatient costs for patients discharged with a primary TKA or THA between October 1, 2009, and September 30, 2011; and (3) hospital-level surgical care improvement project measures available through the Centers for Medicare & Medicaid Services’ (CMS) Hospital Compare (Surgical Care Improvement Project [SCIP] VTE metrics, hip replacement score, and knee replacement score; collected April 1, 2010, to March 31, 2011). We ranked the top 25 teaching and 25 nonteaching hospitals in the database by adding z-scores across the three dimensions, with double weighting of cost. Hospitals were chosen to prioritize the highest overall rank (≤ 10 in teaching/nonteaching categories), minimize inclusion of multiple hospitals from the same healthcare system, and allow inclusion of two high-volume hospitals (rank, 20, 22) and two hospitals from geographically unrepresented areas (rank, 12, 16). Participating hospitals had similar characteristics, but slightly higher performance than those that could not be contacted or declined (mean z-score: participants, 2.8; nonparticipants, 1.8).
To expand our sample beyond the 234 hospitals in the Premier database and identify other potential best practices, three of us (KJB, KMK, AMD) identified 14 hospitals that were nationally recognized as high performers in evidence-based care and patient- and family-centered care. Two hospitals overlapped with hospitals identified from the Premier database; one accepted the invitation and is included in the above sample. We successfully recruited six of 12 remaining hospitals. We were unable to contact two hospitals, three declined stating lack of interest, and one could not be scheduled within the timeframe. Data were not available to compare hospitals that participated with those that declined.
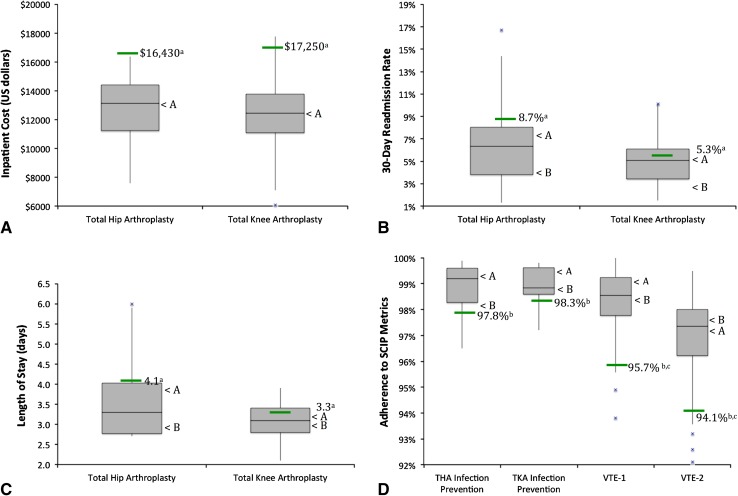
Organizations were diverse in geographic region, teaching status, quality recognition, number of inpatient beds, and annual surgical volume (Table 1). Hospitals selected using the Premier database and author opinion were compared with median performance benchmarks from Premier and CMS comparators; all hospitals exceeded benchmarks in at least 50% of reported metrics (Fig. 2). Owing to differing data sources (database versus self-report), it is difficult to ascertain true differences between hospitals from the two selection methods.
Table 1.
Characteristics of hospitals in the sample
| Characteristic | Hospitals identified via Premier database (n = 10) |
Hospitals identified via author opinion (n = 6) |
All hospitals (n = 16) |
|---|---|---|---|
| Region (number, %) | |||
| Northeast | 3 (30%) | 2 (33%) | 5 (31%) |
| Midwest | 2 (20%) | 0 (0%) | 2 (13%) |
| West | 1 (10%) | 1 (17%) | 2 (13%) |
| South | 4 (40%) | 3 (50%) | 7 (44%) |
| Member of Council of Teaching hospitals (number, %) | 6 (60%) | 3 (50%) | 9 (56%) |
| Recognition for excellence (number, %) | |||
| Blue Cross/Blue Shield Center of Distinction | 4 (40%) | 5 (83%) | 9 (56%) |
| Aetna Institute of Quality - TJA designation | 4 (40%) | 1 (17%) | 5 (31%) |
| Joint Commission - disease-specific certification (TJA) | 5 (50%) | 3 (50%) | 8 (50%) |
| Magnet recognition for nursing excellence | 4 (40%) | 3 (50%) | 7 (44%) |
| Number of hospital inpatient beds (median, range) | 444.5 (185–800) | 277.5 (156–520) | 393 (156–800) |
| Annual surgical volume (median, range) | |||
| Primary THA/year | 297.5 (128–1270) | 482 (273–840) | 428 (128–1270) |
| Primary TKA/year | 462.5 (173–1170) | 687.5 (626–1045) | 658.5 (150–1170) |
TJA = total joint arthroplasty.
Fig. 2A–D.
The distributions of performance among participating hospitals relative to the median performance (green line) among members of the Premier Healthcare Alliance database are shown for (A) inpatient cost, (B) 30-day readmission rate, and (C) length of stay and for (D) CMS Hospital Compare database (SCIP metrics). < A = median performance of hospitals selected from the Premier database; < B = median performance of hospitals selected using author opinion. a = source was 234-Premier Healthcare Alliance member hospitals in the quality improvement database with greater than 150 THAs and greater than 300 TKAs in a 2-year period (October 1, 2009, to September 30, 2011); b = CMS Hospital Compare Surgical Care Improvement Project (SCIP) data, from January 1, 2011, to December 31, 2011; c = data for all surgical types, not specific to TKA and THA; VTE = venous thromboembolism; VTE-1 is a measure of surgery for patients with recommended venous thromboembolism prophylaxis ordered. VTE-2 is a measure of surgery for patients who received appropriate venous thromboembolism prophylaxis within 24 hours before surgery to 24 hours after surgery.
Semistructured Interviews
We conducted semistructured telephone interviews with members of interdisciplinary care teams from selected hospitals and with independently selected patients between March and September 2012. Interdisciplinary care team interviews included nurses from the surgical practice, operating room, postanesthesia care unit, inpatient unit, and home health settings (n = 15); quality improvement personnel (n = 11); midlevel leaders (n = 9); surgeons (n = 9); TJA program coordinators (n = 7); physical therapists (n = 5); care managers (n = 3); senior-level leaders (n = 3); an anesthesiologist (n = 1); and a pharmacist (n = 1). Interviews included one to 13 team members per hospital (median, 3). Patient interviews included one male and one female with a TKA in the previous 2 years. Patients were identified by two of us (AVC, BO) and did not receive care from an interviewed hospital.
