Abstract
Background
Total knee arthroplasty (TKA) can be associated with considerable postoperative pain. Ischemic preconditioning of tissue before inducing procedure-related underperfusion may reduce the postoperative inflammatory response, which further may reduce associated pain.
Questions/purposes
In this prospective, randomized study, we aimed at evaluating the impact of ischemic preconditioning on postoperative pain at rest and during exercise; use of pain medication; levels of systemic prothrombotic and local inflammatory markers; and length of stay and achievement of physical therapy milestones.
Methods
Sixty patients undergoing unilateral TKA under tourniquet were enrolled with half (N = 30) being randomized to an episode of limb preconditioning before induction of ischemia for surgery (tourniquet inflation). Pain scores, analgesic consumption, markers of inflammation (interleukin-6 [IL-6], tumor necrosis factor [TNF]-α in periarticular drainage), and periarticular circumference were measured at baseline and during 2 days postoperatively. Changes in prothrombotic markers were evaluated.
Results
Patients in the preconditioning group had significantly less pain postoperatively at rest (mean difference = −0.71, 95% confidence interval [CI] = −1.40 to −0.02, p = 0.043) and with exercise (mean difference = −1.38, 95% CI = −2.32 to −0.44, p = 0.004), but showed no differences in analgesic consumption. No differences were seen between the study and the control group in terms of muscle oxygenation and intraarticular levels of IL-6 and TNF-α as well as levels of prothrombotic markers. No differences were found between groups in regard to hospitalization length and time to various physical therapy milestones.
Conclusions
Ischemic preconditioning reduces postoperative pain after TKA, but the treatment effect size we observed with the preconditioning routine used was modest.
Clinical Relevance
Given the ease of this intervention, ischemic preconditioning may be considered as part of a multimodal analgesic strategy. However, more study into the impact of different preconditioning strategies, elucidation of mechanisms, safety profiles, and cost-effectiveness of this maneuver is needed.
Introduction
Postoperative pain can be severe after TKA. Current analgesic therapies focus on a combination of regional anesthetic and various oral and parenteral pharmacologic agents. However, studies of nonpharmacologic approaches to alleviate pain postoperatively remain rare [14].
In a previous study focusing on the systemic perioperative inflammatory response in patients undergoing knee arthroplasty, we incidentally found evidence suggesting that patients undergoing a brief period of preconditioning of the operative leg before the inflation of a tourniquet for TKA experienced reduced levels of pain [14]. “Preconditioning” was defined in that study as the inflation of a tourniquet for 5 minutes in the operated limb followed by deflation and reperfusion before reinflation for surgery. Unfortunately, in this study, pain was not the primary outcome, thus rendering the results of limited use. Furthermore, pain data were reviewed restrospectively from chart records, no information on standardized assessment during rest and exercise was available, and no evaluations of the impact of preconditioning on local inflammation and its effect on markers of thrombosis were undertaken. Although we hypothesized that preconditioning may positively influence the level of local inflammation at the site of surgery as suggested by previous experimental evidence [15], no such data were available from our study at the time. Moreover, changes in systemic levels of coagulation activity were not measured in this study, and there is conflicting evidence about the systemic impact of tourniquet maneuvers on coagulation status [10, 18].
Therefore, we designed the current study to evaluate following questions: (1) Can the use of preconditioning reduce postoperative pain scores compared with a control group undergoing TKA under tourniquet ischemia without this intervention? (2) Can preconditioning decrease local inflammation as evaluated by the extent of postoperative periarticular circumference, levels of cytokines found in the intraarticular fluid, and levels of muscle tissue oxygenation in the operative limb, thus potentially providing a hypothetical mechanism for discrepancies in pain levels found previously? (3) Is preconditioning associated with an increase in systemic levels of coagulation markers (prothrombin fragments F1/F2, D-dimer, and thrombin-antithrombin complex [TAT] [11, 17]) between the groups and across various time points? (4) Does preconditioning impact on the length of stay and achievement of physical therapy milestones as found in the previous study?
Materials and Methods
This study was approved by the Hospital for Special Surgery institutional review board. Sixty patients undergoing elective, unilateral primary TKA were enrolled for this study after obtaining written informed consent. Patients with contraindications to tourniquet use (ie, peripheral vascular disease or previous arterial bypass grafts), bleeding disorders, immunocompromise, and those on chronic pain therapy (daily narcotic use for more than 1 month) were excluded from the study. Moreover, patients were considered ineligible whenever their pre- or postoperative analgesia protocol deviated from the standard of care (eg, spinal anesthesia only), when language exclusions were present, in case of no planned use of tourniquet, or when knee procedures other than primary TKA were performed (manipulation, bilateral or revision surgery).
