Abstract
Purpose
This experimental study was undertaken to examine the fixation characteristics of a six-finned acetabular cup in both primary and revision arthroplasty in comparison with two other commonly used cup designs without fins.
Methods
All three cup designs (Ananova® [Intraplant], Plasmacup® NSC [Aesculap]; Exceed ABT™ [Biomet]) were implanted into validated models of normal and revision acetabula. The defect models were designed to simulate a dorso-cranial rim defect of 90° width and 10 mm in depth (moderate rim defect) and a dorso-cranial rim defect of 130° width and 15 mm in depth (severe rim defect). The fixation strength of the three cup designs was tested by cyclically edge-loading the implanted cups using a mechanical testing machine.
Results
The six-finned Ananova implant exhibited greater resistance to foam-cup interface motion than both the Plasmacup and Exceed ABT implants. The largest average differences were observed in the resistance to ultimate spin-out, with Ananova outperforming Exceed ABT and Plasmacup by 26 % and 17 % in the moderate and by 36 % and 38 % in the severe defect models, respectively.
Conclusions
The six-finned Ananova cup was significantly more resistant to edge loading both in the normal acetabulum and in acetabula with moderate to severe dorso-cranial rim defects than cup designs without fins, indicating that it may cover a wider range of clinical indications than conventional press-fit cups and provide clinicians with the confidence that, in primary and simple revision arthroplasty, adequate fixation strength can be obtained.
Keywords: Acetabular implants, Initial stability, Primary and revision arthroplasty, Fin system
Introduction
Press-fit cups have become the gold standard for primary hip replacement [1–3]. Adequate initial stability of press-fit cups is an essential precondition for their osseointegration [4, 5] and is achieved through peripheral load transfer between the implant and the acetabular bone in the three-point contact area of ilium, ischium, and pubis [1]. Mechanical fixation at the cup–bone interface is achieved by oversizing the cup [6–9], which causes the bony acetabulum to deform during impaction of the metal shell [10], allowing the bone to develop a tight grip on the implant [6]. The recommended difference between the cup diameter and the reamer last used ranges from 1 to 2 mm [6–8, 11]. In this way, press-fit cups maximize the peripheral contact between cup and bone and generate sufficient mechanical rigidity for osseointegration to occur [12].
In primary arthroplasty and in cases where a tight press-fit can be obtained, adjunctive screw fixation does not appear to increase mechanical stability [6, 8, 9, 13–16]. Even in revision cases, fixation of press-fit cups without screws has been shown to be possible in well-selected patients [2, 9, 16]. While additional screw fixation may be required in cases of mechanical instability due to osteoporosis or dysplasia [17, 18], screws carry the risk of intrapelvic neurovascular injury [19–21], fretting corrosion [22, 23], loosening of the cup during screw tightening, screw migration, or osteolysis and aseptic loosening [2, 24]. Screw holes may act as conduits for the migration of polyethylene wear debris [25, 26], and removal of screws in revision arthroplasty may lead to intrapelvic injury [27]. By contrast, press-fit cups without screws have been found to show less micromotion [8, 9, 14] and fewer radiolucent lines resulting from an unequal force distribution in the acetabulum caused by the screws [9].
These limitations of screw fixation have encouraged the search for alterative cup designs for acetabular revision. This experimental study was undertaken to examine the fixation characteristics of an acetabular cup with six circumferential fins in both primary and revision arthroplasty in comparison with two other cup designs without fins. Each design was tested in identical synthetic models of the normal and defective acetabulum, with assessment of device performance using specific failure criteria which considered both permanent and total (i.e., permanent and elastic) displacement of the cup-model interface in response to cyclic edge-loading.
Materials and methods
We evaluated the fixation properties of three commercial designs of uncemented acetabular cups: Ananova® (Intraplant, Moedling, Austria), Exceed ABT™ (Biomet, Warsaw, USA), and Plasmacup® (Aesculap, Tuttlingen, Germany). These designs were chosen because they represent typical acetabular components currently used in clinical practice. All three cups are hemispherical in shape with a slight flattening at the pole and are coated with a porous titanium plasma-spray coating. All components were selected to fit into a hemispherical cavity machined to a diameter of 52 mm, as recommended by the manufacturer (Fig. 1). For the Ananova and the Plasmacup models, this corresponded to cup size 52, featuring equatorial diameters of 53.2 mm and 53.3 mm, respectively. For Exceed ABT, the recommended cup size for a reamed diameter of 52 mm was 54, with an equatorial diameter of 54 mm. Therefore a reamed diameter resulted in underreaming of approximately 1 mm for the Ananova and Plasmacup models and of 2 mm for the Exceed ABT model.