We selected interview topics to identify care processes that may contribute to safe, effective, efficient, and patient-centered care. Interdisciplinary care team discussions included (1) typical care experience for a patient and their family; (2) greatest program successes; (3) factors that lead to success; (4) strategy for measuring and tracking care processes and outcomes; and (5) plans to improve care and efficiency. Average duration of interviews was 58 minutes (SD, 15; range, 35–109 minutes). Patient-level discussions were designed to validate concepts identified by care teams and included pleasing and disappointing features of care; factors that contributed to safety, efficiency, or patient and family experience; and advice for providers. Patient interviews lasted 58 and 80 minutes.
We recorded interviews, summarized site attributes, and completed a site checklist to document processes that contributed to a safe, effective, efficient, and patient- and family-centered care experience. We also collected relevant documentation from sites (eg, process and outcome data, TJA care protocols and pathways, and education materials). For each site and patient interview, we mapped flow of care between patients and an interdisciplinary team of providers.
Care Pathway Development
Potential best practices were distilled into a care pathway that starts when a patient has decided to have surgery and has a presurgical visit with their surgeon and ends 12 months after surgery. The care pathway is organized into four care periods: (1) preoperative surgical visit; (2) preoperative preparation and planning for surgery; (3) hospital admission for surgery through hospital discharge; and (4) postdischarge care.
The care pathway highlights processes that apply across the care continuum and in each care period. For each period, it suggests: (1) processes for providing safe, effective, efficient, and patient- and family-centered care; techniques to reduce waste; and techniques to improve communication. It includes system-level processes that apply to how the system of care is designed and patient-level steps that may be applied to most patients; (2) process and outcome measures to monitor; (3) a description of how system-level and patient-level suggestions are mapped to flow of care and provider responsibilities; and (4) additional resources, including links to how-to guides, clinical practice guidelines, meta-analyses, and selected scientific literature.
Multistakeholder Panel Review
After developing a draft care pathway, we convened a 32-member multistakeholder panel to participate in a 1-day workshop focused on reviewing and refining the care pathway. The workshop was sponsored by the Chief Medical Officer of Premier Healthcare Solutions, Inc (RAB); Chief Medical and Scientific Officer of the Institute for Healthcare Improvement (DAG); Vice President of the American Association for Hip and Knee Surgeons (JRL); and Chair of the Council on Research and Quality of the American Academy of Orthopaedic Surgeons (KJB). Sponsors defined meeting objectives, deliverables, and boundaries; selected and recruited participants; and set meeting expectations. Participants were selected based on leadership positions in orthopaedic (n = 4) or anesthesia (n = 1) specialty societies; involvement in organizations specializing in safety and high reliability care (n = 3), lean production/consumption of care (n = 3), and patient experience of care (n=3); membership in an interdisciplinary care team of a hospital selected for interviewing (surgeons, nurses, TJA program directors, and a physical therapist; n = 8); recent receipt of a TJA (n = 1); and participation in the pathway development team (n = 9).
The facilitated workshop included small group breakout sessions with report-outs and opportunities for feedback and consensus building. Sessions focused on gaining consensus on high-level processes and flow; identifying a starter set of metrics to track clinical outcomes, patient experience, cost, and pathway adherence; and developing a demonstration plan to test the impact and feasibility of implementing the care pathway.
The multistakeholder panel input resulted in four classes of revisions to the care pathway. First, language was changed to emphasize general care principles instead of specific care practices to improve generalizability across settings. For example, a recommendation that patients participate in a preoperative education “class” was changed to reflect participation in a preoperative education “process (eg, books, online, video, didactic, class)”. Second, several recommendations were advanced to earlier care periods. For instance, the process of identifying, evaluating, and developing a plan to mitigate surgical risk factors was advanced from the preoperative testing period to the presurgical office visit. Third, recommendations were added to improve specificity in areas that had been insufficiently addressed in semistructured interviews (eg, anesthesiology, postdischarge care). Finally, processes to improve patient engagement and communication were strengthened.
Final Revision
Interview participants, multistakeholder panel members, and three individuals who were unable to attend the multistakeholder panel reviewed the revised care pathway. After further revisions, the proposed care pathway was finalized and made publicly available for testing and context-specific modification [25].
Results
The care pathway suggests 40 processes to improve care, 37 techniques to avoid waste, and 55 techniques to improve communication (total suggestions, 132; Fig. 3; Appendix 1). Overall, 43% (n = 57) of suggestions are aimed at processes that apply to how the system of care is designed, and 57% (n = 75) are steps that may be applied to most patients. A subset (n = 17) of these suggestions applies across all care periods (Table 2), whereas the remainder (n = 115) apply to discrete periods of care (Table 3). The care pathway emphasizes three central themes: standardization and process improvement, interdisciplinary communication and collaboration, and patient and family engagement and education.
Fig. 3.
The distribution of 132 system- and patient-level suggestions across discrete care periods is shown.
Table 2.
Seventeen system- and patient-level suggestions* that apply across all care periods
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Identify an individual (eg, joint program coordinator or nurse coordinator) who is accountable for care delivery and oversees communication with the patient, their family, or caregiver, and care providers |
| Establish standardized, interdisciplinary care protocols that allow little variation across providers but allow customization to specific patient needs |
| Establish a financial arrangement between the hospital and physicians to encourage high-value care by improving quality and decreasing costs (eg, comanagement agreements, service line agreements) |
| Patient-level suggestions |
| Actively engage the patient and their family or caregiver in care discussions from the preoperative surgical appointment through postdischarge care appointments, including in shared decision-making, education, discharge planning, and rehabilitation sessions |
| Follow a risk identification, evaluation, and mitigation process to stratify patients to receive the most beneficial and appropriate level of care |
| Participate in a joint registry such as the American Joint Replacement Registry |
| Tips for reducing waste |
| System-level suggestions |
| Assess staff roles: define roles and responsibilities of the staff/providers that interface with the patient and their family/caregiver before surgery and up to 1 year after surgery; ask yourself, “Is the right person doing the right job, in the right place, at the right time?” |
| Assess information flow: align your information flow with your patient and process flow; ask yourself, “Is the right information available, in the right format, in the right place, at the right time?” |
| Patient-level suggestions |
| Set expectations: specify, set, and manage roles and expectations for care and recovery among patients, their family or caregiver, and clinical care providers; reinforce the expectation that discharge to home is the optimal discharge destination for most patients |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Communication gaps during care transitions: manage communication and care handoffs throughout the care continuum by using standard checklists and creating redundancy in roles or activities |
| Develop communication scripts and protocols for use between the surgical care team and the patient and family/caregiver, primary care providers, consultants, hospital, and postdischarge care providers |
| Consider developing an electronic health record or web portal that can be accessed by patients and providers across the care continuum and can facilitate critical element communication |
| Standardize who communicates with patients and what information is communicated |
| Communicate with and educate the patient and family or caregiver at an appropriate health literacy level and using a culturally sensitive approach |
| Develop a system to learn from and improve the effectiveness, efficiency, safety, and patient and family/caregiver experience of TJA |
| Patient-level suggestions |
| Know your patient: understand what matters to the individual patient and help them achieve their goals; document and communicate the patient’s goals for TJA (eg, decreased pain and stiffness, pursue desired activities) in a care plan that follows the patient across the care continuum and is seen and respected by all providers who interact with the patient; understand that patient circumstances can change during the course of care, and adjust your care to these changes |
| Actively engage patients and their family/caregiver in value-based discussions of care options |
* System-level suggestions are processes that apply to the way the system is designed; patient-level suggestions are steps that may be applied to most or all patients; TJA = total joint arthroplasty.
