Abstract
Background
Fibrous dysplasia of bone is a skeletal dysplasia with a propensity to affect the femur in its polyostotic form, leading to deformity, fracture, and pain. The proximal femur is most commonly involved with a tendency to distal progression, thereby producing the typical shepherd’s crook deformity. However, there are few data on the spectrum and progression of femoral deformities in polyostotic fibrous dysplasia.
Questions/purposes
The purposes of this study were (1) to develop a radiographic classification for polyostotic fibrous dysplasia; (2) to test this classification’s intra- and interobserver reliability; and (3) to characterize the radiographic progression of polyostotic fibrous dysplasia in a population of patients with the condition who were treated with a variety of approaches at several centers.
Methods
We retrospectively reviewed radiographs of 127 femurs from 84 adult patients affected by polyostotic fibrous dysplasia. Fifty-nine femurs had undergone one or more operations. The radiographs were evaluated in the coronal plane for neck-shaft angle and angular deformities along the whole femoral shaft down to the distal epiphysis. Four observers evaluated each film two times at intervals; intra- and interobserver reliability testing was performed using the kappa statistic. Eighty-nine femurs (70%) were available for followup to evaluate for progression at a mean of 10 years (range, 6–20 years).
Results
Six reproducible patterns of deformity were identified in both untreated and operated femurs: type 1 (24%), normal neck-shaft angle with altered shape of the proximal femur; type 2 (6%), isolated coxa valga with neck-shaft angle > 140°; type 3 (7%), isolated coxa vara with neck-shaft angle < 120°; type 4 (20%), lateral bowing of the proximal half of the femur associated with normal neck-shaft angle; type 5 (14%), like in type 4 but associated with coxa valga; and type 6 (29%), like in type 4 but associated with coxa vara. Interobserver and intraoberver kappa values were excellent, ranging from 0.83 to 0.87. In 46 of the 89 femurs (52%) for which longitudinal radiographic documentation was available, there was progressive worsening of the original deformity, although the pattern remained the same; types 1 and 2 tended not to progress, whereas types 3 to 6 did.
Conclusions
A reproducible radiographic classification of polyostotic fibrous dysplasia-associated femoral deformities is proposed, which can serve as a tool for assessing and treating these deformities. After reviewing the radiographs of 127 femurs, we identified six reproducible patterns of femoral deformities.
Level of Evidence
Level III, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Polyostotic fibrous dysplasia is an uncommon skeletal disease caused by somatic activating mutations in the cAMP-regulatory protein Gsα [19, 22]. Normal bone and bone marrow are replaced by abnormal proliferative osteogenic precursors [1, 17, 18], leading to deformity, fracture, functional impairment, and pain [2, 9, 13, 14]. Polyostotic fibrous dysplasia may occur in association with cutaneous hyperpigmentation and hyperfunctioning endocrinopathies, including hyperthyroidism, precocious puberty, growth hormone excess, hypercortisolism, and fibroblast growth factor-23-mediated hypophosphatemia. The combination of polyostotic fibrous dysplasia and one or more extraskeletal manifestations is termed McCune-Albright syndrome.
The proximal femur is one of the most frequently affected sites in individuals with polyostotic fibrous dysplasia [3, 6–8, 10, 21]. Mechanical stress and repeated fractures result in progressive varus and bowing, leading to the classical shepherd’s crook deformity first described by von Reklingausen in 1891 as reported by Ulligan [20]. Management of these deformities is challenging, because they vary from case to case and they often tend to progress despite surgical intervention [3, 5, 7, 10–12]. However, to our knowledge, no radiographic grading system has been validated using intra- and interobserver reliability testing or found to be useful in terms of predicting progression of osseous deformity.
In this study, we performed a retrospective radiographic review in a large cohort of patients with polyostotic fibrous dysplasia to classify the variety of femoral deformities and to correlate the deformity pattern with the surgical correction required. We therefore sought to [1] develop a radiographic classification for polyostotic fibrous dysplasia; [2] test this classification’s intra- and interobserver reliability; and [3] characterize the radiographic progression of polyostotic fibrous dysplasia in a population of patients with the condition who were treated with a variety of approaches at several centers.
Patients and Methods
Demographics
We searched the databases of three treatment centers from 1994 to 2009 to identify all patients with polyostotic fibrous dysplasia treated at those centers during that time. We found 92 patients (138 femurs), of whom 84 (127 femurs) had records complete enough to begin our analysis; to be considered complete, a medical record had to have a good-quality AP radiograph of the hip, the chart notes with diagnosis, and the treatment records of any surgery performed
The treatment centers were the National Institute of Health, Bethesda, MD, USA, and the Department of Orthopaedic Surgery, University of Tor Vergata, Rome, Italy.
Of the 84 patients, 38 were male and 46 female; 43 had bilateral femoral involvement and 41 had unilateral involvement. Fifty-nine patients had McCune-Albright syndrome and 25 polyostotic fibrous dysplasia alone. At the time of the last radiographic examination, all the femurs were skeletally mature. The mean age of the patients at the time of the last radiograph was 30 years (range, 16–52 years). For 89 femurs (57 patients, 70% of the initial study cohort), weightbearing radiographic documentation was available from a mean of 10 years before the final followup radiographs (range, 6–20 years), whereas for 38 femurs, only weightbearing radiographs taken at the final followup were available. In subjects with radiographic documentation spanning several years before the final followup, only the last radiograph of the femur was selected for the classification.