Fig. 1.
Ananova (a), Plasmacup (b), and Exceed ABT (c) acetabular cups, including dimensions (in mm) used in this study
Surrogate model of the acetabulum
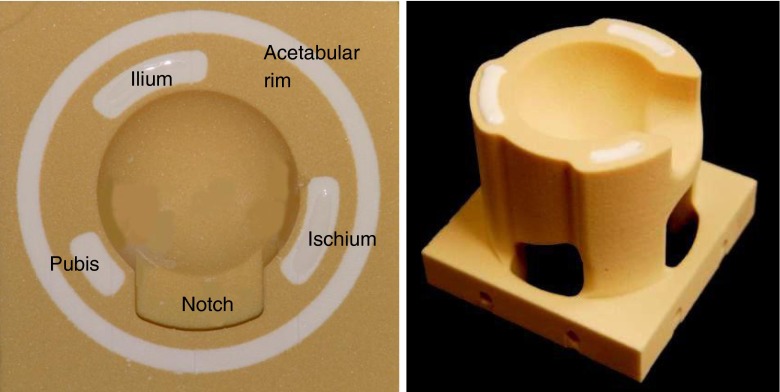
Details of the validated surrogate model of the reamed acetabulum used in this study have been published previously [28]. In brief, the model consists of a composite construct of low-density polyurethane (PU) foam internally reinforced with a rigid polymer (tensile strength, 200 MPa) simulating the native cancellous bone bed (0.1–1.4 g/cm3) and the periacetabular cortex (tensile strength, 166–198 MPa). The model was fabricated with a complex three-dimensional shape replicating the critical anatomic features of the acetabulum [1], including the acetabular notch (Ø 10 mm), the external chamfer of the bony margin (30°), and the reinforcing columns of the ilium, ischium, and pubis (Fig. 2).
Fig. 2.
Photographs of the final surrogate acetabulum design
To replicate an acetabular revision setting, an acetabular defect model was developed simulating morphologic variables known to affect cup fixation and stability. Two forms of Paprosky type IIb dorso-cranial rim defects were modeled: (i) a moderate rim defect of 10 mm in depth, spanning 90° of the acetabular rim, and (ii) a severe rim defect of 15 mm in depth and 130° in width (Fig. 3) [18].
Fig. 3.
Dorso-cranial rim defect illustration in a cross section through the CAD model of the surrogate design. a Moderate defect model with 90° defect in the ischial area. b Severe defect model with 130° defect across the ischial and iliac zones
Testing matrix
Six identical surrogates of each of the three surrogate designs (normal, moderate rim defect, severe rim defect) were prepared by computer-controlled machining (accuracy, ± 0.003 mm), corresponding to a total of 18 tests per cup design. Because six components of each surrogate design were available for testing, each component was tested a total of three times. To counteract the possibility that the data would be skewed by the use of previously implanted components, the assignments of cups and surrogates were controlled. The resulting testing matrix ensured that each surrogate design was tested twice with a new cup, twice with a cup that had been used in one prior test, and twice with a cup that had been used in two prior tests. The effect of repeat testing was minimized through the use of a standard cleaning protocol after each test which consisted of lightly brush-washing each coated component with soap and warm water, followed by drying with compressed air and soaking in an acetone bath for a minimum of 30 minutes. The implant was then blown dry before reuse.
Implantation and micromotion testing
A customized fixture, consisting of an aluminum disk fitting over the rim of each component, was used to ensure an even distribution of load at the shell–surrogate interface during implantation of the shell designs. The fixture was rigidly attached to the cross-head of a biaxial computer-controlled mechanical testing machine (MTS Bionix 858 Axial Torsional Test System, MTS, Minneapolis, MN) and mated with each cup prior to loading. Seating of each cup was standardized through the application of load at a rate of 50 N/s to a maximum of 2,000 N.
