Abstract
Purpose
The purpose of this study was to systematically review the literature and report the clinical and radiographic outcomes of highly-porous acetabular cups in revision settings.
Method
A literature search of four electronic databases of EMBASE, CINAHL-plus, PubMed, and SCOPUS yielded 25 studies reporting the outcomes of 2,083 revision procedures with highly-porous acetabular components. There was lack of high quality evidence (level I and level II studies) and only two studies with level III evidence, while the remainder were all level IV studies. In addition, a majority of the studies had small sample sizes and had short to mid-term follow-up. The mean age of the patients was 65 years (range, 58–72 years) and the mean follow-up was 3.6 years (range, two to six years). Outcomes evaluated were aseptic survivorship, Harris hip scores, migration rates, incidence of peri-acetabular radiolucencies and radiographic restoration of the hip centre.
Results
The mean aseptic survivorship was 97.2 % (range, 80–100 %). The Harris hip scores improved from a mean pre-operative score of 42 points, (range, 29–75 points), to a mean postoperative score of 79 points (range, 69–94 points). The mean incidence of cup migration and prevalence of peri-acetabular radiolucencies was 2.4 % (range, 0–8.8 %) and 4.6 % (range, 0–19 %), respectively, at final follow-up. The vertical hip centre-of-rotation was restored significantly from a mean of 39.2 mm (range, 27.6–50 mm) pre-operatively, to a mean of 24.1 mm (range, 7.4–47 mm), postoperatively.
Conclusion
The short-term clinical and radiographic results of highly-porous metals in revision hip arthroplasty are excellent with a low rate of loosening in the presence of both major and minor bone loss.
Keywords: Highly-porous, Revision, Arthroplasty, Hip, Outcomes, Review
Introduction
Management of acetabular bone loss in revision total hip arthroplasty (THA) can be challenging. The high failure rate of cemented acetabular components in revision THA due to failure of interdigitation of cement into the sclerotic host bone has led to the increased use of cementless fixation [1–5]. Small contained defects can usually be treated with porous hemispherical cups with supplemental bone grafting [6]. However, large uncontained lesions often require extra-large hemispherical cups, impaction grafting, structural allografts, bilobed oblong cups or anti-protrusio rings and reconstruction cages [7–16]. Although the results of cementless fixation appears to be satisfactory in many revision scenarios, less satisfactory outcomes have been reported in some studies when less than 50 % of the weight bearing host bone is available for fixation, which may lead to failure of biological ingrowth [17]. Moreover, sub-optimal outcomes have been reported with reconstruction cages in patients with Paprosky 3A and 3B acetabular defects [18, 19]. These challenges have led to new approaches to manage acetabular defects with highly-porous metals during revision arthroplasty [16].
These newer highly-porous metal implants aim to provide better primary stability, improved physiological stress distribution, and enhanced osseointegration. In addition, the use of highly-porous augments has been proposed as an alternative to structural allografts to provide mechanical support and facilitate bone ingrowth when used in conjunction with the highly-porous acetabular components. These augments may have the added advantage of restoring the hip centre of rotation to near normal which leads to improvement in hip biomechanics. The highly-porous metals presently used are manufactured from tantalum or titanium. They have higher co-efficient of friction, modulus of elasticity, and greater porosity (60–80 %) when compared to conventional cementless porous designs. Structurally, these metals appear to mimic cancellous bone in appearance and have been shown to be quite valuable in acetabular reconstruction [20]. Multiple individual studies have reported low failure rates and improved survivorship with the use of these “metal foams” in revision THA. However, there has been a lack of systematic reviews on the outcomes of these implants for reconstruction of acetabular defects in the revision setting.
Therefore, the aim of this study was to conduct a comprehensive review of the literature and report the outcomes of highly-porous acetabular cups in revision settings. Specifically, we assessed: (1) aseptic cup survivorship, (2) Harris hip scores, (3) migration rates, (4) incidence of peri-acetabular radiolucencies, and (5) restoration of hip-centre of rotation.
