Abstract
Background
Femoral continuous peripheral nerve blocks (CPNBs) provide effective analgesia after TKA but have been associated with quadriceps weakness and delayed ambulation. A promising alternative is adductor canal CPNB that delivers a primarily sensory blockade; however, the differential effects of these two techniques on functional outcomes after TKA are not well established.
Questions/purposes
We determined whether, after TKA, patients with adductor canal CPNB versus patients with femoral CPNB demonstrated (1) greater total ambulation distance on Postoperative Day (POD) 1 and 2 and (2) decreased daily opioid consumption, pain scores, and hospital length of stay.
Methods
Between October 2011 and October 2012, 180 patients underwent primary TKA at our practice site, of whom 93% (n = 168) had CPNBs. In this sequential series, the first 102 patients had femoral CPNBs, and the next 66 had adductor canal CPNBs. The change resulted from a modification to our clinical pathway, which involved only a change to the block. An evaluator not involved in the patients’ care reviewed their medical records to record the parameters noted above.
Results
Ambulation distances were higher in the adductor canal group than in the femoral group on POD 1 (median [10th–90th percentiles]: 37 m [0–90 m] versus 6 m [0–51 m]; p < 0.001) and POD 2 (60 m [0–120 m] versus 21 m [0–78 m]; p = 0.003). Adjusted linear regression confirmed the association between adductor canal catheter use and ambulation distance on POD 1 (B = 23; 95% CI = 14–33; p < 0.001) and POD 2 (B = 19; 95% CI = 5–33; p = 0.008). Pain scores, daily opioid consumption, and hospital length of stay were similar between groups.
Conclusions
Adductor canal CPNB may promote greater early postoperative ambulation compared to femoral CPNB after TKA without a reduction in analgesia. Future randomized studies are needed to validate our major findings.
Level of Evidence
Level III, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Clinical care pathways for patients undergoing joint arthroplasty have been increasingly incorporating continuous peripheral nerve block (CPNB) techniques for postoperative analgesia [1, 8]. Besides offering pain relief, clinical pathways using CPNB have demonstrated a shortened time to functional recovery and decreased postoperative adverse events [8]. Compared to single-injection peripheral nerve block techniques, CPNB involves the percutaneous insertion of an indwelling catheter (i.e., a perineural catheter) in the proximity of a target nerve that acts as a conduit for continuous perineural local anesthetic infusion similar to an epidural catheter [7, 11]. Perineural catheters extend the duration of analgesia provided by peripheral nerve blocks, improving the quality of postoperative recovery while maintaining the selectivity for the operative limb [11].
For major knee surgery including arthroplasty, femoral perineural catheters are well proven to provide effective postoperative analgesia [4, 14]. Recent studies of femoral CPNB have shown that typical perineural local anesthetic infusion doses produce clinically significant quadriceps weakness when administered via catheters inserted using conventional techniques [3, 6]. An alternative perineural catheter site distal to the femoral triangle is the adductor canal. The adductor canal is found in the middle 1/3 of the thigh and runs from the apex of the femoral triangle proximally to the adductor hiatus distally. Because the adductor canal consistently encloses the saphenous nerve and the nerve to the vastus medialis, placement of a catheter within the canal can potentially spare the major motor branches of the femoral nerve while still providing effective pain relief [19, 22]. While the adductor canal technique may preserve quadriceps muscle strength compared to the femoral nerve block in volunteers, the effects, if any, on postoperative rehabilitation and discharge eligibility for actual patients undergoing TKA still remain to be studied [19].
We therefore asked the following questions: After TKA, do patients with adductor canal CPNB versus patients with femoral CPNB (1) achieve greater total ambulation distance on Postoperative Day (POD) 1 and 2 and (2) demonstrate decreased daily opioid consumption, pain scores, and hospital length of stay (LOS)?