The strength of fixation of the three cup designs was measured by applying a compressive edge load to the rim of each implanted shell using a steel pin attached to the hydraulic actuator of the testing machine (Fig. 4). Each cup was cyclically loaded, starting with a preload of 25 N and followed by loading at 50 N/s to a peak value that was increased incrementally by 50 N per loading cycle. After each peak load was reached, the cup was unloaded at 50 N/s and held at 25 N for a rest period of 30 seconds to allow for any viscoelastic recovery of the specimen. The incremental loading procedure was continued until the displacement of the cup at the point of load application reached 4 mm, which was defined as the point of fixation failure (i.e., ultimate spin-out).
Fig. 4.
Edge-loading set-up using a mechanical testing machine
During the loading cycle, the relative displacement of the edge of the cup with respect to the hemispherical cavity was measured with a pair of linear variable differential transducers (LVDTs) with an accuracy of 0.002 mm. One displacement transducer was mounted on the MTS actuator and placed in contact with the upper surface of the surrogate, while the second transducer monitored the displacement of the cross-head with respect to the work surface of the mechanical testing machine (Fig. 4).
Data analysis
The mechanical stability of implant fixation was defined by the edge loads generating:
100 μm of permanent interface displacement after unloading to the 25 N preload
200 μm of permanent interface displacement after unloading to the 25 N preload
200 μm of total (i.e., permanent and elastic) interface displacement during application of the edge load
Ultimate spin-out (4 mm of displacement) of the cup within the reamed cavity of the surrogate
To calculate the edge load satisfying failure criteria A and B, each incremental peak load value was plotted as a function of the corresponding displacement of the interface upon subsequent unloading. The edge loads satisfying failure criteria C and D were determined by interpolating the hysteresis curve relating the applied load to cup displacement. For statistical analysis, a repeated measure ANOVA and Fisher’s PLSD post-hoc test were performed, with the level of significance set at p < 0.05 (Staview for Windows, SAS Inst., 1998).
Results
Fifty-four surrogate acetabula were fabricated and tested in this study, with 18 implantation and micromotion runs completed for the normal acetabular model and for each of the rim-defect models. The six-finned Ananova implant exhibited greater resistance to shell-surrogate interface motion than both the Exceed ABT and Plasmacup implants in all 12 test categories, with statistically significant differences in ten of the 12 categories (Table 1 and Fig. 5). Also, the resistance of the Plasmacup implant tended to be greater than that of the Exceed ABT implant.
Table 1.
Test results of the three cup designs in the normal, moderate rim defect, and severe rim defect surrogate models (mean ± SD) and difference to Ananova in percent (%)
| Cup design | A | B | C | D | ||||
|---|---|---|---|---|---|---|---|---|
| 100 μm of permanent interface displacement | 200 μm of permanent interface displacement | 200 μm of total interface displacement | Ultimate cup spin-out | |||||
| Normal acetabulum | ||||||||
| Ananova | 786.8 ± 17.3 | 837.3 ± 18.3 | 704.8 ± 23.8 | 945.7 ± 26.1 | ||||
| Plasmacup | 713.2 ± 21.2 | (−9 %*) | 733.3 ± 27.2 | (−12 %*) | 529.7 ± 22.7 | (−25 %*) | 758.1 ± 22.3 | (−20 %*) |
| Exceed ABT | 574.7 ± 72.6 | (−27 %*) | 619.7 ± 54.2 | (−26 %*) | 422 ± 41.2 | (−40 %*) | 667 ± 46.9 | (−29 %*) |
| Moderate rim defect model | ||||||||
| Ananova | 783.5 ± 30.6 | 851.7 ± 26.4 | 691.1 ± 27.3 | 948.3 ± 20.5 | ||||
| Plasmacup | 740.4 ± 30.2 | (−15 %**) | 767.6 ± 26.5 | (−10 %*) | 545.6 ± 15.9 | (−21 %*) | 786.5 ± 32.9 | (−17 %*) |
| Exceed ABT | 639.6 ± 60.4 | (−18 %*) | 680.5 ± 63.6 | (−20 %*) | 464.7 ± 44.8 | (−33 %*) | 705.7 ± 65.5 | (−26 %*) |
| Severe rim defect model | ||||||||
| Ananova | 632.1 ± 99.6 | 713.5 ± 93.2 | 598.9 ± 85.6 | 993.5 ± 48.4 | ||||