All patients underwent surgery using a combined spinal and epidural anesthetic (12.5 mg bupivacaine 0.5%). All patients received intraoperative femoral nerve blocks with 30 mL bupivacaine 0.25% with epinephrine 1:200,000 for postoperative pain control as per institutional practice [22]. Patients were monitored according to American Society of Anesthesiologists guidelines [2]. Intraoperative sedation was achieved with 5 mg midazolam and propofol was titrated to achieve sedation while maintaining adequate respirations.
After performance of neuraxial anesthesia and nerve blockade, patients followed the protocols assigned to the control group (no preconditioning, n = 30) or to the experimental group, which consisted of beginning surgery after a period of preconditioning defined as tourniquet inflation for 5 minutes followed by deflation and a 5-minute reperfusion period (n = 30). The preconditioning was performed during prepping and draping to avoid delaying the surgery. Surgeries in all 60 patients were performed under tourniquet inflation to 250 mmHg. Before tourniquet application for preconditioning and before the surgery in both groups, the operative limb was exsanguinated by passive leg raise followed by application of an Esmarch elastic bandage from distal to proximal. All surgeries were performed through a midline incision with a medial parapatellar arthrotomy [14]. All patients received a surgeon-selected cemented posterior-stabilized prosthetic design with patellar resurfacing. The tourniquet was deflated after all implants were cemented.
Postoperatively, patients were observed in a monitored environment and transferred to the ward after meeting criteria for that transfer. Postoperative pain management consisted of an epidural infusion of 10 mcg/ml bupivacaine 0.06%/hydromorphone with a basal rate of 4 mL, a demand dose of 4 mL, and a lockout of 10 minutes. Basal rates were reduced to 2 mL and 0 mL in the morning of postoperative Days 1 and 2, respectively, and epidural catheters were removed at noon on postoperative Day 2. Up to two tablets of oral hydrocodone/acetaminophen (5 mg/325 mg) were made available to all patients every 4 hours. Blocks were not specifically assessed for failure; however, both groups were subject to the same risk of this event.
Patient demographics and perioperative data, including volume of fluid administered during surgery and tourniquet times, were recorded blindly to the group allocation. Postoperative pain was measured using visual analog scale (VAS) scores [8] at 6 hours, 24 hours, and 48 hours postoperatively. Pain scores were also collected before surgery and assessed at each time point at rest and with standardized exercise constituting leg flexion. Overall epidural volume and oral opioid consumption measured in morphine equivalents were assessed.
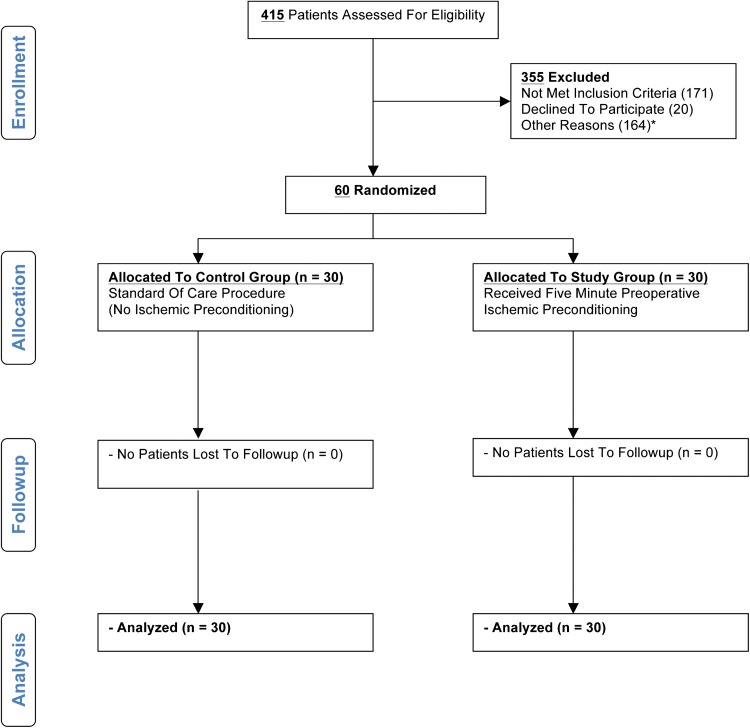
All 60 patients concluded the study (Fig. 1). Demographic and procedure-related variables were similar between groups (Table 1). The average tourniquet time was 6 minutes longer among control subjects, but this variable discrepancy was accounted for in the regression analysis.