Table 3.
Fifteen sample suggestions from discrete care pathway periods (among 132 total)
| Period | Processes to improve care | Techniques to reduce waste | Techniques to avoid communication pitfalls |
|---|---|---|---|
| 1: Preoperative surgical visit | Identify, evaluate, and mitigate risk factors that could delay surgery; conduct a standardized multispecialty evaluation of candidates for TJA to assess comorbid conditions (eg, pulmonary, cardiac, diabetes, renal, anticoagulation, uncontrolled/undiagnosed depression, or infection) and characteristics that may increase risk for complications, extended lengths of stay, or discharge to a stepdown facility (eg, older age, obesity, lower preoperative function); establish level of risk present; establish a plan to mitigate risk | Reduce duplication in history, physical examination, and imaging between surgical practice and hospital | Provide verbal and written communication on risks, benefits, and expectations for care (eg, length of stay, discharge destination, pain, recovery timeline, and expected out-of-pocket and opportunity costs) |
| 2: Preoperative preparation (approximately 4–6 weeks) | Require patients to participate in a preoperative education process (eg, books, online, video, didactic, class) that is customized for patients undergoing TJA; strongly encourage family or caregiver participation; allow exemptions to accommodate patient-specific issues such as attendance at a previous TJA class | Combine patient visits (eg, preoperative testing and education) and dovetail activities (eg, initiate discharge planning and care management and identify necessary home supports during preoperative education) | Encourage patients and family/caregivers to ask questions throughout the care process (eg, give permission to ask “why?”) |
| 3a: Inpatient preparation, operation, and PACU (approximately 6 hours) | Follow surgical site infection prevention protocols, venous thromboembolism prevention protocols, and correct site surgery protocols | Assess patient and material flow and establish staff availability guidelines to ensure on-time surgical starts and minimize patient waiting | Streamline flow and communication using standardized handoffs and communication tools between admissions, preoperative area, OR, PACU, and inpatient floor |
| 3b: Inpatient stay and discharge process (approximately 3 days) | Maximize early mobilization, provide group physical therapy, and involve the family/caregiver in therapy; provide day of surgery physical therapy, when permitted by the patient’s physical condition | Establish a protocol that includes standard criteria for when to request medical consultation and who should receive medical/surgical comanagement | Use a checklist that covers issues to address before discharge, and that identifies when a patient is ready for discharge based on predetermined milestones |
| 4:Postdischarge rehabilitation and followup care (12 months) | Postdischarge care providers should follow a standardized pathway for care and rehabilitation, including therapy, wound monitoring, venous thromboembolism prophylaxis, and surgical and medical followup | Use an algorithm with specific criteria to determine discharge readiness for patients admitted to acute rehabilitation, a skilled nursing facility, or home health services | Ask patients to complete a journal that documents progress toward recovery and helps to engage and hold the patient accountable for their recovery |
TJA = total joint arthroplasty; PACU = postanesthesia care unit; OR = operating room.
Standardization and Process Improvement
Among the 17 suggestions that apply across all care periods, six focus on improving standardization and process improvement. These include standardizing care protocols and staff roles; aligning information flow with patient and process flow; following a risk identification, evaluation, and mitigation process to stratify patients into appropriate care levels; and using registries and electronic systems to track patient outcomes and improve quality. For example, an organization might standardize care across all providers and routinely update protocols to reflect best practices.
Communication and Collaboration
Five of the 17 suggestions that apply across all care periods focus on improving communication and collaboration. These include identifying a role accountable for care delivery and communication; establishing financial arrangements between hospitals and physicians that encourage high-value care; using checklists and scripts to manage communication and care transitions; and using an electronic health record or web portal to facilitate critical element communication. For example, organizations may use electronic transmission of information to enable interdisciplinary communication in and across care settings.
Patient and Family Engagement and Education
The final subset (n = 6) of the 17 suggestions that apply across all care periods focuses on improving patient and family engagement and education. These suggestions include using appropriate health literacy levels and culturally sensitive communication; managing patient and provider expectations for care and recovery, standardizing who delivers information to patients and what information is conveyed, documenting and communicating the patient’s goals for TJA in a care plan that follows the patient across the care continuum, and engaging patients and their families in value-based discussions of care options. For example, an organization might establish a program to engage informal caregivers as active members of the care team.
Discussion
Nearly one million TJAs are performed each year in the United States [21] and substantial variation exists across facilities with respect to quality and efficiency [9, 15, 19, 26, 30, 31]. As payment reforms emphasize high-value, patient-centered care [6], guidelines and care pathways are needed that can consistently guide reliable delivery of safe, effective, efficient, and patient-centered care. The proposed care pathway endeavors to balance safety and effectiveness with dimensions of care not addressed in most other care pathways, such as lean consumption [35], patient-centered care [27], and communication and coordination of care [32, 33]. It addresses care during a period of approximately 14 months (from the presurgical office visit through 12 months after discharge), addresses care delivered by a cross section of providers in multiple settings, and includes 132 suggestions for providing high-value care for primary TJA.