| Plasmacup | 565 ± 23.2 | (−11 %**) | 587.9 ± 23.4 | (−18 %*) | 460.7 ± 25.8 | (−23 %*) | 616.5 ± 24.7 | (−38 %*) |
| Exceed ABT | 537.2 ± 33 | (−15 %*) | 592 ± 29.4 | (−17 %*) | 412.3 ± 31 | (−31 %*) | 639.2 ± 34.8 | (−36 %*) |
*Statistically significant difference (p < 0.05) compared with Ananova
**No statistically significant difference (p < 0.05) compared with Ananova
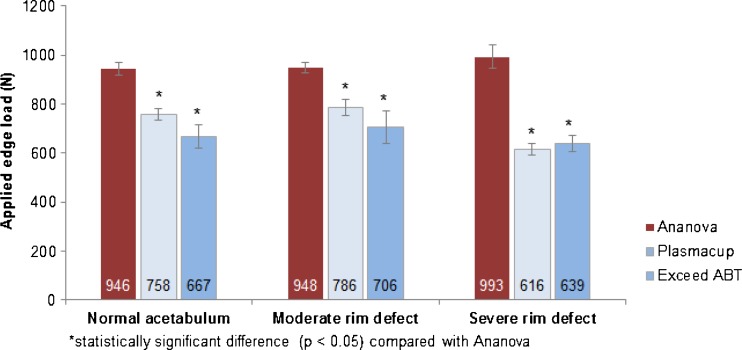
Fig. 5.
Ultimate spin-out results of the three cup designs in the normal, moderate rim defect, and severe rim defect surrogate models (mean ± SD)
In the normal acetabular surrogate, the Ananova cup significantly outperformed each of the two alternative cups in all four test criteria by a margin of 27–40 % for the Exceed ABT and 9–25 % for the Plasmacup. In terms of ultimate spin-out force, the Ananova cup provided 29 % greater resistance to dislodgement than the Exceed ABT design and 20 % greater than the Plasmacup (Fig. 5).
In the moderate and severe rim defect models, the Ananova design significantly outperformed the Exceed ABT in all four measures of fixation stability and the Plasmacup in all measures except the load generating 100 μm of permanent deformation. Again, the largest average differences were observed in the resistance to ultimate spin-out, with Ananova outperforming Exceed ABT and Plasmacup by 26 % and 17 %, respectively, in the moderate defect model, and by 36 % and 38 %, respectively, in the severe defect model (Fig. 5).
When looking at each cup individually, the resistance to displacement and ultimate spin-out between the normal and moderate rim defect models was similar for all four categories (Table 1). By contrast, the resistance to ultimate spin-out in the severe defect model was lower than in the normal and moderate defect models, the only exception being the Ananova cup (Fig. 5), which showed a consistent primary stability across all three models.
Discussion
This study compared the primary stability of three different acetabular cup designs implanted in a composite construction of PU foam with reinforcements simulating the cancellous bone bed, periacetabular cortex, and three-point support. In view of the inherent limitations of cadaveric testing, such as the variability of bone quality and acetabular morphology, we used an acetabular surrogate model to assess the stability of fixation of each cup design under reproducible conditions. The model has previously been validated through comparative testing against cadaveric bone specimens [28].
To allow our study to address the stability of a six-finned press-fit cup in both the normal acetabulum and simple revision situations, we modified the intact surrogate model to simulate rim defects at two levels of severity. In the healthy hip joint, forces during loading are highest in the cranio-medial, posterior-inferior, and anterior-inferior areas of the acetabular cavity, where they are supported by the iliac, ischial, and pubic bones. Peak local pressures are observed in the iliac region [1]. This loading pattern is reflected in both of our defect models, one simulating a dorso-cranial rim defect of 90° in the ischial area, the other replicating a defect covering 130° involving both the ischial and iliac columns, i.e., a significant weakening of the three-point fixation.
The Ananova acetabular cup exhibited significantly better fixation to cyclic edge loading in the normal, moderate rim defect, and severe rim defect models than either of the two alternative cup designs. The superiority of Ananova was most pronounced (17–38 %) in the resistence to ultimate spin-out in each of the three surrogate models, indicating that the additional stabilizing effect of the fins increases with increasing loads. It has been shown that, at lower loads, press fit creates a contact area near the periphery and that increasing loads increases the contact area toward the pole [1, 29]. In the case of the Ananova cup, increasing loads may cause the serrated and slightly skewed fins stretching across the cup shoulder to develop a stronger grip on the periacetabular bone than cups without fins.