Patients and Methods
This retrospective cohort study was reviewed and approved with waiver of informed consent by our affiliated university’s institutional review board and local Veterans Affairs (VA) Research Committee. Our institution is a tertiary VA referral center providing care for all major orthopaedic surgeries with an active joint arthroplasty program. We examined administrative, preoperative, and postoperative data for a series of patients who underwent primary TKA during the course of 1 year (October 2011 to October 2012), 6 months before and 6 months after a clinical pathway revision that replaced femoral CPNBs with adductor canal CPNBs (Fig. 1). Data were collected from VISTA, the VA centralized electronic medical record. Our study inclusion criteria were all patients who underwent unilateral TKA during the study period, had either femoral or adductor canal CPNB, and were admitted postoperatively to the primary surgical ward. Patients were excluded who underwent an additional significant surgical procedure besides unilateral TKA (e.g., bilateral TKAs) or a different regional analgesia technique.
Fig. 1.
A flow diagram shows how patients were selected for this study. After development of the base population, patients were stratified by type of CPNB: adductor canal versus femoral.
During the period of study, 180 patients underwent primary TKA at our practice site, of whom 93% (n = 168) had a continuous peripheral nerve block. In this sequential series, the first 102 patients had a femoral CPNB, and the next 66 had an adductor canal CPNB. The adductor canal group was comparable to the femoral group in key baseline criteria: age (mean ± SD: 66 ± 10 versus 65 ± 9 years; p = 0.44), height, weight, BMI (33 ± 7 versus 33 ± 6 kg/m2; p = 0.76), American Society of Anesthesiologists (ASA) classification, and surgery time (Table 1). There was no difference in the median catheter placement time. Based on prior literature and discussions among our research group, we determined that a clinically meaningful effect size for the adductor canal cohort would be twice the ambulation distance compared to the femoral group [14, 19]. Our sample size calculation was as follows: using a two-sided alpha error of 0.05 and an allocation ratio of 0.66 for the adductor group, we determined that a total of 88 patients would be required to achieve 80% power.
Table 1.
Patient characteristics
| Variable | Femoral CPNB group (n = 102) | Adductor canal CPNB group (n = 66) | p value |
|---|---|---|---|
| Age (years)* | 66 (10) | 65 (9) | 0.44 |
| Sex (male/female) (number of patients) | 98/4 | 61/5 | 0.32 |
| Height (m)* | 1.7 (0.1) | 1.7 (0.1) | 0.72 |
| Weight (kg)* | 101 (21) | 101 (23) | 0.98 |
| BMI (kg/m2)* | 33 (7) | 33 (6) | 0.76 |
| ASA class (number of patients) | 0.83 | ||
| 1 | 0 | 0 | |
| 2 | 17 | 10 | |
| 3 | 85 | 56 | |
| 4 | 0 | 0 | |
| 5 | 0 | 0 | |
| Time for CPNB placement (minutes) | 15 (5) | 16 (5) | 0.24 |
| Surgeon (number of patients) | 0.62 | ||
| 1 | 27 | 26 | |
| 2 | 38 | 22 | |
| 3 | 8 | 4 | |
| 4 | 11 | 3 | |
| 5 | 10 | 9 | |
| 6 | 6 | 0 | |
| 7 | 1 | 0 | |
| 8 | 1 | 2 | |
| Surgery time (minutes)* | 105 (27) | 105 (18) | 0.9 |
* Values are expressed as mean, with SD in parentheses; CPNB = continuous peripheral nerve block; ASA = American Society of Anesthesiology.
The perioperative management of all patients followed our institutional TKA clinical pathway. Apart from the change from femoral to adductor canal CPNB, the clinical pathway did not vary in any other aspect during the period of study. Preoperatively, all patients underwent insertion of a perineural catheter, either in the adductor canal or in proximity to the femoral nerve [22, 26]. These procedures were performed either by an attending regional anesthesiologist or a clinical regional anesthesia and acute pain medicine fellow supervised one-on-one by an attending regional anesthesiologist. Patients received moderate sedation during the procedure titrated to comfort while maintaining verbal responsiveness. All catheters were placed using a technique described previously [22, 25]. In brief, the target nerve was visualized in short axis with a high-frequency 6- to 13-MHz ultrasound transducer (HFL38, M-Turbo®; FUJIFILM Sonosite, Bothell, WA, USA), and the placement needle was guided in-plane toward the target nerve. Approximately 20 mL mepivacaine 1.5% was injected into the appropriate compartment to surround the target nerve via the placement needle; then a nonstimulating flexible epidural-type catheter (Arrow® FlexTip Plus®; Teleflex Medical, Research Triangle Park, NC, USA) was advanced up to 3 cm beyond the placement needle tip. Catheter tip location was confirmed by injecting 0.5 mL of air via the catheter under ultrasound [20]. After catheter placement, onset of sensory anesthesia in the target nerve distribution was confirmed before the patients’ transition to the operating room.