Materials and methods
Definition and search strategy for identification of studies
Since an exact definition for the newer highly-porous metals have not been well-defined in the literature, we considered these metals as those that have higher porosity (over 50–80 %) and larger pore diameter (above 400 microns) than conventional porous implants. According to the PRISMA guidelines, electronic databases of EMBASE, CINAHL plus, PubMed, and SCOPUS were searched to identify reports published in literature from January 1990 to February 2013 that documented the outcomes of highly-porous metals in cementless acetabular fixation for revision total hip arthroplasty. The criteria used in the initial search included the following terms: “highly-porous,” “porous,” “porous-coated,” “trabecular,” “trabecular-metal,” “tantalum,” and “titanium,” “tritanium” and was combined with the terms “cementless,” “un-cemented,” “acetabulum,” “cup,” “hip,” “replacement,” “arthroplasty” and “revision.”
The bibliographies of all retrieved reports were explored to find additional studies which were overlooked after the initial search. Studies published in the English literature that reported on demographic, clinical and radiographic data were included in the final analysis to measure the outcome metrics. The content was critically analysed to avoid including the same patient population when multiple reports were published by the same author. When such a situation was encountered, the study with the larger group of patients and/or the longer follow-up was included in the analysis. Review studies and case reports of single patients on highly-porous metals that did not report on the clinical outcomes were excluded from this review. Reports on the use of highly-porous materials for the management of acetabular defects following tumour resection were also excluded from this review. Biomechanical, histological, migration-analysis, or in vitro studies that did not report on clinical outcomes were also disregarded during the outcome analysis.
Eligibility criteria for inclusion of studies
The following were the inclusion criteria for this review: (1) studies on highly-porous acetabular components in revision settings, (2) studies that had a minimum of two-year mean follow-up, and (3) reported on clinical (e.g. survivorship, Harris hip scores, complications) and radiographic outcome metrics (e.g. component-migration, radiolucencies, osteolysis). The exclusion criteria were (1) studies describing the outcomes on conventional porous-coated acetabular components in revision scenarios, (2) non-English language studies or abstracts, (3) studies reporting on the outcomes of highly-porous coated acetabular implants in primary hip arthroplasty, (4) biomechanical, histological reports on highly-porous metals not reporting clinical outcomes and, (5) case reports or reviews on highly-porous metals.
Assessment of methodological quality of the studies
Two authors (SB and KI) performed the initial literature search independently and all studies included in the final analysis were selected after a consensus decision. A third author’s (RP) opinion was sought when a consensus decision could not be reached. Two authors (SB and KI) conducted a quality assessment individually for each of the studies selected for final analysis. Quality assessment of the selected reports was made by using the 12-point Methodological Index for Non-randomised Studies (MINORS) criteria, which has been reported to have high test–retest, inter-observer reliability and external and internal validity [21, 22]. A modified 23-point Rometsch et al. and Huisstede et al. quality assessment scale for observational studies was also used to analyse the methodological quality of the studies in this review [23, 24].
Data collection
All studies included in this review were analysed for study type, publication year, and level of evidence. The data from individual reports on revision hip arthroplasty were further sub-divided on the basis of demographic characteristics (e.g. age, gender, sample-size, and patient characteristics), metal-type (tantalum versus titanium), surgical approach and clinical and radiographic outcomes. Acetabular defects were classified according to the Paprosky’s classification [25]. These were then further sub-classified into major and minor bone loss cohorts for the purpose of the review. Paprosky type 1 to type 2B were included in the minor bone loss group while Paprosky type 2C to 3B were include in the major bone loss group. Harris hip scores were recorded for evaluation of functional outcomes. Vertical or horizontal translation more than five millimetres between the initial and final radiographs was defined as cup migration in the radiographic analysis. The presence of more than two millimetres of implant-bone gap or progressive peri-acetabular radiolucency in the three radiographic zones of DeLee and Charnley were used as defining criteria for assessment of loosening [26]. For the purpose of the review, the vertical hip centre was measured from the inter-tear drop line and centre of the femoral head; while the horizontal hip centre was the measured distance between the femoral head and the perpendicular drawn from the inter-tear drop line at the tear drop as described by Callaghan et al. [27, 28].