The most pronounced difference in the resistance to spin-out was seen in the severe defect model, where the finned design displayed no loss of fixation strength compared to the 36–38 % reduction in the strength of the other two designs tested. This suggests that the stabilizing effect of the fins becomes more important in defect situations. This is in agreement with the results by Baleani et al., who compared both a two-finned and a 12-finned cup design with a cup without fins implanted in PU foam using either press or exact fit [30]. The addition of fins on the cup rim enhanced primary cup stability under all test conditions, but the stabilizing effect of the fins was most evident under exact-fit conditions simulating a situation in which optimal press-fit cannot be achieved.
Widmer et al. found that peripheral press-fit is best obtained along the diagonal axis between the superior-anterior rim of the iliac bone and the posterior-inferior rim of the ischial bone, with the iliac and ischial bones taking about 55 % and 25 % of the total load on the articular surface, respectively [1]. In our moderate defect model, which simulated a dorso-cranial rim defect of 90° involving the ischial bone only, lever-out forces were similar to those seen in the normal model, suggesting that a limited defect along the main iliac–ischial axis does not substantially affect the primary stability of the cup. In our severe defect model, however, the defect covered more than one third of the rim circumference across both the ischial and iliac zones. Here, the resistance to edge loading was markedly lower than in the normal and moderate defect models—the sole exception being the six-finned cup, which showed a consistently high resistance and a significantly higher resistance than either of the alternative cups. Thus, a rim defect across the very axis offering the best conditions for press-fit fixation [1] substantially compromises the stability of conventional press-fit cups, an effect not seen when the six-finned cup was used.
All three cups used in this study feature a hemispherical design with a flattening at the pole. Contact between the implant and the bone in the polar region is nonphysiologic and compromises the three-point support [1]. Loading a hemispherical cup without a flattened pole increases the load transfer in the dome and decreases the load transfer at the rim, indicating that press-fit stability decreases as load increases [29]. With cups featuring a flattened pole, peripheral load transfer continues to be higher than load transfer in the dome as loads increase, optimising peripheral acetabular stresses [29] and, as a result, initial cup fixation [1, 24]. With the Plasmacup exhibiting the most and Exceed ABT the least pronounced pole flattening, the isolated effect of this design feature on the difference between Ananova and the other two cup models is likely to have been negligible.
The defect situations tested in this study weaken peripheral cup fixation. Whereas the circumferential fin system of the Ananova cup maintained a high level of primary stability in these situations, the absence of fins in the two other models is thought to explain their lower primary stability. While Exceed ABT exhibited the lowest primary stability in the normal acetabulum, its loss of primary stability in defect situations was less than of the Plasamcup model. This difference may have been due to the less pronounced pole flattening of the Exceed ABT resulting in a greater contact area between cup and bone.
The superiority of the finned cup design appears to be due to the presence of the six radial fins spaced 60 degrees apart. Whereas too many fins may bear negatively on the press-fit characteristics by distributing equatorial stresses, too few fins may result in a lack of bone contact in cases where the rim is defective. With six equispaced fins, a press-fit cup is able to achieve supplementary fixation from at least four fins, even in the presence of defects extending over more than 120 degrees of the acetabular margin.
Ries et al., in their photoelastic model evaluating periacetabular stresses at the time of implantation of four different cup designs, found that a four-finned cups had lower peripheral than dome stresses and that the fins appeared to separate the periacetabulum into quadrants that reduced the peripheral stress [29]. The authors speculated that the fins, rather than the peripheral press-fit, would provide initial cup stability. This observation may have been due not only to the four slots that were created in the model to accommodate the fins, but also to both the shape and the location of the fins, which were placed almost flush with the cup edge, protruded beyond the cup diameter, and may indeed have cut through the substrate, thereby reducing the press-fit effect. By contrast, Baleani et al. [30] found that the fins added to and enhanced the cup’s press-fit effect.