There are several limitations to this care pathway. First, the overall care pathway represents a proposal that has not been tested in a clinical setting. Although some suggestions are evidence-based practices, others may not be supported by an evidence base. This compilation of potential best practices has not been validated as effective in its full form or implemented by any one group. It is unknown whether the pathway can be fully implemented, will improve the quality and value of care, or will generalize to diverse care settings. We have attempted to offset these limitations by surveying different kinds of hospitals, creating a diverse multistakeholder panel, and providing reference to a select set of evidence-based practices. We placed recommendations in the context of established principles of the science of improvement [13, 14, 22]; however, organizations may require coaching to prioritize and implement suggestions. Verbal scripts, checklists, standard order sets, and other materials for patients, providers, and administrators may strengthen the ability of an organization to operationalize and implement suggestions. Moreover, we recognize that implementing a large number of suggestions will be challenging and therefore have structured the pathway to include discrete improvement categories and care periods that allow providers to implement suggestions that correspond to their priorities without undertaking full implementation of the pathway. To identify implementation priorities, an organization may find it valuable to complete a matrix that couples care pathway suggestions with expected ease and cost of implementation; impact on clinical, safety, satisfaction, and cost metrics; and alignment with strategic objectives. Finally, we have not evaluated cost of implementation. Although our process for selecting hospitals targeted those that had achieved high-quality care at a low cost, testing is needed to determine the cost associated with implementing these suggestions.
There are also limitations to the methods used to develop the pathway. First, although we used several mechanisms to elicit input from patients and patient advocates (eg, interviews, multistakeholder panel, and ad hoc advisory discussions), there would be value in having greater participation of patients who had recently undergone TJA (we included three patients) and in establishing a formal advisory role of one or more patients throughout the project. Second, identification of high-performing organizations was limited by available data. There are no national databases available to monitor quality and cost associated with TJA programs over the episode of care. As such, we used author opinion to supplement available data from the Premier and CMS Hospital Compare databases. Finally, we encountered some resistance from hospitals regarding sharing proprietary information, however most organizations welcomed the opportunity to contribute to a pathway that would be freely available to the orthopaedic community.
Common themes in the care pathway align with recommendations found in the literature [4, 7, 8, 13, 14, 16, 18, 24, 27, 33] and include standardization and process improvement, communication and collaboration, and patient engagement and education. Research has shown that standardization and use of established process improvement methods can improve clinical outcomes, safety, and efficiency of TJA [4, 7, 8, 13, 14, 16, 33]. For instance, process standardization and adherence to evidence-based guidelines can shorten lengths of hospital stay, improve clinical outcomes, and reduce negative outcomes (including death, readmission, reoperation, or surgical complications) independent of hospital or surgeon procedure volume [7]. Communication across members of interdisciplinary care teams has been recognized as a critical element for successful care transformation, yet this area has been largely neglected in care pathways [33]. Finally, patient-centeredness is increasingly recognized as a necessary attribute of healthcare quality [18, 27], and patient and family engagement can lead to improved TJA clinical outcomes [13, 14, 24].
TJA processes are evolving, and progressive health systems are actively testing initiatives to improve delivery of high-value care. We used a multistakeholder approach to develop a TJA care pathway that outlines suggestions that might improve care. Care pathway suggestions are designed to be transferable to diverse settings, and suggestions are specifically broad to accommodate local characteristics, culture, and resource availability. The pathway we proposed should be evaluated in high- and low-volume settings to determine its effectiveness, feasibility, cost of implementation, and need for context-specific adaptation. To ensure high-value services across the care continuum, TJA programs should endeavor to standardize care processes and may draw on suggestions identified herein.
Acknowledgments
We thank Julia Rowe Taylor for assistance with project oversight; Jane Roessner and Val Weber for editorial assistance; Vanessa Chan for help with manuscript preparation; members of the 16 interdisciplinary care teams and two patients for their participation in semistructured interviews; and the 32 members of our multistakeholder panel for their work to refine and improve the care pathway. We also acknowledge that this work arose from efforts by the Dartmouth population health team and individuals at Dartmouth-Hitchcock Medical Center (ECN, KMK) to incorporate the Institute of Medicine’s quality aims into their care pathway development efforts. Dartmouth-Hitchcock’s GreenCare approach uses a similar conceptual framework, process flow diagrams to illustrate roles and tasks over the entire episode, integration of tasks into team roles and into the electronic medical record, and use of patient-reported outcomes. The current project offered the opportunity to adopt and extend Dartmouth’s approach to pathway development and to engage a diverse cross section of clinicians and health systems in developing a potentially generalizable care pathway.
Appendix 1. Suggestions (n = 132) included in the care pathway
Table 4.
Processes that apply across the continuum of care (n = 17)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Identify an individual (eg, joint program coordinator or nurse coordinator) who is accountable for care delivery and oversees communication with the patient, their family or caregiver, and care providers |
| Establish standardized, interdisciplinary care protocols that allow little variation across providers but allow customization to specific patient needs |
| Establish a financial arrangement between the hospital and physicians to encourage high-value care by improving quality and decreasing costs (eg, comanagement agreements, service line agreements) |
| Patient-level suggestions |
| Actively engage the patient and their family or caregiver in care discussions from the preoperative surgical appointment through postdischarge care appointments, including in shared decision-making, education, discharge planning, and rehabilitation sessions |
| Follow a risk identification, evaluation, and mitigation process to stratify patients to receive the most beneficial and appropriate level of care |
| Participate in a joint registry such as the American Joint Replacement Registry |
| Tips for reducing waste |
| System-level suggestions |
| Assess staff roles: define roles and responsibilities of the staff/providers that interface with the patient and their family/caregiver before surgery and up to 1 year after surgery; ask yourself, “Is the right person doing the right job, in the right place, at the right time?” |
| Assess information flow: align your information flow with your patient and process flow; ask yourself, “Is the right information available, in the right format, in the right place, at the right time?” |
| Patient-level suggestions |
| Set expectations: specify, set, and manage roles and expectations for care and recovery among patients, their family or caregiver, and clinical care providers; reinforce the expectation that discharge to home is the optimal discharge destination for most patients |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Communication gaps during care transitions: manage communication and care handoffs throughout the care continuum by using standard checklists and creating redundancy in roles or activities |
| Develop communication scripts and protocols for use between the surgical care team and the patient and family/caregiver, primary care providers, consultants, hospital, and postdischarge care providers |
| Consider developing an electronic health record or web portal that can be accessed by patients and providers across the care continuum and can facilitate critical-element communication |
| Standardize who communicates with patients and what information is communicated |
| Communicate with and educate the patient and family or caregiver at an appropriate health literacy level and using a culturally sensitive approach |
| Develop a system to learn from and improve the effectiveness, efficiency, safety, and patient and family/caregiver experience of TJA |
| Patient-level suggestions |
| Know your patient: understand what matters to the individual patient and help them achieve their goals. Document and communicate the patient’s goals for TJA (eg, decreased pain and stiffness, pursue desired activities) in a care plan that follows the patient across the care continuum, and is seen and respected by all providers who interact with the patient. Understand that patient circumstances can change during the course of care, and adjust your care to these changes |
| Actively engage patients and their family/caregiver in value-based discussions of care options |
Note This Care Pathway begins when the patient, family/caregiver, and doctor have decided on surgery and ends 12 months after surgery. It assumes that this process was preceded by a well-informed, shared decision-making process and by appropriate nonoperative treatment options
Table 5.