Radiographic Evaluation
The femoral radiographs were examined twice by four of the authors (EI, PF, AC, FDM) with an interval of 6 weeks. Of the four authors who read the radiographs, three were orthopaedic surgeons and one was a pathologist. The readers who were blinded to each other had postfellowship professional experience ranging from 10 to 40 years. The following parameters were evaluated: [1] location of fibrous dysplasia (FD) lesions within the femur; FD was identified by the presence of at least one of the following features: (a) typical “ground glass” appearance, (b) increased or decreased radiodensity, (c) bone cysts, or (d) enlargement and/or irregularity of the femoral profile; [2] presence and extent of the shaft cortices and the medullary canal; [3] neck-shaft angle measurement on the AP view; the angle was measured between the axis of the femoral neck and the anatomical axis of the femur. When a lateral bowing deformity was present in the proximal femoral shaft below the lesser trochanter (shepherd’s crook deformity), the neck-shaft angle was identified by the intersection of the axis of the femoral neck and the anatomical axis of the trochanteric segment located above the deformity. The neck-shaft angle of the normal femur in the unilateral cases served as controls; [4] proximal and distal femoral deformities: bowing deformities of the femoral shaft in the coronal plane were identified as the angle formed by the axis of the two adjacent segments of the femur encompassing the deformity. Distal juxtaarticular deformities were identified by drawing one line parallel to the articular surface of the femoral condyles, a second line intersecting the first line with 87° of lateral angulation and coincident with the midpoint of the intercondylar notch (representing the mechanical axis of the femur), and a third line representing the anatomical axis of the distal femoral shaft. The angle formed by the two femoral axes normally ranges from 3° to 7°. Any increase (valgus) or decrease (varus) with respect to that range was considered a juxtaarticular deformity of the distal femur [4, 15, 16]; [5] progression of the lesion(s) from the first to the most recent film; [6] operated cases and fixation devices applied; and [7] intraobserver and interobserver agreement for parameters 3 and 4.
Description of Classification System
We identified six patterns of deformity of the proximal part of the femur according to the neck-shaft angle measurement and the presence or absence of lateral bowing of the proximal femoral shaft. In the first three types, lateral bowing of the femur was absent; in type 1, the neck-shaft angle was normal, ranging from 120° to 140° (Fig. 1); in type 2, the neck-shaft angle measured more than 140° (coxa valga) (Fig. 2), whereas in type 3, the neck-shaft angle measured less than 120° (coxa vara) (Fig. 3). In types 4, 5, and 6, lateral bowing of the proximal part of the femoral shaft was present, associated, respectively, to a normal neck-shaft angle (type 4) (Fig. 4), coxa valga (type 5) (Fig. 5), and coxa vara (type 6) (Fig. 6).
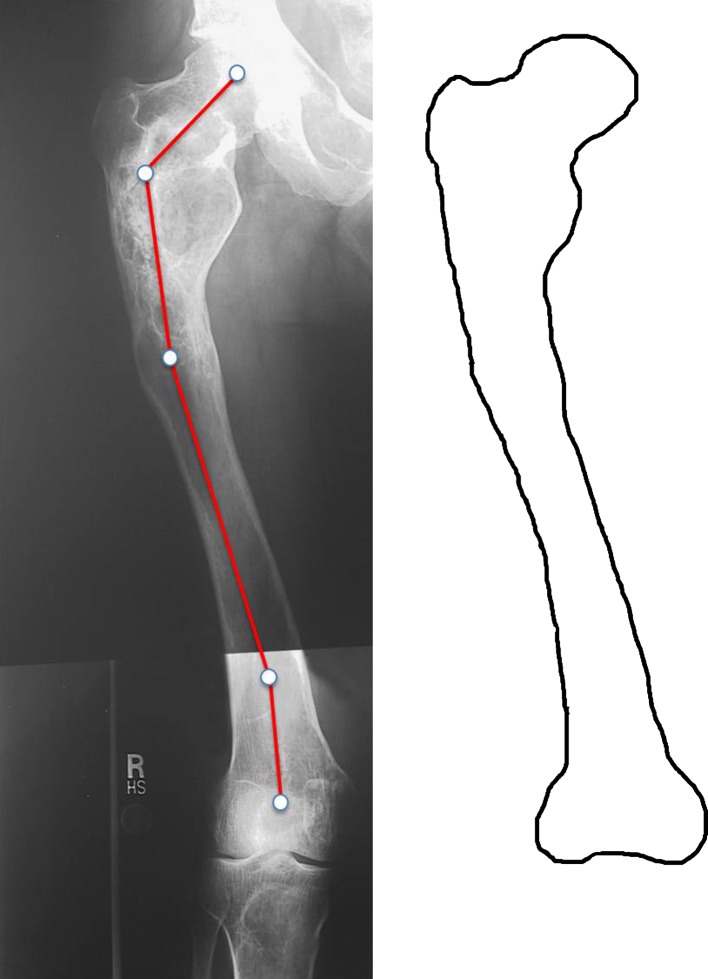
Fig. 1.