Fig. 1.
This figure shows the CONSORT flow diagram with information about enrollment and followup. *Other reasons for exclusion are: patient was part of another study (data integrity concerns) or timing of surgery not suitable for data collection.
Table 1.
Demographics and procedure-related variables of the preconditioning and control groups
| Variable | Preconditioning (n = 30) |
Control (n = 30) |
p value | ||
|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||
| Sex | |||||
| Male | 11 | 36.7 | 16 | 53.3 | 0.1945 |
| Female | 19 | 63.3 | 14 | 46.7 | |
| ASA | |||||
| 1 | 1 | 3.3 | 1 | 3.3 | 0.8779 |
| 2 | 23 | 76.7 | 21 | 70.0 | |
| 3 | 6 | 20.0 | 8 | 26.7 | |
| Median | IQR | Median | IQR | p value | |
|---|---|---|---|---|---|
| Age (years) | 66.5 | 10.8 | 72.5 | 13.3 | 0.0927 |
| Body mass index (kg/m2) | 26.8 | 5.9 | 28.1 | 7.2 | 0.9062 |
| Intraoperative estimated blood loss (mL) | 200 | 50.0 | 200 | 0.0 | 0.0246 |
| Fluid intake (mL) | 1700 | 500.0 | 1800 | 575.0 | 0.8061 |
| Tourniquet time (minutes) | 47.5 | 13.3 | 54 | 17.5 | 0.0319 |
ASA = American Society of Anesthesiologists; IQR = interquartile range.
To investigate the potential impact of preconditioning on the local inflammatory response, a number of surrogate markers were examined. First, intraarticular fluid was aspirated from the drain at the end of surgery and examined for levels of tumor necrosis factor-α (TNF-α) and for interleukin-6 (IL-6) as described previously [14]. For this purpose, samples were centrifuged at 3000 rpm for 5 minutes and the supernatant frozen at < −18° F. For analysis, solid-phase, enzyme-labeled, chemiluminescent sequential immunometric assays (Immulite; Siemens, Los Angeles, CA, USA) were used. Results are reported in pg/mL. Second, periarticular circumference (defined as thigh circumference at the midpoint between the patella and anterosuperior iliac spine) was measured at baseline and at 48 hours postsurgery [13]. Third, muscle tissue oxygenation was measured using a novel noninvasive near-infrared spectroscopy device at baseline and at 48 hours postoperatively [20].
To test if the use of preconditioning would adversely affect the patients’ coagulation status, we measured systemic levels of the procoagulatory markers prothrombin fragments F1/F2, D-dimer, and TAT at the following time points: (1) preoperatively; (2) after tourniquet release; and (3) 3 hours postoperatively [11].
Patient length of hospitalization as well as time to reaching physical therapy milestones (independent transfer from sitting position to wheelchair and back, patient able to ambulate 40 feet with a crutch or cane, full knee extension) was compared between groups.
The patients and assessors were blinded to the study intervention. Surgeons and anesthesiologists were reminded not to reveal the study treatment to patients for followup care. The assessors were not present when the study treatment was used, and they were not allowed to view patients’ operative notes including tourniquet times in the medical charts.
Statistics
An earlier work examining the impact of limb preconditioning [14] found that patients in the control group had a median VAS score of 2.7 (± 1.5) on average at 48 hours after TKA as compared with 1.6 (± 1.4) in the intervention group. Using this result, we anticipated a requirement of 30 patients per group to detect a statistically significant difference in pain scores with 80% power and 5% Type I error. Descriptive statistics include median and interquartile range for continuous variables and proportion for discrete variables. Continuous and discrete demographic and perioperative variables as well as values for laboratory tests were compared between groups using Mann-Whitney-Wilcoxon rank test and Fisher’s exact test, respectively. Regression analyses based on the generalized estimating equations (GEE) method [23] were conducted to compare the changes over time in VAS scores at rest and with exercise between groups while controlling for age, sex, American Society of Anesthesiologists classification, tourniquet time, and body mass index. Levels of systemic markers of coagulation were compared in separate GEE models, adjusting for the same potential confounders mentioned before. The GEE method is able to take into account correlations between repeated measures and does not require the outcome to follow a normal distribution, leading to a robust parameter estimation. Because of these appealing properties, the GEE method has been recommended for analysis of longitudinal data in a variety of research, including studies in anesthesiology and pain research [12].