Period 1: preoperative surgical office visit (24 suggestions)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Develop and maintain a shared decision-making process so that patients can make well-informed decisions about care options (eg, surgical approach, anesthesia choices, and discharge disposition) |
| Patient-level suggestions |
| Identify, document, and communicate the patient’s personal goal for surgery |
| Educate the patient and family/caregiver about expectations for the continuum of the care experience, including: (1) preoperative preparation, home-based exercises, and home safety; (2) surgical preparation, operation, and immediate recovery; (3) inpatient rehabilitation, pain, and expected length of stay; (4) discharge options and postdischarge rehabilitation/recovery; and (5) long-term followup care; provide written or video documentation for the patient and family/caregiver |
| Identify, evaluate, and mitigate risk factors that could delay surgery; conduct a standardized multispecialty evaluation of candidates for TJA to assess comorbid conditions (eg, pulmonary, cardiac, diabetes, renal, anticoagulation, uncontrolled/undiagnosed depression, or infection) and characteristics that may increase risk for complications, extended lengths of stay, or discharge to a stepdown facility (eg, older age, obesity, lower preoperative function); establish level of risk present; establish a plan to mitigate risk |
| Use a surgical site infection prevention checklist to help identify, evaluate, and mitigate risk from anemia (hemoglobin < 9 g/dL), poor nutrition (albumin < 3 g/dL), uncontrolled diabetes (HbA1c > 8%), obesity (BMI > 40 kg/m2), and smoking |
| Encourage presurgery physical conditioning, when appropriate |
| Encourage value-added prosthesis(-es) selection, based on anatomy and activity level of the patient |
| Use a standard checklist and verbiage to document medical necessity for TJA in office notes and hospital admission history (ie, radiographic findings, physical examination, disease history, failure of nonoperative treatment) |
| Tips for reducing waste |
| System-level suggestions |
| Reduce duplication in history, physical examination, and imaging between surgical practice and hospital |
| Define roles and responsibilities of the staff/providers that interface with the patient (eg, registration, joint program coordinator, physician’s assistant, anesthesia providers, nurses, residents, surgeon) |
| Patient-level suggestions |
| Ask patient to complete preoperative assessment forms before surgical office visit (eg, history and physical, health-related quality of life, functional health status) |
| Use a checklist to assess family/caregiver support capabilities and need for assistance; encourage home discharge; educate patient on appropriateness of home discharge versus inpatient care |
| If appropriate, initiate referrals to postdischarge services to help facilitate discharge (eg, home health, outpatient or inpatient rehabilitation, skilled nursing) |
| Tips for avoiding communication pitfalls |
| Patient-level suggestions |
| Use a longitudinal surgical consent process that is patient-centered, accurate, timely, surgeon-led and surgeon-entered; consider preoperative electronic entry and verification |
| Provide patient with potential questions to discuss with surgical care team before the surgical office visit |
| Provide verbal and written communication on risks, benefits, and expectations for care (eg, length of stay, discharge destination, pain, recovery timeline, and expected out-of-pocket and opportunity costs) |
| Create a written, bidirectional engagement agreement or contract between the patient and surgical care team regarding prework by the patient, including exercise, home inspection, and risk mitigation |
| Alert patient that they should contact the surgical office within 14 days of the surgery to identify any emergent health concerns that could affect the ability to proceed with surgery |
| Develop and use a patient communication form that includes questions the surgeon needs answered (including patient goals) and the information needed on the day of preoperative testing |
| Use an operating room scheduling checklist that includes all critical elements of the surgical episode and is used consistently by all surgical team members to ensure consistent critical element communication, include planned surgical approach, implant specifics, medical comorbidities, postoperative plan, anticoagulation management plan, and patient-specific variables (eg, adverse reactions to medications likely to be used, problems/concerns with previous surgeries, allergies, and latex or metal sensitivities) |
| Engage patients in weight loss efforts to encourage them to take ownership of their health; encourage participation in a wellness program, if available |
| Consider connecting new patients with experienced patients through written and verbal communication (eg, handouts, phone calls, face-to-face meetings) |
| Follow a standard protocol to communicate with the patient and referring physician, including sending a copy of a letter and clinical notes for every new patient visit, and clinical notes from established care visits |
| Follow a standard protocol to schedule all anticipated patient appointments, including preoperative joint education, preoperative testing and blood work, surgical date, and followup surgeon and primary care physician visits |
Note The preoperative surgical office visit includes the last surgical visit before surgery, and the care and consultations that are initiated during this period; this visit typically occurs 4 to 6 weeks before surgery
Table 6.