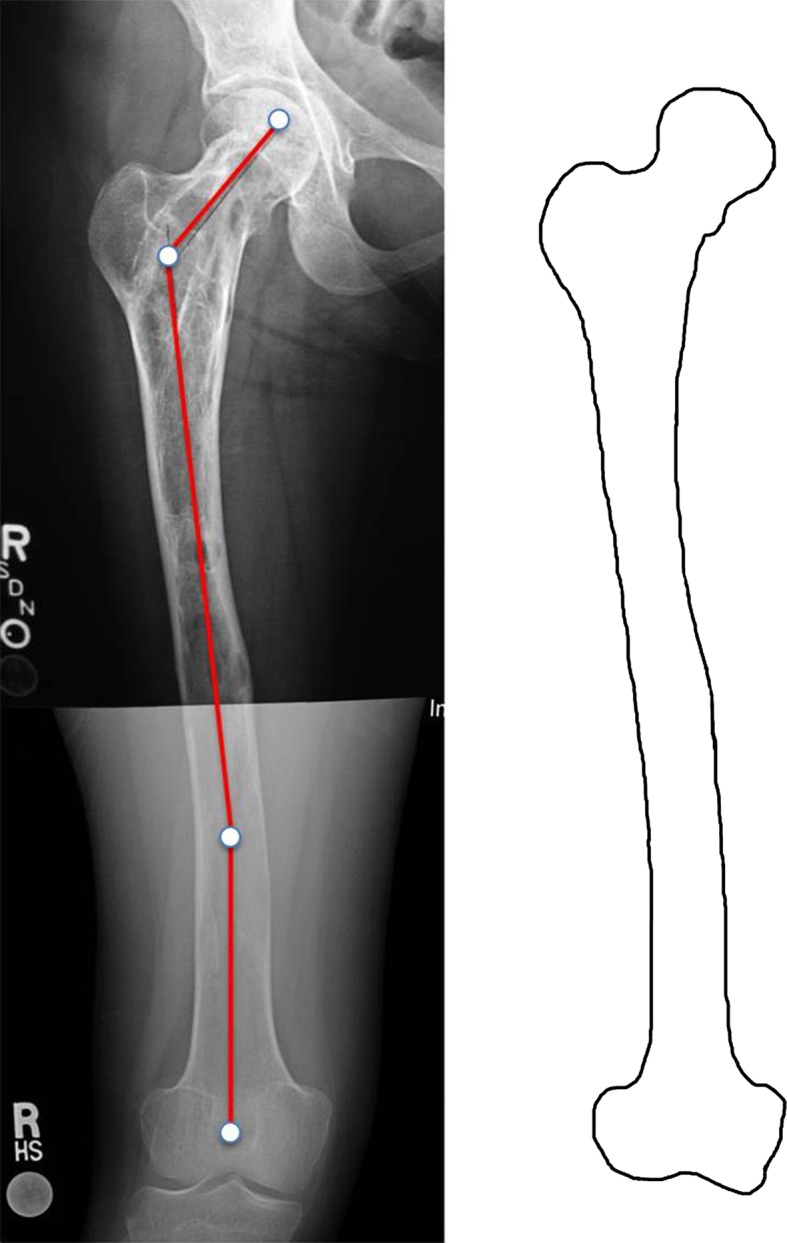
Type 1 deformity in a 25-year-old woman. Fibrous dysplasia is present throughout the entire femur. The proximal end is irregularly shaped. The neck-shaft angle is normal (135°), but a distal shaft 16° valgus deformity is present. Shaft cortices are present, although thinner than normal. Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right.
Fig. 2.

Type 2 deformity in a 31-year-old man. Round-shaped areas of ground glass appearance encircled by a radiodense rim are scattered within the femoral neck and trochanteric and subtrochanteric regions. The neck-shaft angle is valgus (152°). Shaft cortices are normally represented. Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right.
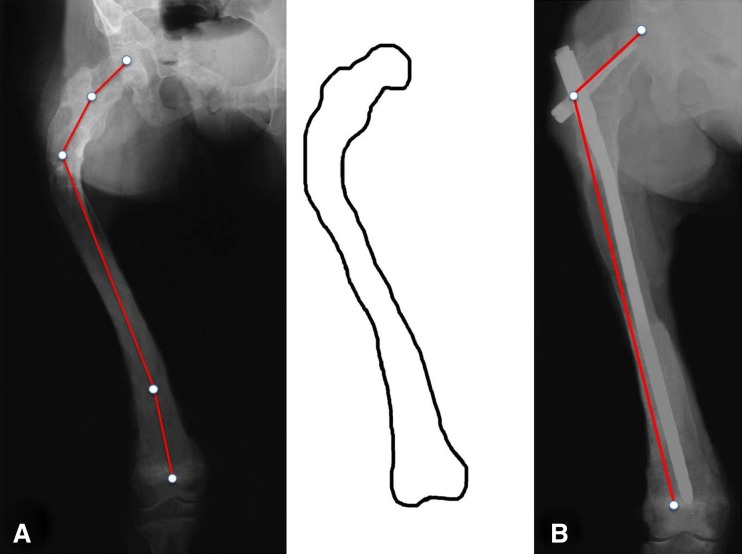
Fig. 3.
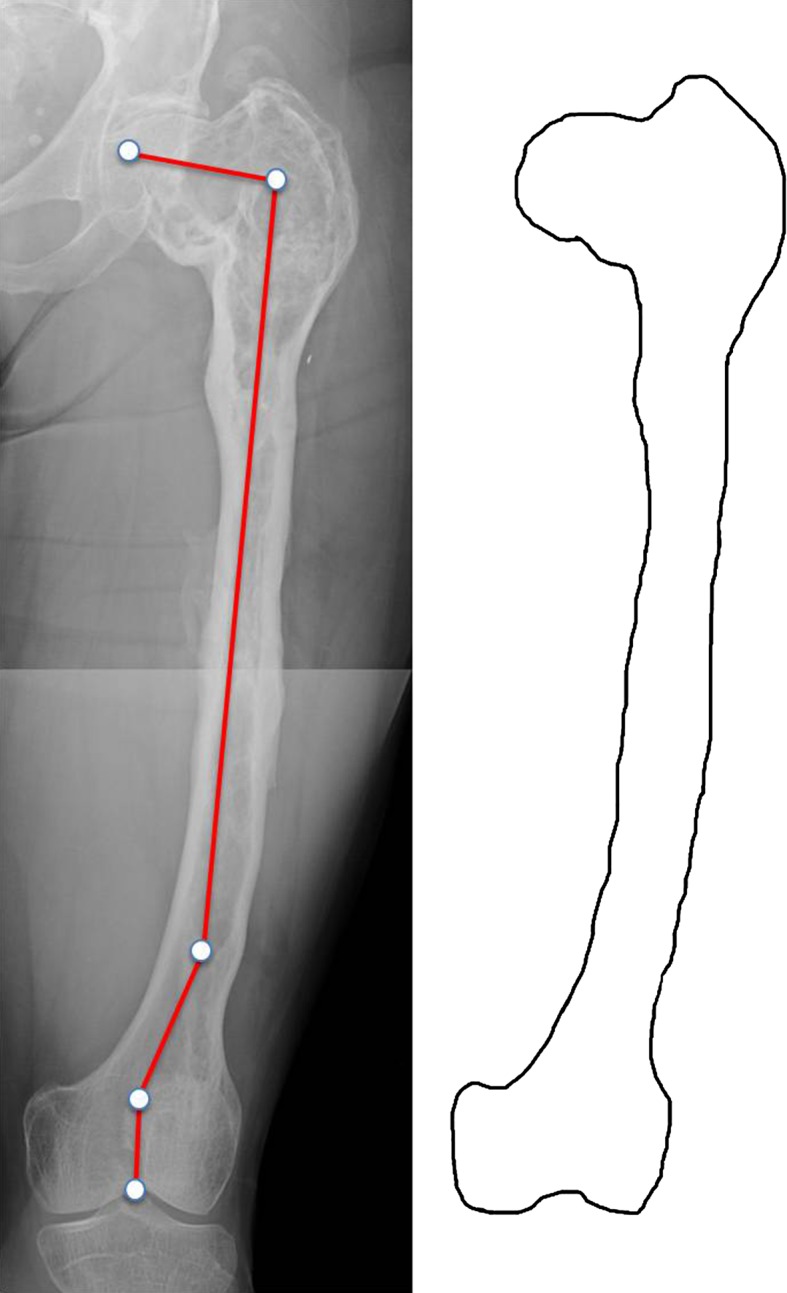
Type 3 deformity in a 30-year-old woman. The entire femur is affected by FD. The neck-shaft angle is varus (100°). A distal shaft 10° varus deformity as well as a distal juxtaarticular valgus deformity is present. Shaft cortices are irregular but present. Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right.