Intraoperatively, all patients received general anesthesia, but there was no standardization of anesthetic technique. Similarly, there was no restriction on the selection and dosing of intraoperative opioids; however, at our institution, opioid options are limited to fentanyl, morphine, and hydromorphone. All patients had tricompartment knee arthroplasty with patellar resurfacing and PCL-substituting implants under tourniquet control. A standard medial parapatellar approach (not a mini-incision) was used. Some surgeons routinely everted the patella while others did so only occasionally. Implants were either cemented or had hybrid fixation (uncemented femoral component). At the conclusion of surgery, all patients received periarticular injections of epinephrine-containing ropivacaine 0.2% (150 mL) with ketorolac 30 mg divided equally within the posterior capsule, retinacular layer, and subcutaneous tissue per routine [25]. Postoperatively, in the postanesthesia care unit, each perineural catheter was attached to a FDA-approved portable infusion device (ON-Q C-bloc with ONDEMAND™; I-Flow Corp, Lake Forest, CA, USA) set to deliver an infusion of ropivacaine 0.2% (basal rate of 6 mL/hour; patient-controlled bolus of 5 mL; 30-minute lockout interval). Patients were prescribed scheduled oxycodone, acetaminophen, and diclofenac plus additional oral oxycodone and intravenous morphine for breakthrough postoperative pain inadequately treated with the perineural ropivacaine infusion/bolus. None of the patients were prescribed intravenous opioid patient-controlled analgesia per protocol. Full weightbearing was allowed on the first postoperative morning, after the drain and urinary catheter were removed. Continuous passive motion was not used. Routine postoperative care on the surgical ward included a standardized regimen for physical therapy. Starting on POD 1, the patients underwent twice-daily physical therapy sessions consisting of transfers and ambulation with progression to stair climbing. Patients ambulated with the assistance of a front wheel walker but without the aid of a knee immobilizer on the operative limb.
Our primary outcome was total ambulation distances (meters) on POD 1 and 2 defined by the sum of the ambulation distances for the two physical therapy sessions on each day. Secondary outcomes included total daily opioid consumption (morphine milligram equivalents), pain scores at rest (numeric rating scale 0–10 where 0 = no pain and 10 = worst possible pain), and hospital LOS (days). We also compared falls occurring during POD 1 and 2 between our two groups.
Descriptive statistics were performed, and normality was assessed by the Kolmogorov-Smirnov test. Continuous variables were analyzed using Student’s t-test (normal distributions) or the Wilcoxon rank-sum test (nonnormal distributions); categorical variables were compared using the chi-square test or Fisher’s exact test when applicable (n < 5 in any field). We further examined the association between the primary outcome on POD 1 and CPNB technique by performing crude and adjusted ordinary least-squares linear regression. Potential confounders such as age, BMI, ASA status, perineural catheter insertion time, surgery time, and surgeon were forced into the model. For our secondary outcomes, we conducted repeated-measures ANOVA using a within-subject covariance structure of compound symmetry. All p values were two-sided, and a p value of less than 0.05 was considered statistically significant. We considered any statistically significant findings regarding our secondary outcomes as preliminary. STATA® 12.1 (STATA Corp, College Station, TX, USA) was used for all analyses.