Period 2: preoperative preparation and planning (22 suggestions)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Standardize preoperative screening tests–tailor to patient-specific risk factors (eg, comorbid conditions and abnormalities found in routine testing) and limit to medically necessary procedures (see Period 1). |
| Patient-level suggestions |
| Implement a patient expectation management process where patients are actively engaged in the care process and in the discharge planning process before admission; set expectations about pain, mobilization (day of surgery), and discharge disposition (home as preferred option for most patients) |
| Require patients to participate in a preoperative education process (eg, books, online, video, didactic, class) that is customized for patients undergoing TJA; strongly encourage family or caregiver participation; allow exemptions to accommodate patient-specific issues such as attendance at a previous TJA class |
| Screen all patients for Staphylococcus aureus–methicillin-resistant (MRSA) and methicillin-sensitive (MSSA)–before surgery, allowing enough time for those who screen positive to be decolonized with five days of intranasal mupirocin and 5 days of chlorhexidine soap before surgery |
| Instruct all patients to bathe with chlorhexidine gluconate soap 3 days (times) before surgery; ensure patients understand how to procure the soap and review directions for bathing with the product |
| Use a sleep apnea screening tool/checklist, with preoperative testing when positive |
| Build a checklist of critical/high-risk medications to monitor in the perioperative period, such as diabetes medications, anticoagulants, beta-blockers, antirheumatologic medications, and pain medications |
| Tips for reducing waste |
| System-level suggestions |
| Reduce duplication of information collection (eg, patient history) between surgical practice and hospital |
| Evaluate patient and family/caregiver flow and wait time during preadmission testing |
| Establish and follow a standardized blood management protocol |
| Define roles and responsibilities of the staff/providers that interface with the patient (eg, registration, access coordinator, joint program coordinator, physician assistants, anesthesia providers, nurses, residents, surgeon) |
| Patient-level suggestions |
| Combine patient visits (eg, preoperative testing and education) and dovetail activities (eg, initiate discharge planning and care management and identify necessary home supports during preoperative education) |
| Evaluate home environment and social support needs; arrange for expected postdischarge services and equipment |
| Educate patient and family/caregiver on exercises that should be done before, during, and after surgery |
| Engage in preoperative anesthesia planning and education to minimize use of opiate narcotics; establish whether the patient is opioid-naïve; provide patient with anesthesia choices and expectations; educate patient that “complete” pain relief (< 3/10) can have side effects and prolong the hospital stay |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Establish mechanism to ensure that preoperative preparation is complete and that care providers (surgical and inpatient) are informed of upcoming patients, their potential risk factors, and equipment and staffing needs |
| Establish a standard protocol for communication among the surgical practice, hospital, primary care physician, and medical consultants |
| Patient-level suggestions |
| Identify and document the patient’s medical surrogate or durable power of attorney |
| Encourage patients and family/caregivers to ask questions throughout the care process (eg, give permission to ask “why?”) |
| Instruct patients on how they will be notified if they screen positive for MRSA/MSSA |
| Address fall prevention with the patient and family/caregiver and identify steps to prepare the home before surgery |
| Provide telephone check-in to remind patient and family/caregiver of preoperative preparation |
Note The preoperative preparation and planning period should be completed within 30 days of surgery, allowing for as much time between testing and surgery as possible to optimize care and mitigate risks
Table 7.
Period 3a: inpatient experience: preparation, operation, and postanesthesia care unit (PACU) (22 suggestions)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Encourage the use of dedicated surgical care teams that consist of the surgeon, midlevel team member(s), and specialized arthroplasty circulating nurse and scrub technician; if the volume of the hospital permits, include a dedicated anesthesia provider in the surgical care team |
| Increase value by: (2) negotiating implant costs with vendors; (2) forming a value analysis team; and (3) developing an orthopaedic service line with expectations for value (quality/cost) |
| Patient-level suggestions |
| Follow communication protocols to ensure patient safety; use surgical pauses and checklists (eg, preincision, postsurgery); consider adding redundancy in documentation during timeouts or pauses |
| Follow analgesia protocols that maximize pain relief, minimize nausea, and decrease length of recovery time • Consider pain management consultation for high-risk patients • Allow for preoperative analgesic customization, particularly for high-risk patients Establish a multimodal perioperative pain management protocol; when possible, encourage use of regional anesthesia and/or regional nerve blocks, preemptive analgesics, and antiemetics |
| Follow blood management and transfusion guidelines. |
| Follow hyperglycemia observation, management and treatment guidelines |
| Follow surgical site infection prevention protocols, venous thromboembolism prevention protocols, and correct site surgery protocols |
| Tips for reducing waste |
| System-level suggestions |
| Assess patient and material flow and establish staff availability guidelines to ensure on-time surgical starts and minimize patient waiting |
| Limit the number of surgical staff in the operating room |
| Consider using an implant time-out before opening the prosthesis package to confirm that the proper implant is present |
| Consider the value and cost-benefit tradeoffs of different technology, equipment, and implants |
| Reduce excess materials from the operating room (eg, drapes, cloths, instruments) |
| Identify the value proposition of different care models based on the location of services, the time of service delivery, the expertise of personnel, and the value (eg, quality/cost) of the service |
| Maximize the efficiency in a one-operating room model before considering the use of two operating rooms for select surgeons |
| Define roles and responsibilities of the staff/providers that interface with the patient (eg, registration, joint program coordinator, transporter, physician assistants, nurses, resident, anesthesia provider, surgeon, PACU staff) |
| Patient-level suggestions |
| Standardize patient positioning on the operating room table |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Follow a “culture of safety” where all staff members are empowered to identify potential safety issues |
| Consider endorsing TeamSTEPPS® (Agency for Healthcare Research and Quality, Rockville, MD, USA) as an “optimal” surgical team communication education format |
| Streamline flow and communication using standardized handoffs and communication tools between admissions, preoperative area, operating room, PACU, and inpatient floor |
| Patient-level suggestions |
| Communicate with the patient and family/caregiver about expectations, level of anxiety, and current and next steps of care |
| Provide updates to family/caregiver at preidentified times and address standard and emergent topics; consider use of face-to-face contact, communication boards, pagers, or other means of communication |
| Actively engage patients and their family/caregiver in value-based discussions of care options |
Note The preparation, operation, and PACU period typically is completed within 6 hours; it begins when the patient arrives at the hospital for surgery and ends when the patient is discharged to the inpatient floor
Table 8.