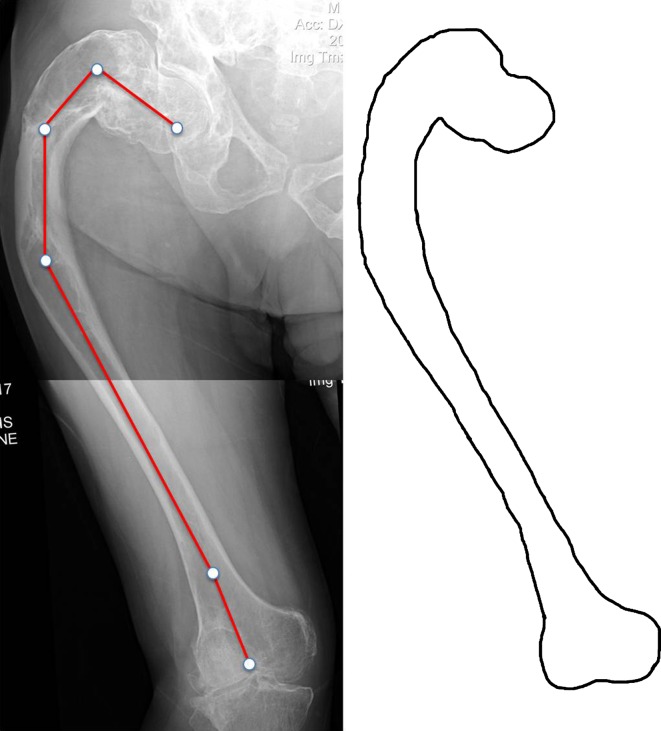
Fig. 4.
Type 4 deformity in a 44-year-old woman. The entire femur is affected by FD with marked alteration of the femoral shape. Despite the apparent weakness of the cervicotrochanteric region in which a cystic lesion is present, the neck-shaft angle is normal, measuring 125°. Proximal lateral (shepherd’s crook) and distal medial bowing of the femoral shaft are present. Shaft cortices are absent. Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right.
Fig. 5A–B.
(A) Type 5 deformity in a 17-year-old girl. The entire femur is involved. The neck-shaft angle is valgus (160°). There is lateral bowing of the proximal femur (shepherd’s crook) and medial bowing of the distal femur. Shaft cortices are absent. Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right. (B) Five years after correction of the deformity by multiple osteotomies and stabilization with a cervicodiaphyseal nail.
Fig. 6.
Type 6 deformity in a 40-year-old woman. The entire femur is affected by FD. Lateral bowing of the proximal femur is present at two levels (shepherd’s crook) as well as medial bowing of the distal femur. The neck-shaft angle is varus (100°). Permission received from Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, for reproducing the scheme on the right.
Statistical Analysis: Intra- and Interobserver Agreement
We validated our grading system by calculating the kappa score for both intra- and interobserver agreements. The femoral radiographs were examined twice by four of the authors (EI, PF, AC, FDM) with an interval of 6 weeks. Note that Cohen’s kappa is a measure of association (correlation or reliability) between two measurements of the same individual when the measurements are categorical. Kappa is often used to study the agreement of two raters such as judges or doctors. Each rater classifies each individual into one of the categories. The statistically significant kappa test indicates that we should reject the null hypothesis that the ratings are independent (ie, kappa = 0) and accept the alternative that agreement is better than one would expect by chance. Rules of thumb for kappa are that values < 0.40 indicate low association; values between 0.40 and 0.75 indicate medium association; and values > 0.75 indicate a high association between the two raters.
Treatments Used and Progression of Disease
The general indications for surgery were femoral fractures, femoral deformities, and painful femurs without either fracture or deformity. The indications for surgery were similar in the two centers in which surgical treatment was performed. Fifty-nine femurs (46%) had one or more operations by the time of the last followup radiograph, whereas 68 femurs (54%) were under observation at the time of followup. No patient surgically treated received any other medical treatment. Surgical treatment consisted of open reduction and internal fixation in femoral fractures, osteotomy and internal fixation in deformities, and only internal fixation in painful femurs. Both hip screw-plates and hip blade-plates had been applied in 23 femurs, flexible intramedullary nails (Ender, titanium elastic nails and Steinmann or Rush pins) in 16 femurs, rigid unlocked or interlocked diaphyseal intramedullary nails (Kűntscher; Gross and Kempf [Stryker Trauma, Kiel, Germany]) in six femurs, and cervicodiaphyseal interlocked intramedullary nails (UFN [Synthes Trauma, Oberdorf, Switzerland], PFN [Synthes Trauma], Zickel [Howmedica] and others) had been implanted in 14 femurs.
Progression of deformity was evaluated by four of us (EI, PF, AC, FDM) in all 59 surgically treated femurs, and in 30 femurs not operated on, on serial AP radiographs of the femur taken periodically for a minimum of 6 years to a maximum of 20 years from surgery (mean, 10 years).