In contrast to both fin systems used in these previous studies [29, 30], the fins of the component we evaluated are recessed below the face of the cup, do not protrude beyond the outer cup diameter, are aligned with the curvature of the cup, and have serrated rather than plain edges. This design is thought to reduce the likelihood of the fins cutting through the substrate, conceivably combining optimal press-fit with full engagement of the fins whenever the periacetabular bone stock is intact and providing additional stability when optimal press fit cannot be obtained.
Our study has a number of limitations. First, it was performed under controlled laboratory conditions, and the results cannot, therefore, be directly transferred into clinical practice. Although our model was designed to simulate the anatomy of natural bone, the mechanical, viscoelastic, and adaptive properties of the periactabular bone cannot be completely replicated in an in vitro model. Second, whereas optimal cup implantation can be achieved in a surrogate model, positioning of the cup in clinical cases of poor bone quality may be more difficult, which is why our results may overestimate the differences observed between the cup designs. Finally, we did not assess the effect of fins on the rotational stability of the cup. However, this may be expected to be even more pronounced than the tangential lever-out stability. Baleani et al. [30] found that rotational stability was more than two times that of the frontal stability determined in their study.
In conclusion, our results indicate that the finned press-fit cup evaluated in this study is significantly more resistant to cyclic and asymmetric edge loading both in the normal acetabulum and in acetabula with moderate to severe dorso-cranial rim defects than the cup designs without fins. The largest mean difference (about 35 %) between the six-finned design and the two alternative cups was seen in the resistance to ultimate spin-out in the severe defect model, suggesting that the superiority of the finned design is most pronounced with increasing loads and when an optimal press-fit cannot be obtained. The consistently strong fixation of the six-finned cup in both normal and rim defect surrogate models, although still awaiting confirmation from clinical studies, suggests that the finned cup is likely to cover a wider range of clinical indications than conventional press-fit cups, providing the surgeon with the confidence that adequate fixation strength can be obtained without the additional use of screws.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Widmer KH, Zurfluh B, Morscher EW. Load transfer and fixation mode of press-fit acetabular sockets. J Arthroplasty. 2002;17(7):926–935. doi: 10.1054/arth.2002.34526. [DOI] [PubMed] [Google Scholar]
- 2.Elke R, Berli B, Wagner A, Morscher EW. Acetabular revision in total hip replacement with a press-fit cup. J Bone Joint Surg Br. 2003;85(8):1114–1119. doi: 10.1302/0301-620X.85B8.13590. [DOI] [PubMed] [Google Scholar]
- 3.Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop. 2011;35(2):289–298. doi: 10.1007/s00264-010-1198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini CM, Galbusera F, Ceroni RG, Raimondi MT. Loss in mechanical contact of cementless acetabular prostheses due to post-operative weight bearing: a biomechanical model. Med Eng Phys. 2007;29(2):175–181. doi: 10.1016/j.medengphy.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;208:108–113. [PubMed] [Google Scholar]
- 6.Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372–376. doi: 10.1302/0301-620X.74B3.1587880. [DOI] [PubMed] [Google Scholar]
- 7.Adler E, Stuchin SA, Kummer FJ. Stability of press-fit acetabular cups. J Arthroplasty. 1992;7(3):295–301. doi: 10.1016/0883-5403(92)90052-R. [DOI] [PubMed] [Google Scholar]
- 8.Kwong LM, O’Connor DO, Sedlacek RC, Krushell RJ, Maloney WJ, Harris WH. A quantitative in vitro assessment of fit and screw fixation on the stability of a cementless hemispherical acetabular component. J Arthroplasty. 1994;9(2):163–170. doi: 10.1016/0883-5403(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 9.Roth A, Winzer T, Sander K, Anders JO, Venbrocks RA. Press fit fixation of cementless cups: how much stability do we need indeed? Arch Orthop Trauma Surg. 2006;126(2):77–81. doi: 10.1007/s00402-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 10.Larsson S, Elloy M, Hansson LI. Fixation of trochanteric hip fractures. A cadaver study of static and dynamic loading. Acta Orthop Scand. 1987;58(4):365–368. doi: 10.3109/17453678709146356. [DOI] [PubMed] [Google Scholar]