Period 3b: inpatient experience: inpatient stay and discharge process (27 suggestions)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Use dedicated care providers (eg, nurses, physical therapists, occupational therapists) to care for patients undergoing TJA; increase the percentage of care providers with specialized training in orthopaedic care |
| Create a dedicated space for patients recovering from TJA (eg, joint replacement unit, or concentrating all patients undergoing TJA (or “clean” surgery cases) within a specific area) |
| Shift to afternoon/evening rehabilitation staffing to allow for day of surgery physical therapy sessions |
| Standardize postoperative care (eg, type/timing of dressing change(s), fall risk assessments); tailor care to patient-specific risk factors (eg, comorbid conditions/special needs, identified in Period 1) |
| Identify at-risk patients who may need more thorough postdischarge followup (to prevent readmission) |
| Patient-level suggestions |
| Maximize early mobilization, provide group physical therapy, and involve the family/caregiver in therapy; provide day of surgery physical therapy, when permitted by the patient’s physical condition |
| Use an appropriate comanagement approach (eg, anesthesia, internal medicine, and orthopaedic providers) for patients identified as eligible in preoperative risk evaluation and stratification (see Period 1) |
| Tips for reducing waste |
| System-level suggestions |
| Establish a protocol that includes standard criteria for when to request medical consultation and who should receive medical/surgical comanagement |
| Train nurses to help patients into and out of bed. |
| Consider the value and cost-benefit tradeoffs of different technology and equipment |
| Demand resources and staffing capacity to match the level of patient acuity |
| Patient-level suggestions |
| Reinforce patient and staff expectations that discharge to home is optimal for most patients |
| Avoid use of patient-controlled analgesia by transitioning to oral medications; minimize opiate use |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Avoid disparity in level of staff attention to patients between day/night and weekday/weekend shifts |
| Identify a dedicated person to facilitate discharge planning (eg, case manager, discharge nurse) |
| Use daily, goal-directed interdisciplinary team rounding to help all team members (including patient and family or caregiver) know status and expectations; rounding should occur within a scheduled time window and address patient goals, pain management, therapy goals, and discharge planning |
| Incorporate bedside shift reports, hourly rounding, and leader rounding into care processes |
| Patient-level suggestions |
| Develop and use a fall prevention checklist, including patient and family instructions for fall prevention |
| Use a checklist that covers issues to address before discharge and that identifies when a patient is ready for discharge based on predetermined milestones |
| Use standardized handoff communication between care settings (eg, floor to postdischarge setting) |
| Communicate and document in daily rounds the “plan for the day” and “plan for the stay”; provide a written summary of the updated care plan to the patient and the family/caregiver |
| Reinforce patient and family/caregiver education in an ongoing manner throughout the inpatient stay; identify and address gaps in knowledge; reinforce expectations for roles and responsibilities of patient, family/caregiver, and providers; link verbal education to written educational materials |
| Educate the patient and family/caregiver on the differences between complications needing medical/surgical followup and those that are uncomfortable but expected |
| Determine patient’s ability to manage postoperative care and arrange needed home supports |
| For patients discharged to home, discuss and document who to call with medical and surgical questions |
| Include necessary and time-sensitive appointments with the healthcare system in the discharge plan; the discharge plan should address postdischarge care for surgical and comorbid medical conditions |
| Train patients to track their progress (in a journal or electronic health record patient portal, if available) |
Note The inpatient stay and discharge process period typically is completed within 3 days; it begins when the patient arrives at the inpatient floor and ends when the patient is discharged from acute care
Table 9.
Period 4: postdischarge rehabilitation and followup care (20 suggestions)
| Processes for providing safe, effective, efficient, and patient- and family-centered care |
| System-level suggestions |
| Postdischarge care providers should follow a standardized pathway for care and rehabilitation, including therapy, wound monitoring, venous thromboembolism prophylaxis, and surgical and medical followup |
| Identify at-risk patients who may need more thorough postdischarge followup to prevent readmission (continued from Period 3b). |
| Develop and follow an anticoagulation protocol, including a dedicated postoperative management team |
| Patient-level suggestions |
| For patients discharged to home health services, arrange home health visit to occur within 24 hours |
| Continue postdischarge communication with patients and family/caregiver, including issues around health and recovery and what went well or could have been improved with the care experience |
| Tips for reducing waste |
| System-level suggestions |
| Consider the value and cost-benefit tradeoffs of different technology and equipment |
| Identify the proper interval for patient followup; use patient-reported outcome measures and assessments from other providers to help determine the frequency of followups |
| Track outcomes and implant life in a joint registry. |
| Patient-level suggestions |
| Use an algorithm with specific criteria to determine discharge readiness for patients admitted to acute rehabilitation, a skilled nursing facility, or home health services |
| Provide continuity of physical therapy between inpatient and outpatient settings |
| Provide patients with transportation options to facilitate access to outpatient care, and reduce the need for skilled nursing or home health services |
| Tips for avoiding communication pitfalls |
| System-level suggestions |
| Develop a contractual arrangement with acute care, skilled nursing facility, home health, and outpatient therapy providers to ensure that standard care and communication protocols are followed |
| Define customer service level and clarify whom the patient or family/caregiver should contact with questions (eg, some settings offer 24/7 telephone access to a surgical care team member) |
| Identify an individual who is responsible for coordinating care among providers (eg, joint program coordinator) |
| Standardize care transition and handoff communication among the hospital staff, surgical care team, and postdischarge care providers; and between the surgical care team and primary care physician for up to 1 year postsurgery (eg, communication checklist and templates, transfer of rehabilitation and medical notes, notice of discharge); include standardized electronic communication between sites, if possible |
| Patient-level suggestions |
| Followup with patient within 24 to 48 hours after hospital discharge, using a communication checklist |
| Consider connecting new patients to experienced patients through written and verbal communication (eg, a “joint buddy”) |
| Document patient and provider goals for physical therapy (eg, range of motion, gait, and desired activities); educate patient and family/caregiver in the process and specific milestones for achieving personal goals; assess delays in reaching goals |
| Ask patients to complete a “journal” that documents progress toward recovery and helps to engage and hold the patient accountable for their recovery |
| Provide patients with a list of frequently asked questions and a guideline of other specific questions that may be appropriate for asking the medical (hospital/primary care physician) or surgical care team |
Note In this Care Pathway, the postdischarge rehabilitation and followup care period lasts for 12 months after the patient is discharged from the hospital following the initial surgery; however, ongoing monitoring typically continues throughout the patient’s life to assess for deterioration of the implant
Footnotes
The institution of the authors (ADVC, DAG, ECN, BO, FAF) has received funding from Premier Healthcare Alliance, Inc, Washington, DC, USA, through a research partnership with the Institute for Healthcare Improvement.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
The primary work for this project was performed at the Institute for Healthcare Improvement main office (Cambridge, MA, USA) and in Washington DC, Hanover, NH, and Lebanon, NH, USA.
References
- 1.American Academy of Orthopaedic Surgeons (AAOS). Clinical Practice Guidelines. Rosemont, IL: AAOS. Available at: http://www.aaos.org/research/guidelines/guide.asp. Accessed October 28, 2013.
- 2.American Academy of Orthopaedic Surgeons (AAOS). Guideline on the Diagnosis of Periprosthetic Joint Infections of the Hip and Knee. Rosemont, IL: AAOS. Available at: http://www.aaos.org/Research/guidelines/PJIguideline.asp. Accessed October 28, 2013.