Results
Classification System
Thirty (24%) of the 127 affected femurs were classifies as type 1 with a normal neck-shaft angle. In these femurs, areas of abnormal bone were primarily located in the trochanteric area, extending for a short distance proximally into the femoral neck and distally into the femoral shaft. The proximal femoral profile was altered in those cases. Eight femurs with coxa valga (6%) were classified as type 2. In these femurs, the pattern of proximal femoral involvement was similar to those with a normal neck-shaft angle, involving primarily the trochanteric area. Nine femurs with coxa vara (7%) were classified as type 3. In these femurs, both the femoral neck and the intertrochanteric area were fully involved with FD, but the femoral shaft below the lesser trochanter was either uninvolved or mildly involved.
In 80 cases (63%), there was lateral bowing of the proximal femoral shaft; 25 affected femurs (20%) were classified as type 4; in these cases, the lateral bowing was associated with a normal neck-shaft angle. Eighteen femurs (14%) were classified as type 5; in these cases, the lateral bowing was associated with a coxa valga; and 37 femurs (29%) were classified as type 6, in which the lateral bowing was associated with a coxa vara.
Distal deformities were present in 48 femurs (38%). In six femurs, a valgus deformity was observed between the diaphysis and the metaphysis. Twenty-seven femurs were affected with a distal juxtaarticular valgus deformity (Fig. 1), whereas in three, a valgus deformity was present at both levels (Figs. 5, 6). In nine femurs, a varus deformity was present between the diaphysis and the distal metaphysis, and in three cases, it was associated with a juxtaarticular valgus deformity (Fig. 3). Juxtaarticular valgus deformity was observed in five cases of type 1, two cases of type 3, eight cases of type 4, six cases of type 5, and nine cases of type 6 deformities. No case of type 2 presented this associated deformity.
In 21 femurs (16%) there were circumscribed areas of FD interspersed with normal bone in the proximal femur (Fig. 1), whereas in 19 (15%), there was diffuse involvement from the femoral neck extending downward into the shaft. In all those cases, femoral shaft cortices were present, although they were thinner than normal in some cases (Figs. 1–3). Eighty-seven femurs (69%) were completely involved from the neck to the condyles and the shaft cortices were absent (Figs. 4–6). In 24 femurs, an expansive lesion was located within the trochanteric area, altering the profile of the proximal part of the bone (Figs. 4–6). Thirty femurs had cystic lesions in the intertrochanteric or subtrochanteric areas (Figs.1, 2). Five femurs presented a soap bubble appearance with small, rounded cysts filling the entire bone (Fig. 7).
Fig. 7.
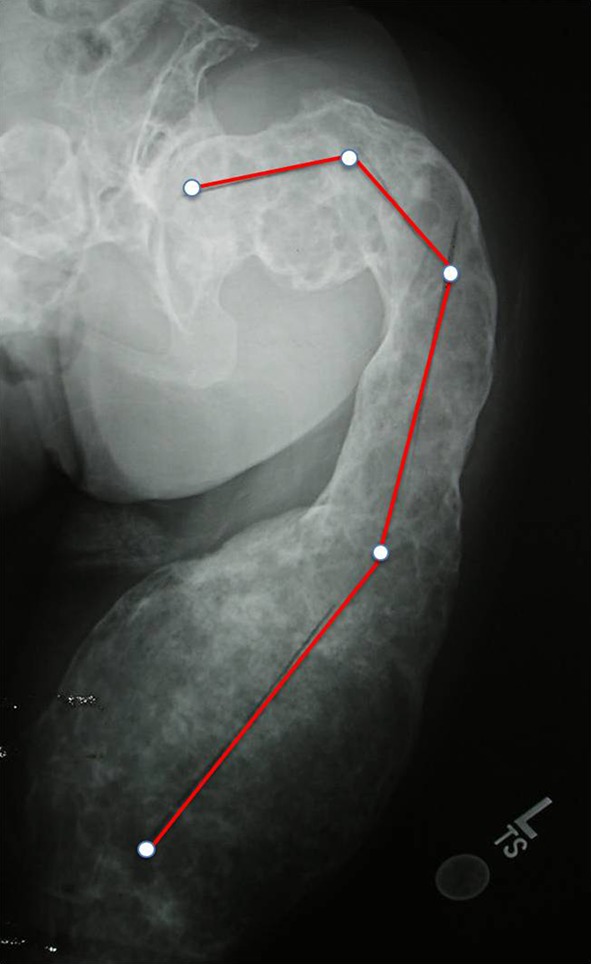
Very severe type 4 deformity in a 30-year-old woman with diffuse FD cystic involvement, which gives the femur a soap bubble-like appearance, suggesting severe bone weakness.
In femurs with prevalently proximal involvement, the medullary canal was almost entirely present in the distal shaft (Figs. 1, 2). In femurs with total FD involvement, the medullary canal was absent (Figs. 4–6). Cortices were either thin or absent in areas of femoral shaft involvement, whereas they appeared to present normal thickness in areas with a normal medullary canal (Figs. 1–6).
Intra- and Interobserver Agreement
Intra- and interobserver agreement both were excellent using the grading system proposed here. The kappa score for interobserver agreement was 0.855, whereas the kappa score for intraobserver agreement was 0.851, 0.871, 0.852, and 0.833. The highest percentage of erroneous classification was for types 1, 2, and 3 (in cases with mild shepherd’s crook deformity) versus types 4, 5, and 6.
Progression of Disease
Of the 30 femurs still untreated at followup radiographic examination, 18, classified as type 1 and 2, did not progress to a more severe pattern. Two femurs were type 3, and in one of these there was worsening of coxa vara. In the remaining 10 femurs, four type 4 and six type 6, the deformity remained the same but its severity progressed.