Results
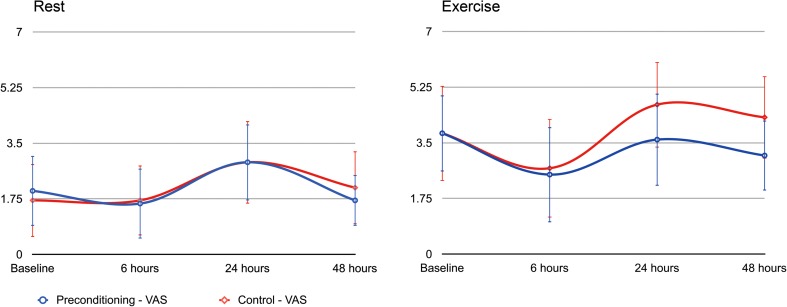
Patients in the preconditioning group had less postoperative pain at rest (p = 0.043) and with exercise (p = 0.004), even after controlling for covariates (sex, age, body mass index, American Society of Anesthesiologists classification, and tourniquet time). The adjusted mean VAS score was 0.71 points (95% confidence interval [CI] = −1.40 to −0.02) lower during rest and 1.38 points (95% CI = −2.32 to −0.44) lower during exercise in the preconditioning group than in control subjects, respectively. The biggest differences in VAS scores were seen at 24- and 48-hour measurements during exercise, presumably because these periods represented observations made after resolution of the femoral nerve block (Fig. 2). With the numbers available, we observed no differences in terms of the total amount of epidural patient-controlled analgesia volume (140.5 ± 68.4 mL versus 160.8 ± 70.1 mL; p = 0.432) or oral morphine equivalents (76.9 ± 37.1 mg versus 81.8 ± 39.9 mg; p = 0.111) used.
Fig. 2.
This figure depicts the unadjusted pain scores obtained at baseline and 6, 24, and 48 hours postoperatively, respectively, at rest and with light exercise. VAS = visual analog scale.
There were no differences in levels of inflammatory markers between the groups. The postsurgical intraarticular levels of TNF-α and IL-6 were not different between the groups (p = 0.526 and p = 0.763, respectively) nor was the postoperative increase in periarticular circumference (2.3 ± 3.2 cm and 1.5 ± 1.1 cm, respectively; p = 0.602) or muscle tissue oxygenation at 48 hours (75% ± 22.4% versus 69% ± 18.5%; p = 0.584).
All systemic markers of procoagulation significantly increased between baseline (preoperatively) and 3 hours postoperatively (Table 2), but there were no differences between the study groups. No differences were found between groups in regard to hospitalization length (108 ± 39 hours versus 100 ± 19 hours; p = 0.8656) or in terms of the time to various physical therapy milestones (Table 3).
Table 2.
Systemic markers of coagulation*
| Marker | Time | Preconditioning | Control | p value | ||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| Prothrombin fragments F1/F2 | Preoperatively | 252.1 | 244.5 | 344.7 | 217.9 | 0.1827 |
| Tourniquet release | 421.7 | 617.5 | 519.8 | 303.4 | ||
| 3 hours postoperatively | 848.0 | 1176.0 | 954.1 | 1540.7 | ||
| D-dimer | Preoperatively | 693.5 | 306.5 | 574.6 | 300.4 | 0.9749 |
| Tourniquet release | 986.5 | 598.0 | 1027.6 | 551.3 | ||
| 3 hours postoperatively | 8625.3 | 5999.2 | 7481.4 | 6194.2 | ||
| Thrombin-antithrombin complex (TAT) | Preoperatively | 7.3 | 28.0 | 8.4 | 12.6 | 0.2125 |
| Tourniquet release | 35.3 | 86.0 | 22.8 | 28.7 | ||
| 3 hours postoperatively | 69.0 | 66.2 | 59.6 | 74.2 | ||
* All systemic markers of coagulation significantly increased between preoperatively and 3 hours postoperatively (whole cohort: prothrombin fragments F1/F2: p = 0.0011; D-dimer: p < 0.0001; TAT: p = 0.0021). However, there were no differences between the preconditioning and control groups concerning the magnitude of this effect; IQR = interquartile range.