Results
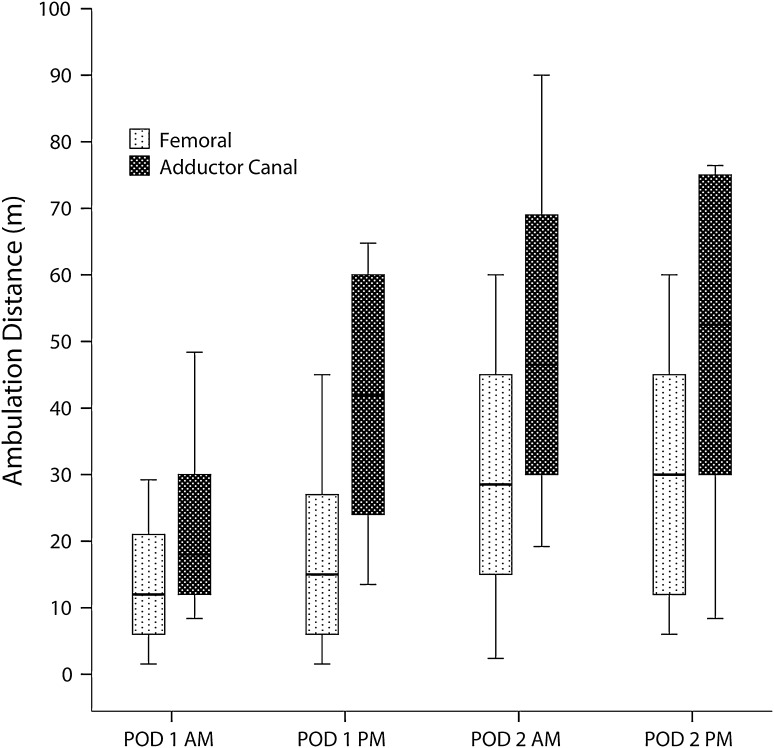
The total ambulation distance was higher in the adductor canal group than in the femoral group on POD 1 (median [10th–90th percentiles]: 37 m [0–90 m] versus 6 m [0–51 m]; p < 0.001) (Fig. 2). The adductor canal group also achieved greater ambulation distance on POD 2 (60 m [0–120 m] versus 21 m [0–78 m]; p = 0.003). Adjusted linear regression confirmed the statistically significant association between use of adductor canal catheters and ambulation distance on POD 1 (B = 23; 95% CI = 14–33; p < 0.001) and POD 2 (B = 19; 95% CI = 5–33; p = 0.008). While increasing BMI was associated with less ambulation on POD 1 in the adjusted linear regression, the effect of BMI on ambulation was small (B = − 0.81; 95% CI = − 1.55 to −0.07; p = 0.03). None of the other potentially confounding variables we analyzed, including surgeon (B = − 0.6; 95% CI = − 6.0 to 4.8; p = 0.82), appeared to influence ambulation.
Fig. 2.
A graph shows ambulation distance determined at each physical therapy session with two sessions each day, one in the morning and one in the afternoon. Horizontal lines represent medians; boxes represent 25th to 75th percentiles; whiskers represent 10th to 90th percentiles.
There were no differences between groups in our secondary outcomes for total postoperative opioid use on POD 1 or 2, pain at rest, or hospital LOS (Table 2). In addition, there were four falls in the femoral group and none in the adductor canal group (p = 0.15).
Table 2.
Secondary outcomes
| Variable | Femoral CPNB group (n = 102) | Adductor canal CPNB group (n = 66) | p value |
|---|---|---|---|
| Total opioid usage* | 0.72 | ||
| POD 1 | 64 (45) | 69 (39) | |
| POD 2 | 53 (38) | 51.2 (31) | |
| Pain score at rest† | 0.09 | ||
| POD 1 AM | 3 (3) | 4 (3) | |
| POD 1 PM | 3 (2) | 4 (3) | |
| POD 2 AM | 3 (3) | 3 (2) | |
| POD 2 PM | 2 (2) | 2 (2) | |
| Length of stay (days) | 4 (3) | 5 (5) | 0.80 |
Values are expressed as mean, with SD in parentheses; * morphine milligram equivalents; † numeric rating scale of 0 to 10 where 0 = no pain and 10 = worst possible pain; CPNB = continuous peripheral nerve block; POD = postoperative day.
Discussion
Since the NIH issued a consensus statement in 2003 identifying the need for evidence-based approaches for rehabilitation after TKA, studies have focused on functional outcomes after TKA [5, 14, 15, 23, 29]. Our study builds on prior research to further emphasize the important role of clinical analgesic pathways to promote early postoperative ambulation for patients with TKA [8, 9, 11]. In this single-center retrospective analysis of sequential patients undergoing TKA over 1 year, patients receiving adductor canal CPNB achieved greater ambulation distance on POD 1 and 2 but showed no differences in opioid consumption, pain scores, or hospital LOS compared with patients receiving femoral CPNB.