Of the 23 femurs stabilized with a hip screw-plate or blade-plate, 18 had recurrence of the deformity (Fig. 8), whereas in five, the deformity remained stable. In the latter cases, femoral shaft cortices were present (types 1–3). Of the 16 femurs stabilized with elastic intramedullary nails, the deformity recurred in all of them (Fig. 9). In those cases, shaft cortices were either absent or poorly represented (types 4–6). Of the 20 femurs stabilized with rigid intramedullary nails, the deformity remained stable in the 14 cases in which a cervicodiaphyseal device had been applied (Fig. 10). In the other six cases, in which only a rigid diaphyseal intramedullary nail had been applied, the diaphyseal deformities kept their correction but the femoral neck deformity shifted into varus in all cases (type 3) (Fig. 11).
Fig. 8A–B.
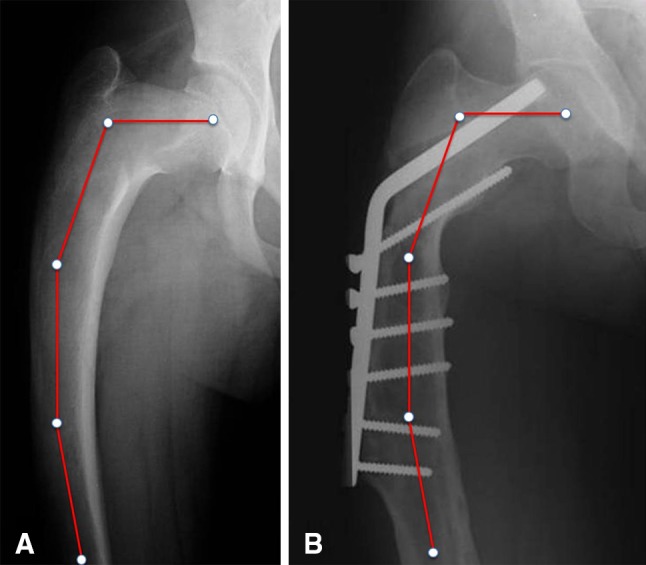
Type 6 femoral deformity with diffuse femoral involvement and shaft cortices poorly represented in an 18-year-old boy treated by multiple osteotomies and blade-plate stabilization. Screw loosening, medial bending of the femoral shaft below the end of the plate, and recurrence of the varus deformity of the femoral neck previously corrected to 130° with impending cutout of the cervical blade are evident 6 years after surgery.
Fig. 9.
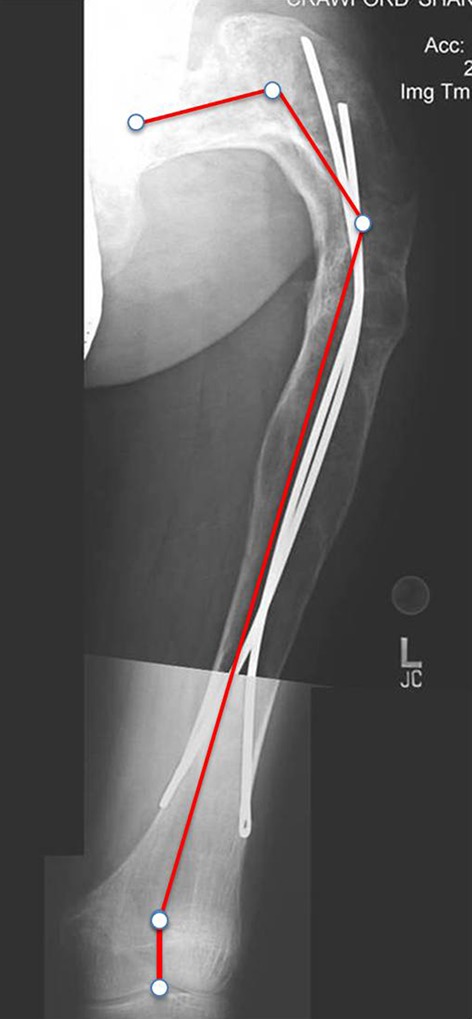
Type 6 femoral deformity in a 28-year-old woman after Enders nailing. Elastic intramedullary nailing is not suitable for maintaining correction when the femur is diffusely affected by FD. Juxtaarticular valgus deformity is also present.
Fig. 10A–B.
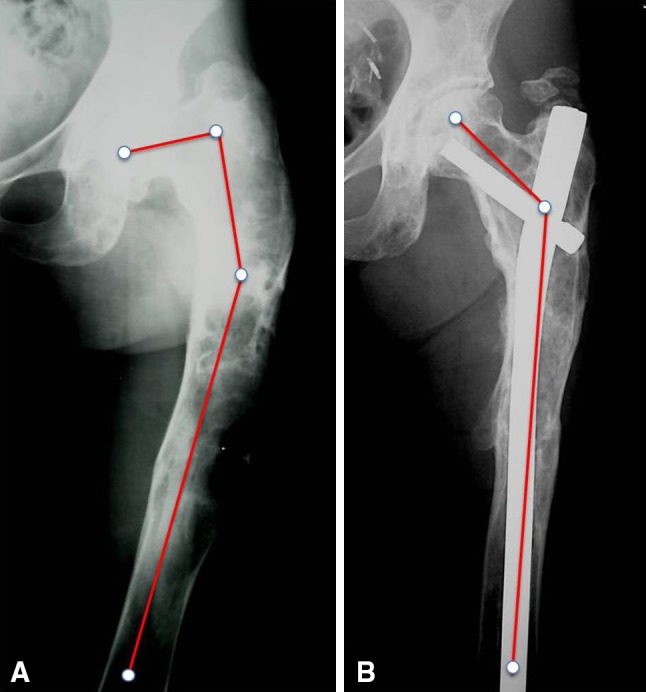
(A) Type 4 femoral deformity corrected by multiple osteotomies and stabilized with a Zickel nail in a 29-year-old man. (B) The same case illustrated in A. The correction is maintained 20 years later.