Statistical analysis of the data
All data extracted were integrated into an Excel spreadsheet (Excel, Microsoft Corporation, Redmond, Washington) for the final analysis. As most of the reports included in the review provided level IV evidence, statistical analysis to determine whether the outcomes were significantly different between these newer highly-porous designs and the conventional porous-coated cups was not performed. Statistical software (GraphPad 6.0, Inc., La Jolla, California) was used to calculate the mean, confidence interval and t-test for significance for each outcome metric. Test for normality of data sets between individual studies was performed using the D’Agostino and Pearson’s omnibus normality test. Chi-square test and Student t-test for calculating statistical significance between means and proportions were used for comparing the survivorships between the minor and the major bone-loss groups. Pearson’s correlation statistical analysis was used to measure correlation between the quality of the studies and component survivorship. A p-value of less than 0.05 was considered to be statically significant.
Results
Study identification
We identified 2,871 reports using our search criteria. Of these, 502 reports concerned porous-coated or highly-porous coated implants. Of these, 376 studies were excluded from this review, as they were linked to conventional porous implants. There were 21 basic science, histopathology or review studies that did not report on clinical outcomes that were excluded from the review. Fifty-nine studies on highly-porous implants that were unrelated to hip arthroplasty were also excluded. Two reports on endoprosthetic reconstruction following tumour excision using these implants were excluded from this review. The remaining 44 studies concerned primary or revision THA. Of the 44 studies reporting on clinical outcomes, there were 16 reports on primary hip arthroplasty which were excluded from the review. Out of the 28 remaining studies on revision THA, there were only 25 studies that had a minimum of two-year mean follow-up, and these were included in the final analysis [29–56].
Study characteristics
This review encompassed 2,083 revision hip arthroplasties. There were 45 % men and 55 % women who had a mean age of 65 years (range, 58–71 years) (Table 1) and the mean follow-up was 3.6 years (range, two to six years). Aseptic loosening (60.6 %), instability (6.8 %) and osteolysis (6.6 %) were the three most common diagnoses followed by infection (4.6 %) and post-traumatic (1.2 %) causes, after excluding a variety of miscellaneous causes which constituted 20.1 % of all acetabular revisions (see Appendix). There were 598 hips (55 %) with major bone loss and 485 hips (45 %) with minor acetabular bone loss (Appendix). Paprosky type 3A (33 %; n = 354 hips) and type 2A defects (18 %; n = 197 hips) were found to be the two most common types of acetabular defects in 1,083 hips undergoing revision hip arthroplasties and these were followed by type 2B (15 %; n = 163 hips), type 1 (11.5 %; n = 125 hips), type 3B (11.4 %; n = 124 hips), and type 2C (11 %; n = 120 hips) defects. In 66 % of patients a multi-holed “revision” acetabular component was used, while the modular and the monoblock design was used in 22 % and 12 % of the cases (n = 1,018), respectively. Metal augments were used concurrently with the highly-porous acetabular components in 26 % of the 1,152 reported revisions, and bone grafting was used in 60 % of the patients (n = 789). There was only one study reporting the use of titanium-based highly-porous metals for acetabular reconstruction while the remaining studies were on tantalum [40]. None of the studies except one analysed in this review reported on complications related to metal debris at final follow-up.
Table 1.