- 11.MacKenzie JR, Callaghan JJ, Pedersen DR, Brown TD. Areas of contact and extent of gaps with implantation of oversized acetabular components in total hip arthroplasty. Clin Orthop Relat Res. 1994;298:127–136. [PubMed] [Google Scholar]
- 12.Morscher EW. Current status of acetabular fixation in primary total hip arthroplasty. Clin Orthop Relat Res. 1992;274:172–193. [PubMed] [Google Scholar]
- 13.Schmalzried TP, Wessinger SJ, Hill GE, Harris WH. The Harris-Galante porous acetabular component press-fit without screw fixation. Five-year radiographic analysis of primary cases. J Arthroplasty. 1994;9(3):235–242. doi: 10.1016/0883-5403(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 14.Won CH, Hearn TC, Tile M. Micromotion of cementless hemispherical acetabular components. Does press-fit need adjunctive screw fixation? J Bone Joint Surg Br. 1995;77(3):484–489. [PubMed] [Google Scholar]
- 15.Zilkens C, Djalali S, Bittersohl B, et al. Migration pattern of cementless press fit cups in the presence of stabilizing screws in total hip arthroplasty. Eur J Med Res. 2011;16(3):127–132. doi: 10.1186/2047-783X-16-3-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udomkiat P, Dorr LD, Wan Z. Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year follow-up. J Bone Joint Surg Am. 2002;84-A(7):1195–1200. doi: 10.2106/00004623-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Templeton JE, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen-year follow-up study. J Bone Joint Surg Am. 2001;83-A(11):1706–1711. doi: 10.2106/00004623-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9(1):33–44. doi: 10.1016/0883-5403(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 19.Keating EM, Ritter MA, Faris PM. Structures at risk from medially placed acetabular screws. J Bone Joint Surg Am. 1990;72(4):509–511. [PubMed] [Google Scholar]
- 20.Wasielewski RC, Galat DD, Sheridan KC, Rubash HE. Acetabular anatomy and transacetabular screw fixation at the high hip center. Clin Orthop Relat Res. 2005;438:171–176. doi: 10.1097/01.blo.0000165855.76244.53. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick JS, Callaghan JJ, Vandemark RM, Goldner RD. The relationship of the intrapelvic vasculature to the acetabulum. Implications in screw-fixation acetabular components. Clin Orthop Relat Res. 1990;258:183–190. [PubMed] [Google Scholar]
- 22.Cipriano CA, Issack PS, Beksac B, Della Valle AG, Sculco TP, Salvati EA. Metallosis after metal-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2008;37(2):E18–E25. [PubMed] [Google Scholar]
- 23.Jasty M, Bragdon C, Jiranek W, Chandler H, Maloney W, Harris WH. Etiology of osteolysis around porous-coated cementless total hip arthroplasties. Clin Orthop Relat Res. 1994;308:111–126. [PubMed] [Google Scholar]
- 24.Wilson-MacDonald J, Morscher E, Masar Z. Cementless uncoated polyethylene acetabular components in total hip replacement. Review of five- to 10-year results. J Bone Joint Surg Br. 1990;72(3):423–430. doi: 10.1302/0301-620X.72B3.2341441. [DOI] [PubMed] [Google Scholar]
- 25.Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627–1635. doi: 10.2106/00004623-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Berman AT, Avolio A, Jr, DelGallo W. Acetabular osteolysis in total hip arthroplasty: prevention and treatment. Orthopedics. 1994;17(10):963–965. doi: 10.3928/0147-7447-19941001-17. [DOI] [PubMed] [Google Scholar]
- 27.Galat DD, Petrucci JA, Wasielewski RC. Radiographic evaluation of screw position in revision total hip arthroplasty. Clin Orthop Relat Res. 2004;419:124–129. doi: 10.1097/00003086-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Jamieson ML, Russell RD, Incavo SJ, Noble PC. Does an enhanced surface finish improve acetabular fixation in revision total hip arthroplasty? J Arthroplasty. 2011;26(4):644–648. doi: 10.1016/j.arth.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Ries MD, Salehi A, Shea J. Photoelastic analysis of stresses produced by different acetabular cups. Clin Orthop Relat Res. 1999;369:165–174. doi: 10.1097/00003086-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Baleani M, Fognani R, Toni A. Initial stability of a cementless acetabular cup design: experimental investigation on the effect of adding fins to the rim of the cup. Artif Organs. 2001;25(8):664–669. doi: 10.1046/j.1525-1594.2001.025008664.x. [DOI] [PubMed] [Google Scholar]