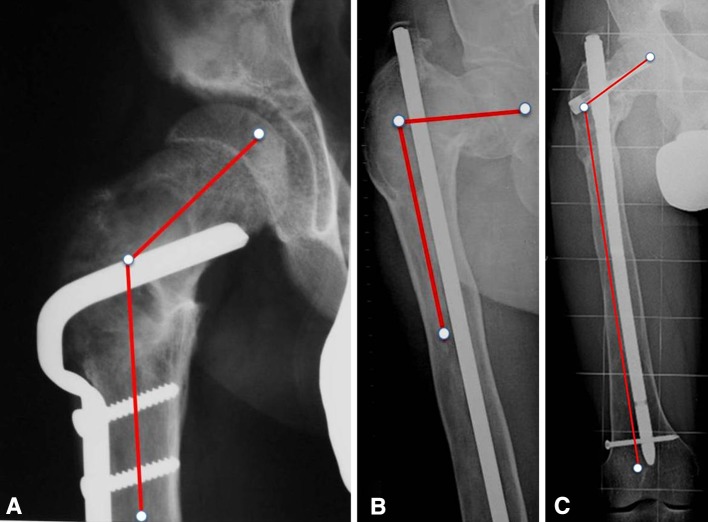
Fig. 11A–C.
(A) Type 1 femoral deformity in a 17-year-old boy after open reduction and internal fixation of an intertrochanteric fracture. The neck-shaft angle measured 130°. One year later, a femoral shaft fracture occurred below the plate and it was stabilized with a Kuntscher nail. (B) Four years later, radiographs showed type 3 deformity with 80° neck-shaft angle. The deformity was corrected by an osteotomy stabilized with an interlocking cervicodiaphyseal nail. (C) Ten years later, the correction was maintained and the neck-shaft angle measured 120°.
The proposed classification system was useful in terms of predicting progression. The original deformity did not worsen in types 1 and 2, whereas types 3 to 6, which were either virgin cases or cases operated on with peripheral plates or elastic intramedullary nails, became worse as did the cases in which a rigid intramedullary nail had been applied after correction of a type 4 or 6 deformity. In the latter, a type 3 deformity was observed that worsened in time. Overall, in 51 of the 89 femurs (57%) for which longitudinal radiographic documentation was available from an average of 10 years before the last followup examination, there was progressive worsening of the original deformity, although the pattern remained the same.
Discussion
Fibrous dysplasia, although uncommon in the population, is a condition that most major referral centers will take care of as part of their tumor practices. To our knowledge, there has been no prior systematic attempt to classify the spectrum of proximal femoral deformities in polyostotic fibrous dysplasia. Through a retrospective review of the largest reported cohort of patients with FD femoral involvement, we were able to identify six distinct patterns of femoral deformity based on a combination of the neck-shaft angle and the presence or absence of proximal shaft lateral bowing. This proposed classification thus may serve as a tool for both evaluating the severity of the disease and planning realignment osteotomies in complex cases.
There were a number of limitations inherent in our study design. First, the deformities were evaluated only in the coronal plane, because our system was based on the AP radiograph only. Therefore, we could have underestimated recurrence/progression of the deformity in the sagittal plane. However, the few lateral view radiographs available in our material always showed the presence of anterior bowing of the femoral shaft. This finding has been confirmed by our surgical practice and reported by others [11]. Also, the analyses were retrospective and limited to radiographs. Thus, we did not have access to the subjects’ medical histories or clinical aspects, which may have impacted the progression of their femoral deformities. It is also important to note that we lost some patients to followup, and so the percentage of patients who progressed could be low-end estimates. Some patients might have had disease progression and reoperation by other centers if they were lost to followup. In addition, we have related the progression of the disease to our classification system and to the treatment performed, not considering other predictors of progression.We also note that we do not have sufficient numbers to compare different treatment approaches in the different types of deformities to determine what surgical approaches are best for each. Finally, the limited number of patients for whom radiographs taken during growth were available did not allow us to draw any conclusions regarding either the pattern of deformity or treatment in children.
Our classification may be useful for indicating surgical treatment, but we caution readers that our sample was not sufficiently large to compare different treatments within subtypes, and there were no controls used; the recommendations here are intuitive in nature based on the mechanics of the deformities in question. Future studies will need to determine the efficacy of these recommendations. Types 1 and 2 deformities very often undergo stabilization for pain as a result of microfractures, although they do not progress in terms of deformity. The neck-shaft angle does not need correction because it is normal in type 1 and in a biomechanically stable valgus position in type 2. Type 3 deformity expressed by coxa vara can be treated using valgus intertrochanteric osteotomy, because it causes Trendelenburg gait and pain, and it may worsen, as observed in some of our cases with long-term followup. Both types 4 and 5 deformities may benefit from correction by femoral shaft osteotomy alone, because the femoral neck-shaft angle is normal in type 4 and valgus in type 5. If the proximal lateral femoral bowing encompasses a long segment of the femoral shaft, two osteotomies might be necessary to correct the deformity. In type 6 deformity, a valgus intertrochanteric osteotomy should be added to fix the varus deformity of the femoral neck. All the distal deformities, whenever present, should be corrected by additional osteotomies centered at the apex of the deformity [15, 16]. Preoperative planning for correction of the deformities should be very accurate because the presence of opposite deformities along the femur, very often proximal lateral bowing and distal medial bowing, might result in correction of the proximal varus lateral bowing and worsening of the distal medial bowing or juxtaarticular valgus deformity, whenever present. In those cases, a concomitant distal osteotomy with opposite correction should be planned.
We found high intra- and interobserver reliability using this classification. Similar intra- and interobserver intraclass correlation coefficient values were obtained by Feldman et al. [4] in lower limb deformity measurements in the frontal plane to plan surgical correction. The high degree of intra- and interobserver agreement in our study suggests that this classification system will be reproducible in other centers. Not surprisingly, the extent of femoral involvement was predictive of both the types of deformities and the progression of the deformities over time. In subjects with the disease limited to the proximal femur, the predominant deformity patterns were types 1, 2, and 3. Subjects with total femoral involvement presented most frequently with types 4, 5, or 6 deformities, which had a trend toward worsening in time. The most frequent distal femoral deformity was valgus, occurring at either the distal metaphysis or the distal epiphysis (juxtaarticular). The latter deformity seemed to be related to overgrowth of the medial condyle. Varus deformity was rarely observed at the level of the distal femoral shaft. Distal femoral deformities were most frequently associated with types 4, 5, and 6 deformities. It is likely that both mechanical and biological factors (ie, asymmetrical growth of the distal femoral physis) underlie distal femoral deformities.