Table 3.
Hospitalization and physical therapy milestones*
| Preconditioning | Control | p value | |||
|---|---|---|---|---|---|
| Variable | Median | IQR | Median | IQR | |
| Length of hospital stay (hours) | 100.6 | 44.4 | 99.4 | 36.0 | 0.8656 |
| Extension ROM < 10 (days) | 1.0 | 0.8 | 1.0 | 1.0 | 0.7364 |
| Ambulation 40ft (days) | 2.0 | 1.0 | 3.0 | 1.0 | 0.8422 |
| Independent transfer (days) | 3.5 | 1.3 | 4.0 | 1.0 | 1.0000 |
* No difference was found between groups in regard to hospitalization length (108 ± 39 hours versus 100 ±19 hours; p = 0.8656) and the time to various physical therapy milestones; IQR = interquartile range.
Discussion
Inadequate pain control after TKA can decrease patient satisfaction with the experience and in the worst case result in permanent stiffness [4, 21]. Although many pharmacological and procedural interventions have been investigated to determine their ability to impact on pain, few data exist on noninvasive, nonpharmacological approaches to achieve this goal [14].
In this study we found that ischemic preconditioning before TKA reduced patient-reported postoperative pain compared with a control group already receiving intense multimodal pain management after TKA without any adverse impact on levels of factors associated with procoagulation. No significant differences were found in the overall consumption of analgesics between the group treated with preconditioning and the control group (no preconditioning) or in terms of length of stay and physical therapy milestones, suggesting that the treatment effect size associated with preconditioning is relatively modest. However, the 5-minute time period was chosen based on previous experience, and different preconditioning strategies may lead to other and/or more pronounced effects.
A number of limitations must be considered when interpreting our data. Although our study was powered to identify differences in postoperative pain levels between groups, the size of our study sample was likely inadequate to determine differences in the secondary outcomes, particularly those targeted to evaluate local inflammation. Furthermore, although we attempted to use previously described methods to evaluate inflammatory changes [9, 14, 16], their validity and clinical relevance remain uncertain. However, we attempted to collect data on the basis of a proof of concept design rather than provide definitive conclusions. Thus, they may be used as pilot data for a larger, perhaps multicenter study. In addition, our data have to be interpreted in the context of our practice concerning the use of multimodal analgesia including neuraxial, per-oral, and peripheral nerve block techniques. It is known that regional anesthetic techniques can positively influence the perioperative inflammatory response, and thus (although speculative) the effects of preconditioning may be underestimated in this setting [3]. Studies in which patients could be randomized without the effect of regional anesthetics may be able to elucidate this issue further.
Preconditioning was associated with a decrease in postoperative pain scores at rest and with exercise compared with control subjects in this study. However, the effect size was small, between 0.7 and 1.4 points on a 10-cm VAS. Although in the latter case this presents an approximately 30% difference, commonly used as a threshold [6], it remains questionable if such small decreases are clinically significant. Even so, this finding is especially remarkable, because patients at our institution already receive epidural analgesia with a femoral nerve block and oral analgesics. Our results are in concordance with previous investigations suggesting that preconditioning may be associated with a decrease in postoperative pain after knee surgery. Orban et al. [16] investigated the effect of administration of N-acetyl-cysteine, an antioxidant, and preconditioning of the lower extremity in patients undergoing knee ligamentoplasty. Although those authors were not able to show differences in markers of local muscle tissue damage (myoglobin and creatinine phosphatase levels as well as muscular functional parameters) compared with a control group, they detected lower narcotic use in the treatment group. Given the fact that this study was limited to patients undergoing ligamentoplasty, tourniquet times, and thus tissue reperfusion damage to the muscle, which is relatively resistant to ischemic injury, may have been limited and not applicable to patients undergoing TKA [16]. In our own pilot study of 34 patients that was primarily targeted to identify if preconditioning had an effect on the levels of systemic inflammatory markers, we found that the control group had higher median pain scores compared with the treatment (preconditioning) group. However, the assessment of pain scores postoperatively was only a secondary outcome and no systematic approach to its evaluation and potential link to local markers of inflammation was studied [14]. The mechanism(s) by which preconditioning might lead to reduced pain scores remains elusive. It has been suggested that postoperative inflammation and swelling of the operated extremity may contribute to the degree of pain [7]. Further reperfusion injury after tourniquet ischemia for knee surgery may play a role in the degree of the local inflammatory response and therefore pain [14, 16]. Studies in animals suggest that applying the principal of preconditioning may decrease the level of inflammation by virtue of reduced tissue damage [9]. Schoen et al. [19] reported that ischemic preconditioning before prolonged application of a hind limb tourniquet in rats resulted in reduced inflammatory cell response, reduced rates of apoptotic cell death, and improved microcirculation. However, preconditioning also led to a greater extent of nerve degeneration, loss of pain sensation, and motoric function than tourniquet application alone.