This study had a number of limitations. First, conclusions regarding causality should be approached cautiously because the study was retrospective. However, our study focused on the immediate postoperative period when the analgesic and motor effects of CPNB catheters are most relevant [10]. Second, there may have been selection bias based on patient characteristics or scheduling variability that may have affected how patients were treated in the clinical setting [2]. We attempted to minimize this bias by including consecutive surgical patients within the broad time frame of 1 year. Our patients showed no differences in key baseline characteristics. We also examined patients who were managed as part of a clinical pathway in which all other aspects of the clinical pathway (e.g., physical therapy regimen, nursing care, analgesic medications) remained constant during the study period. Moreover, it is worth noting that this study focused on actual patients undergoing TKA, instead of healthy volunteers, and the comparison of a newer technique to an established standard (i.e., femoral CPNB) [19]. Third, our results occurred within the context of a particular, established clinical pathway and therefore may not generalize well to hospitals practicing without a similar clinical pathway [1]. Fourth, our study comes from a single, university-affiliated VA medical center with factors typical to that practice setting (including a predominantly male patient population and the involvement of residents in patient care) and so may not be generalizable to every institution. However, the length of observation under conditions of routine clinical practice and management by multiple surgeons, a single surgery type, and a cohort consisting of a sequential series of patients support the external validity of our results. Fifth, we noted that, while our femoral group showed a wide range (10th–90th percentiles) of ambulation distance (0–51 m), the median was 6 m. While we adjusted for this in our linear models and while other studies have seen similarly substantial delays in ambulation owing to residual motor effects of a femoral nerve block of this magnitude [19], it certainly is possible to mobilize patients more effectively who have motor nerve blockade using knee immobilizers and assistive devices for ambulation. The fact that knee immobilizers are not included in our postoperative physical therapy protocol for patients undergoing TKA may have increased the apparent effect size of the adductor canal block. Sixth, our study did not evaluate quadriceps strength, so no firm conclusions can be made between preservation of muscle strength and functional outcomes. Finally, our study was not designed, nor powered, to examine differences in the secondary outcomes. Therefore, the results of these analyses should be interpreted as preliminary and not conclusive.
We found that the use of adductor canal CPNB was associated with an increase in ambulation distance on POD 1 and 2 compared to femoral CPNB. This increase is clinically relevant, given the desire for early and effective rehabilitation after TKA. Early ambulation after TKA has been shown to help decrease deep venous thrombosis of the legs, enhance muscle strength and gait control, and decrease hospital LOS [21, 30]. Although the analgesic benefits of CPNB in the setting of joint arthroplasty have been shown previously, clinical studies demonstrate that perineural local anesthetic infusions exert varying degrees of analgesia and motor block at different anatomic sites [11, 12, 14, 15, 17]. Recent studies of femoral nerve and lumbar plexus catheters have shown that typical perineural local anesthetic infusion doses produce clinically significant quadriceps weakness when administered via catheters inserted using conventional techniques [3, 6, 16]. Further, there have been concerns raised regarding a potential link between femoral nerve blocks and patient falls [6, 13]. We observed four in-hospital falls in our femoral group and none in our adductor canal group; however, our study was not powered to detect a difference in this uncommon complication between the treatment groups, so no statistical difference was observed. In-hospital falls can lead to prolonged hospital stays with higher costs and are associated with more frequent postoperative complications, including serious organ system dysfunction and death [27]. Since current local anesthetic medication options do not offer selectivity of sensory over motor nerves, it is important to optimize available technical options, including catheter insertion location, to maximize patient functional rehabilitation and other perioperative outcomes and to minimize important side effects such as muscle weakness [1, 18].