An analysis of nonoperated and operated femurs suggests that surgery may be beneficial for types 3 to 6, which had the greatest risk of progression. Appropriate choices for surgical devices need to be made to minimize the likelihood of recurrence of the original pattern of deformity.
In conclusion, we present a systematic classification of proximal femoral deformities based on the largest reported cohort of subjects of which we are aware with polyostotic fibrous dysplasia involving the femur. Femoral deformities can be classified into six types based on the neck-shaft angle and the presence or absence of lateral shaft bowing. Types 1 and 2 deformities usually do not progress and they may need stabilization when they become painful, whereas types 3 to 6 deformities may recur even with treatment. Type 3 may be corrected using an intertrochanteric osteotomy to fix coxa vara; types 4 and 5 may be corrected by one or more proximal femoral shaft osteotomies, whereas type 6 may benefit from an intertrochanteric osteotomy to fix coxa vara and one or more osteotomies to correct shepherd’s crook deformity. Whenever present, all the associated distal deformities should be corrected accordingly by the appropriate osteotomy.
Acknowledgments
We thank Prof Paolo Bianco, Department of Molecular Medicine of the University of Rome La Sapienza, Rome, Italy, and Prof Mara Riminucci for their scientific support; Roberto Lala MD, head of the Department of Pediatric Endocrinology of Regina Margherita Hospital Turin, Turin, Italy, who provided several cases of McCune-Albright syndrome; C. Chebli MD, and M. Kelly RN, MS, from the National Institutes of Health, Bethesda, MD, USA, for case review; and Matteo Benedetti Valentini MD, from the Department of Orthopaedic Surgery of the University of Tor Vergata, Rome, Italy, who provided drawings of the six types of the proposed classification.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of Rome Tor Vergata, Rome, Italy.
References
- 1.Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101:1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins MT, Riminucci M, Bianco P. Fibrous dysplasia. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7. Washington, DC, USA: American Society for Bone and Mineral Research; 2008. pp. 423–427. [Google Scholar]
- 3.Enneking WF, Gearen PF. Fibrous dysplasia of the femoral neck. Treatment by cortical bone-grafting. J Bone Joint Surg Am. 1986;68:1415–1422. [PubMed] [Google Scholar]
- 4.Feldman DS, Henderson ER, Levine HB, Schrank PL, Koval KJ, Patel RJ, Spencer DB, Sala DA, Egol KA. Interobserver and intraobserver reliability in lower-limb deformity correction measurements. J Pediatr Orthop. 2007;27:204–208. doi: 10.1097/01.bpb.0000242441.96434.6f. [DOI] [PubMed] [Google Scholar]
- 5.Freeman BH, Bray EW, 3rd, Meyer LC. Multiple osteotomies with Zickel nail fixation for polyostotic fibrous dysplasia involving the proximal part of the femur. J Bone Joint Surg Am. 1987;69:691–698. [PubMed] [Google Scholar]
- 6.Funk FJ, Jr, Wells RE. Hip problems in fibrous dysplasia. Clin Orthop Relat Res. 1973;90:77–82. [PubMed] [Google Scholar]
- 7.Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am. 1998;80:648–658. doi: 10.2106/00004623-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Harris WH, Dudley HR, Jr, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg Am. 1962;44:207–233. [PubMed] [Google Scholar]
- 9.Hoppenfield S, Zeide MS, editors. The Orthopaedic Dictionary. JB Lippincott Co: Philadelphia, PA, USA; 1994. [Google Scholar]
- 10.Ippolito E, Bray EW, Corsi A, De Maio F, Exner UG, Robey PG, Grill F, Lala R, Massobrio M, Pinggera O, Riminucci M, Snela S, Zambakidis C, Bianco P. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B. 2003;12:155–177. doi: 10.1097/01.bpb.0000064021.41829.94. [DOI] [PubMed] [Google Scholar]
- 11.Keijser LC, Van Tienen TG, Schreuder HW, Lemmens JA, Pruszczynski M, Veth RP. Fibrous dysplasia of bone: management and outcome of 20 cases. J Surg Oncol. 2001;76:157–166; discussion 167–168. [DOI] [PubMed]
- 12.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop. 2007;1:3–17. doi: 10.1007/s11832-007-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein L. Polyostotic fibrous dysplasia. Arch Surg. 1938;36:874–898. doi: 10.1001/archsurg.1938.01190230153012. [DOI] [Google Scholar]
- 14.Lichtenstein L, Jaffe HL. Fibrous dysplasia of bone: a condition affecting one, several or many bones, the graver cases of which may present abnormal pigmentation of skin, premature sexual development, hyperthyroidism, and still other extraskeletal abnormalities. Arch Pathol. 1942;33:777–797. [Google Scholar]
- 15.Paley D, Tetsworth K. Mechanical axis deviation of the lower limbs. Preoperative planning of uniapical angular deformities of the tibia or femur. Clin Orthop Relat Res. 1992;280:48–64. [PubMed] [Google Scholar]
- 16.Paley D, Tetsworth K. Mechanical axis deviation of the lower limbs. Preoperative planning of multiapical frontal plane angular and bowing deformities of the femur and tibia. Clin Orthop Relat Res. 1992;280:65–71. [PubMed] [Google Scholar]
- 17.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–1600. [PMC free article] [PubMed] [Google Scholar]
- 18.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, Bianco P. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249–258. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulligan ME, editor. Classic Radiologic Signs: An Atlas and History. Castenton Hall, NY, USA: Parthenon Publishing Group; 1997. [Google Scholar]
- 21.Warrick CK. Polyostotic fibrous dysplasia; Albright’s syndrome; a review of the literature and report of four male cases, two of which were associated with precocious puberty. J Bone Joint Surg Br. 1949;31:175–183. [PubMed] [Google Scholar]
- 22.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]