Given these data, we sought to study local markers of inflammation previously used in various studies [9, 14, 16] and compare them between groups. No differences were found. Reasons may include the use of an extensive regional anesthetic protocol at a relatively short preconditioning time, thus at least in theory leaving open the possibility that changes in these factors may yield different results.
Similarly, no differences were seen in systemic levels of coagulation activation. Although evidence is emerging that even mild episodes of limb ischemia might elicit pathologic changes like increased leukocyte adhesiveness, endothelial damage, and activation of a procoagulatory state with possible risk of thrombogenesis [10], the clinical relevance of these findings remains unknown. In a recent clinical study, Reikerås et al. found significant local thrombogenic and fibrinolytic activity in the operative leg after tourniquet deflation but no significant systemic impact [18]. Moreover, it was previously suggested that a hypercoagulative state is more likely to appear in TKA cases in which no tourniquet is used [1]. In this study we did not specifically screen for clinically relevant clotting events, but it remains doubtful that we would have been able to detect any such outcomes with such a limited preconditioning routine.
No differences in outcomes concerning length of stay or achievement of physical therapy milestones were found. This is in contrast to our previous publication [14], in which we identified a reduction in the length of stay among the preconditioning group. The reasons for this discrepancy have to remain speculative because it seems feasible that patients with better pain control may be discharged earlier because postoperative pain has been shown to be an important determinant of discharge readiness [5]. In this context it must be noted that a number of initiatives were introduced in our institution with the goal to reduce length of stay since the time of our last investigation. These cumulative changes may therefore have reduced the relative impact of pain management on this outcome.
We found that preconditioning achieves a modest reduction in postoperative pain after TKA. Although the mechanism(s) of this action remain(s) elusive, the simplicity of this intervention should be considered as an adjunct in patients undergoing TKA to alleviate pain postoperatively. The widespread adoption of this practice, however, requires that more extensive studies substantiate our findings, evaluate safety and cost-effectiveness as well as evaluate outcomes in more diverse practice settings. Furthermore, different preconditioning routines may yield different result and require further investigation.
Acknowledgments
We thank Dr J. Matthias Walz and Dr Babs Soller, University of Massachusetts Medical School, and Reflectance Medical, Inc (Westborough, MA, USA) for providing the near-infrared oximetry monitoring device. We thank the Department of Anesthesiology, University of Massachusetts Medical School, for providing some equipment used for this study. We thank the Weill Medical College of Cornell University Clinical and Translational Science Center Core Laboratory for conducting all laboratory analyses.
Footnotes
The authors’ institution has received funding from the Anna-Maria and Stephen Kellen Physician-Scientist Career Development Award provided by the Hospital for Special Surgery (SGM) and Mr Glenn Bergenfield and the Simon Foundation (AGDV). The laboratory that conducted the analyses, Weill Medical College of Cornell University Clinical and Translational Science Center (CTSC) Core Laboratory, received funding through CTSC grant UL1 TR000457-06.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Analysis of Nationwide Inpatient Sample data files was performed at Weill Medical College of Cornell University, New York, NY, USA.
References
- 1.Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res. 2000;371:169–177. doi: 10.1097/00003086-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists. ASA Physical Status Classification System. Available at: www.asahq.org/Home/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System. Accessed July 2012.