We found no difference between the two catheter techniques in terms of our secondary outcomes: pain scores, postoperative opioid consumption, and hospital LOS. In theory, the selective adductor canal block should provide a smaller distribution of sensory anesthesia and analgesia compared to the femoral nerve block since the femoral nerve often divides extensively as it passes the inguinal ligament. Despite this concern for potentially inferior analgesia with continuous adductor canal blocks, direct comparison of pain scores did not demonstrate differences between the two groups, and there were no differences in total opioid use between groups on POD 1 or 2. We can only speculate that, if our study had been specifically powered to examine postoperative pain scores on POD 1 and 2, then we may have detected a difference. Adequate analgesia and functional achievement during physical therapy are included in our institutional criteria for discharge eligibility. Although patients receiving adductor canal CPNBs ambulated further each postoperative day compared to patients receiving femoral CPNBs with similar analgesia, there was no difference in hospital LOS. Previous studies have shown that a single adjustment in regional anesthetic technique alone may not be sufficient to impact actual LOS, and other factors may play an important role [28, 31]. Since this study was not designed with sufficient power for these secondary outcomes, we should consider our results suggestive only [24]. Our study builds on prior work demonstrating analgesic efficacy of adductor canal CPNB to placebo and is one of the first to compare these two CPNB techniques directly with regard to functional outcomes in actual patients undergoing TKA [22].
In summary, we found the perioperative inclusion of adductor canal CPNB for patients undergoing TKA is associated with an increase in total ambulation distance on POD 1 compared to similar patients receiving femoral CPNBs within the same clinical analgesic pathway. The more distal catheter insertion site in the adductor canal does not negatively affect analgesic efficacy when a multimodal analgesic approach is employed. Future randomized studies should be performed to validate our major findings.
Acknowledgments
The authors thank Dr. Ronald Pearl, Chair, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University, for his general support of this research and Dr. Todd Wagner, Associate Director, Center for Health Care Evaluation, Veterans Affairs Palo Alto Health Care System, for his guidance on statistical methods.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Veterans Affairs Palo Alto Health Care System (Palo Alto, CA, USA).
References
- 1.Barbieri A, Vanhaecht K, Van Herck P, Sermeus W, Faggiano F, Marchisio S, Panella M. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32. doi: 10.1186/1741-7015-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 3.Charous MT, Madison SJ, Suresh PJ, Sandhu NS, Loland VJ, Mariano ER, Donohue MC, Dutton PH, Ferguson EJ, Ilfeld BM. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115:774–781. doi: 10.1097/ALN.0b013e3182124dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, Criswell A. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16:436–445. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 5.Cushner F, Agnelli G, FitzGerald G, Warwick D. Complications and functional outcomes after total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY) Am J Orthop. 2010;39:22–28. [PubMed] [Google Scholar]
- 6.Feibel RJ, Dervin GF, Kim PR, Beaule PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. J Arthroplasty. 2009;24:132–137. doi: 10.1016/j.arth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Grant SA, Nielsen KC, Greengrass RA, Steele SM, Klein SM. Continuous peripheral nerve block for ambulatory surgery. Reg Anesth Pain Med. 2001;26:209–214. doi: 10.1053/rapm.2001.22256. [DOI] [PubMed] [Google Scholar]
- 8.Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, Hanssen AD, Horlocker TT. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. [PubMed] [Google Scholar]
- 9.Hebl JR, Kopp SL, Ali MH, Horlocker TT, Dilger JA, Lennon RL, Williams BA, Hanssen AD, Pagnano MW. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):63–70. doi: 10.2106/JBJS.E.00491. [DOI] [PubMed] [Google Scholar]