- 3.American Academy of Orthopaedic Surgeons (AAOS). Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. Rosemont, IL: AAOS. Available at: http://www.aaos.org/Research/guidelines/VTE/VTE_full_guideline.pdf. Accessed October 28, 2013. [DOI] [PubMed]
- 4.Barbieri A, Vanhaecht K, Van Herck P, Sermeus W, Faggiano F, Marchisio S, Panella M. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32. doi: 10.1186/1741-7015-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone and Joint Canada. Hip and Knee Replacement Toolkit: A Living Document. In: Waddell JP, Frank C, eds. Toronto,Canada: Bone and Joint Canada. Available at: http://www.boneandjointcanada.com. Accessed October 28, 2013.
- 6.Bozic KJ. Value-based healthcare and orthopaedic surgery. Clin Orthop Relat Res. 2012;470:1004–1005. doi: 10.1007/s11999-012-2267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92:2643–2652. doi: 10.2106/JBJS.I.01477. [DOI] [PubMed] [Google Scholar]
- 8.Cima RR, Brown MJ, Hebl JR, Moore R, Rogers JC, Kollengode A, Amstutz GJ, Weisbrod CA, Narr BJ, Deschamps C, Surgical Process Improvement Team Mayo Clinic, Rochester Use of lean and six sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg. 2011;213:83–92. doi: 10.1016/j.jamcollsurg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Cram P, Lu X, Kaboli PJ, Vaughan-Sarrazin MS, Cai X, Wolf BR, Li Y. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305:1560–1567. doi: 10.1001/jama.2011.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119:1442–1452. doi: 10.1161/CIRCULATIONAHA.107.742775. [DOI] [PubMed] [Google Scholar]
- 11.Dartmouth-Hitchcock Medical Center. Dartmouth-Hitchcock “Green Care”. Available at: http://lebanon.dhortho.org/dartmouth-hitchcock-green-care/. Accessed October 28, 2013.
- 12.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5:1–20. [PubMed] [Google Scholar]
- 13.DiGioia AM, 3rd, Greenhouse PK. Care experience-based methodologies: performance improvement roadmap to value-driven health care. Clin Orthop Relat Res. 2012;470:1038–1045. doi: 10.1007/s11999-011-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGioia AM, 3rd, Greenhouse PK, Levison TJ. Patient and family-centered collaborative care: an orthopaedic model. Clin Orthop Relat Res. 2007;463:13–19. [PubMed] [Google Scholar]
- 15.Friedman RJ, Gallus AS, Cushner FD, Fitzgerald G, Anderson FA, Jr, Global Orthopaedic Registry Investigators Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin. 2008;24:87–97. doi: 10.1185/030079907X242746. [DOI] [PubMed] [Google Scholar]
- 16.Harders M, Malangoni MA, Weight S, Sidhu T. Improving operating room efficiency through process redesign. Surgery. 2006;140:509–514; discussion 514–516. [DOI] [PubMed]
- 17.Institute for Healthcare Improvement. Welcome to Project JOINTS. Available at: http://www.ihi.org/ProjectJOINTS. Accessed October 28, 2013.
- 18.Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001. [PubMed] [Google Scholar]
- 19.Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, Colin C, Ghali WA, Burnand B, IMECCHI Group Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307:294–303. doi: 10.1001/jama.2011.2029. [DOI] [PubMed] [Google Scholar]
- 20.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59:481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 22.Langley GL, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2. San Francisco, CA: Jossey-Bass Publishers; 2009. [Google Scholar]
- 23.Map of Medicine. Elective knee surgery. Available at: http://healthguides.mapofmedicine.com/choices/map-open/elective_knee_surgery1.html. Accessed October 28, 2013.
- 24.Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: what role does patient preconditioning play? J Bone Joint Surg Am. 2007;89:1920–1927. doi: 10.2106/JBJS.F.01153. [DOI] [PubMed] [Google Scholar]
- 25.Premier Inc, Institute for Healthcare Improvement. Integrated Care Pathway for Total Joint Arthroplasty. Charlotte, NC: Premier, Inc. and Cambridge, MA: Institute for Healthcare Improvement; 2013. Available at: www.premierinc.com and www.ihi.org. Accessed October 28, 2013.
- 26.Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173:427–432. doi: 10.1001/jamainternmed.2013.460. [DOI] [PubMed] [Google Scholar]
- 27.Shaller D, Shaller Consulting. Patient-Centered Care: What Does it Take? The Commonwealth Fund. Available at: http://www.commonwealthfund.org/usr_doc/Shaller_patient-centeredcarewhatdoesittake_1067.pdf. Accessed November 7, 2013.
- 28.Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff (Millwood). 2011;30:1708–1717. doi: 10.1377/hlthaff.2010.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SooHoo NF, Lieberman JR, Farng E, Park S, Jain S, Ko CY. Development of quality of care indicators for patients undergoing total hip or total knee replacement. BMJ Qual Saf. 2011;20:153–157. doi: 10.1136/bmjqs.2009.032524. [DOI] [PubMed] [Google Scholar]
- 30.Soohoo NF, Tang EY, Krenek L, Eagan M, McGlynn E. Variations in the quality of care delivered to patients undergoing total knee replacement at 3 affiliated hospitals. Orthopedics. 2011;34:356. doi: 10.3928/01477447-20110317-08. [DOI] [PubMed] [Google Scholar]
- 31.Tomek IM, Sabel AL, Froimson MI, Muschler G, Jevsevar DS, Koenig KM, Lewallen DG, Naessens JM, Savitz LA, Westrich JL, Weeks WB, Weinstein JN. A collaborative of leading health systems finds wide variations in total knee replacement delivery and takes steps to improve value. Health Aff (Millwood). 2012;31:1329–1338. doi: 10.1377/hlthaff.2011.0935. [DOI] [PubMed] [Google Scholar]
- 32.Van Herck P, Vanhaecht K, Deneckere S, Bellemans J, Panella M, Barbieri A, Sermeus W. Key interventions and outcomes in joint arthroplasty clinical pathways: a systematic review. J Eval Clin Pract. 2010;16:39–49. doi: 10.1111/j.1365-2753.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 33.Vanhaecht K, Bellemans J, De Witte K, Diya L, Lesaffre E, Sermeus W. Does the organization of care processes affect outcomes in patients undergoing total joint replacement? J Eval Clin Pract. 2010;16:121–128. doi: 10.1111/j.1365-2753.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanhaecht K, Panella M, van Zelm R, Sermeus W. An overview on the history and concept of care pathways as complex interventions. Int J Care Pathways. 2010;14:117–123. doi: 10.1258/jicp.2010.010019. [DOI] [Google Scholar]
- 35.Womack JP, Jones DT. Lean consumption. Harvard Business Review. 2005;83:58–68, 148. [PubMed]