- 3.Bagry H, De la Cuadra Fontaine JC, Asenjo JF, Bracco D, Carli F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med. 2008;33:17–23. doi: 10.1016/j.rapm.2007.06.398. [DOI] [PubMed] [Google Scholar]
- 4.Chang CB, Cho WS. Pain management protocols, peri-operative pain and patient satisfaction after total knee replacement: a multicentre study. J Bone Joint Surg Br. 2012;94:1511–1516. doi: 10.1302/0301-620X.94B11.29165. [DOI] [PubMed] [Google Scholar]
- 5.Duellman TJ, Gaffigan C, Milbrandt JC, Allan DG. Multi-modal, pre-emptive analgesia decreases the length of hospital stay following total joint arthroplasty. Orthopedics. 2009;32:167. doi: 10.3928/01477447-20090301-08. [DOI] [PubMed] [Google Scholar]
- 6.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Ju H, Yang B, An H. Effects of a selective cyclooxygenase-2 inhibitor on postoperative inflammatory reaction and pain after total knee replacement. J Pain. 2008;9:45–52. doi: 10.1016/j.jpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty SA. Pain measurement tools for clinical practice and research. AANA J. 1996;64:133–140. [PubMed] [Google Scholar]
- 9.Harkin DW, Barros D’Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg. 2002;35:1264–1273. doi: 10.1067/mva.2002.121981. [DOI] [PubMed] [Google Scholar]
- 10.Hughes SF, Hendricks BD, Edwards DR, Bastawrous SS, Roberts GE, Middleton JF. Mild episodes of tourniquet-induced forearm ischaemia-reperfusion injury results in leukocyte activation and changes in inflammatory and coagulation markers. J Inflamm (Lond). 2007;4:12. doi: 10.1186/1476-9255-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G, Cervellin G, Franchini M, Favaloro EJ. Biochemical markers for the diagnosis of venous thromboembolism: the past, present and future. J Thromb Thrombolysis. 2010;30:459–471. doi: 10.1007/s11239-010-0460-x. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med. 2012;37:99–105. doi: 10.1097/AAP.0b013e31823ebc74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105:815–821. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memtsoudis SG, Valle AG, Jules-Elysse K, Poultsides L, Reid S, Starcher B, Ma Y, Sculco TP. Perioperative inflammatory response in total knee arthroplasty patients: impact of limb preconditioning. Reg Anesth Pain Med. 2010;35:412–416. doi: 10.1097/AAP.0b013e3181e82e8e. [DOI] [PubMed] [Google Scholar]
- 15.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.RES.66.4.913. [DOI] [PubMed] [Google Scholar]
- 16.Orban JC, Levraut J, Gindre S, Deroche D, Schlatterer B, Ichai C, Grimaud D. Effects of acetylcysteine and ischaemic preconditioning on muscular function and postoperative pain after orthopaedic surgery using a pneumatic tourniquet. Eur J Anaesthesiol. 2006;23:1025–1030. doi: 10.1017/S026502150600086X. [DOI] [PubMed] [Google Scholar]
- 17.Páramo JA. Prothrombin fragments in cardiovascular disease. Adv Clin Chem. 2010;51:1–23. doi: 10.1016/S0065-2423(10)51001-1. [DOI] [PubMed] [Google Scholar]
- 18.Reikerås O, Clementsen T. Time course of thrombosis and fibrinolysis in total knee arthroplasty with tourniquet application. Local versus systemic activations. J Thromb Thrombolysis. 2009;28:425–428. doi: 10.1007/s11239-008-0299-6. [DOI] [PubMed] [Google Scholar]
- 19.Schoen M, Rotter R, Gierer P, Gradl G, Strauss U, Jonas L, Mittlmeier T, Vollmar B. Ischemic preconditioning prevents skeletal muscle tissue injury, but not nerve lesion upon tourniquet-induced ischemia. J Trauma. 2007;63:788–797. doi: 10.1097/01.ta.0000240440.85673.fc. [DOI] [PubMed] [Google Scholar]
- 20.Soller BR, Ryan KL, Rickards CA, Cooke WH, Yang Y, Soyemi OO, Crookes BA, Heard SO, Convertino VA. Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit Care Med. 2008;36:176–182. doi: 10.1097/01.CCM.0000295586.83787.7E. [DOI] [PubMed] [Google Scholar]
- 21.Vince KG. The stiff total knee arthroplasty: causes and cures. J Bone Joint Surg Br. 2012;94:103–111. doi: 10.1302/0301-620X.94B11.30793. [DOI] [PubMed] [Google Scholar]
- 22.YaDeau JT, Cahill JB, Zawadsky MW, Sharrock NE, Bottner F, Morelli CM, Kahn RL, Sculco TP. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg. 2005;101:891–895. doi: 10.1213/01.ANE.0000159150.79908.21. [DOI] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]