- 10.Ilfeld BM. Continuous peripheral nerve blocks in the hospital and at home. Anesthesiol Clin. 2011;29:193–211. doi: 10.1016/j.anclin.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–925. doi: 10.1213/ANE.0b013e3182207778. [DOI] [PubMed] [Google Scholar]
- 12.Ilfeld BM, Ball ST, Gearen PF, Le LT, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Meyer RS. Ambulatory continuous posterior lumbar plexus nerve blocks after hip arthroplasty: a dual-center, randomized, triple-masked, placebo-controlled trial. Anesthesiology. 2008;109:491–501. doi: 10.1097/ALN.0b013e318182a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–713. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–484. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilfeld BM, Moeller LK, Mariano ER, Loland VJ, Stevens-Lapsley JE, Fleisher AS, Girard PJ, Donohue MC, Ferguson EJ, Ball ST. Continuous peripheral nerve blocks: is local anesthetic dose the only factor, or do concentration and volume influence infusion effects as well? Anesthesiology. 2010;112:347–354. doi: 10.1097/ALN.0b013e3181ca4e5d. [DOI] [PubMed] [Google Scholar]
- 17.Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96:1089–1095. [DOI] [PubMed]
- 18.Ilfeld BM, Yaksh TL. The end of postoperative pain—a fast-approaching possibility? And, if so, will we be ready? Reg Anesth Pain Med. 2009;34:85–87. doi: 10.1097/AAP.0b013e3181962547. [DOI] [PubMed] [Google Scholar]
- 19.Jaeger P, Nielsen ZJ, Henningsen MH, Hilsted KL, Mathiesen O, Dahl JB. Adductor canal block versus femoral nerve block and quadriceps strength: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Anesthesiology. 2013;118:409–415. doi: 10.1097/ALN.0b013e318279fa0b. [DOI] [PubMed] [Google Scholar]
- 20.Kan JM, Harrison TK, Kim TE, Howard SK, Kou A, Mariano ER. An in vitro study to evaluate the utility of the “air test” to infer perineural catheter tip location. J Ultrasound Med. 2013;32:529–533. doi: 10.7863/jum.2013.32.3.529. [DOI] [PubMed] [Google Scholar]
- 21.Labraca NS, Castro-Sanchez AM, Mataran-Penarrocha GA, Arroyo-Morales M, Sanchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25:557–566. doi: 10.1177/0269215510393759. [DOI] [PubMed] [Google Scholar]
- 22.Lund J, Jenstrup MT, Jaeger P, Sorensen AM, Dahl JB. Continuous adductor-canal-blockade for adjuvant post-operative analgesia after major knee surgery: preliminary results. Acta Anaesthesiol Scand. 2011;55:14–19. doi: 10.1111/j.1399-6576.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 23.Mahomed NN, Liang MH, Cook EF, Daltroy LH, Fortin PR, Fossel AH, Katz JN. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J. Rheumatol. 2002;29:1273–1279. [PubMed] [Google Scholar]
- 24.Mariano ER, Ilfeld BM, Neal JM. “Going fishing”—the practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med. 2007;32:183–185. doi: 10.1016/j.rapm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Mariano ER, Kim TE, Funck N, Walters T, Wagner MJ, Harrison TK, Giori N, Woolson S, Ganaway T, Howard SK. A randomized comparison of long-and short-axis imaging for in-plane ultrasound-guided femoral perineural catheter insertion. J Ultrasound Med. 2013;32:149–156. doi: 10.7863/jum.2013.32.1.149. [DOI] [PubMed] [Google Scholar]
- 26.Mariano ER, Loland VJ, Sandhu NS, Bellars RH, Bishop ML, Afra R, Ball ST, Meyer RS, Maldonado RC, Ilfeld BM. Ultrasound guidance versus electrical stimulation for femoral perineural catheter insertion. J Ultrasound Med. 2009;28:1453–1460. doi: 10.7863/jum.2009.28.11.1453. [DOI] [PubMed] [Google Scholar]
- 27.Memtsoudis SG, Dy CJ, Ma Y, Chiu YL, Della Valle AG, Mazumdar M. In-hospital patient falls after total joint arthroplasty: incidence, demographics, and risk factors in the United States. J Arthroplasty. 2012;27(823–828):e1. doi: 10.1016/j.arth.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napier RJ, Spence D, Diamond O, O’Brien S, Walsh T, Beverland DE. Modifiable factors delaying early discharge following primary joint arthroplasty. Eur J Orthop Surg Traumatol. 2012 July 22 [Epub ahead of print]. [DOI] [PubMed]
- 29.NIH Consensus Statement on total knee replacement. NIH Consens State Sci Statements. 2003;20:1–34. [PubMed] [Google Scholar]
- 30.Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of postoperative venous thromboembolism. J Bone Joint Surg Br. 2007;89:316–322. doi: 10.1302/0301-620X.89B3.18196. [DOI] [PubMed] [Google Scholar]
- 31.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]