Patient demographics and aseptic survivorship with highly-porous metals
| Author/year | No. of hips | Men/women | Mean age (range) | Follow-up in years (range) | Porous material | Aseptic cup survivorship (%) |
|---|---|---|---|---|---|---|
| Revision THA | ||||||
| Nehme et al. (2004) [31] | 16 | 4/12 | 63.6(34–86) | 2.7(2–3.3) | Tantulum | 93.7 |
| Unger et al. (2005) [32] | 59 | 18/42 | 64.2 (27–85) | 3.5(1.2–5.7) | Tantalum | 98.3 |
| Sporer et al. (2006) [33] | 13 | 3/10 | 63 (47–88) | 2.6 (1–3) | Tantalum | 100 |
| Weeden et al. (2007) [34] | 43 | 17/25 | 65.4(45–86) | 2.8 (2–4) | Tantalum | 100 |
| Flecher et al. (2008) [35] | 23 | 7/16 | 58.2 (34–84) | 2.9(2 to 4.2) | Tantalum | 100 |
| Sporer et al. (2008) [36] | 28 | 13/15 | 64(36–89) | 3.1(1–4) | Tantalum | 100 |
| Kim et al. (2008) [37] | 46 | 15/31 | 64(23–85) | 3.3(2–4.3) | Tantalum | 98.8 |
| Van Kleunen et al. (2009) [38] | 97 | 40/50 | 59 (27–87) | 3.8 (2–6.6) | Tantalum | 100 |
| Lakstein et al. (2009) [39] | 53 | 29/24 | 63 (29–86) | 3.8 (2–5.9) | Tantalum | 96 |
| Seigmeth et al. (2009) [41] | 34 | 15/19 | 64(37–97) | 2.8 (2–4.6) | Tantalum | 94.1 |
| Malkani et al. (2009) [42] | 22 | 9/16 | 71.7 ± 10.5 | 3.3 (2.3–4.6) | Tantalum | 100 |
| Simon et al. (2009) [43] | 53 | NR | 63.8 ± 20.6 | 2.1 ± 0.7 | Tantalum | 100 |
| Lingaraj et al. (2009) [44] | 23 | 7/15 | 67 (38–81) | 3.4 (2–5.2) | Tantalum | 100 |
| Flecher et al. (2010) [45] | 72 | 30/41 | 60 (34–84) | 4 (2–6) | Tantalum | 100 |
| Kosashvilli et al. (2010) [46] | 15 | NR | 67(34–89) | 4 (2–6) | Tantalum | 80 |
| Fernandez-Fairen et al. (2010) [47] | 263 | 113/150 | 69.5(39–84) | 6.1(5–7) | Tantalum | 100 |
| Jafari et al. (2010) [48] | 81 | 155/128 | 66(26–88) | 3(2–5.3) | Tantalum | 94 |
| Ballester-Alfaro et al. (2010) [29] | 19 | NR | 63 | 2.2(1.5–3.6) | Tantalum | 100 |
| Hasart et al. (2010) [49] | 38 | NR | NR | 2.1 | Tantalum | 94.8 |
| Lachiewicz et al. (2010) [56] | 39 | NR | 65.1(41–79) | 3.3(2–7) | Tantalum | 97.5 |
| Skytta et al. (2011) [52] | 827 | NR | 69.1 (16–94) | 3 | Tantalum | 98 |
| Davies et al. (2011) [30] | 46 | 22/24 | 66.7(39–85) | 4.2(2.3–6.3) | Tantalum | 100 |
| Del Gaizo et al. (2012) [53] | 37 | NR | 60(36–80) | 5(2.2–8.8) | Tantalum | 97.3 |
| Sterheim et al. (2012) [54] | 53 | 24/29 | 62.4(42–80) | 6(5–8.5) | Tantalum | 92.5 |
| Sterheim et al. (2012) [54] | 49 | 27/22 | 64.4(37–89) | 6(5–9) | Tantalum | 100 |
| Abolghasemian et al. (2013) [55] | 34 | 14/20 | 69.3 (46–86) | 5.4(2.2–8.9) | Tantalum | 91.1 |
NR not reported
Study design
Of the 25 studies included in this review there were two studies that had a control group (level III evidence) while the remaining 23 studies had level IV evidence. There were four reports that analysed the radiographic and clinical outcomes prospectively and there were four multi-centre studies included in this review. Eighteen studies (72 %) reported that more than 95 % of patients were available at final follow-up while 80 % (20 out of 25) of studies provided a reason for drop out of patients.
Description of study population
All studies reported on baseline demographics and cup survivorship. There were eight studies (32 %) that reported on the outcomes of more than 50 patients in their study group. Ten studies (40 %) had preset inclusion and exclusion criteria for selection of their study population. There were 20 studies (80 %) that reported on acetabular component migration while 88 % (n = 22) of studies reported a description of the medical and surgical complications. D’Agostino and Pearson’s omnibus normality test for individual data sets revealed no significant differences in the mean age (p = 0.9), mean follow-up (p = 0.22), pre-operative Harris hip scores (p = 0.07), and distribution of Paprosky 3a and 3b acetabular defects (p = 0.38 and p = 0.69, respectively) among the various 25 reports included in the analysis. However, significant differences were found in the gender distribution, pre-operative diagnoses for revision, and the incidence of minor bone loss among the study populations in the various reports (p = <0.001).
Quality assessment
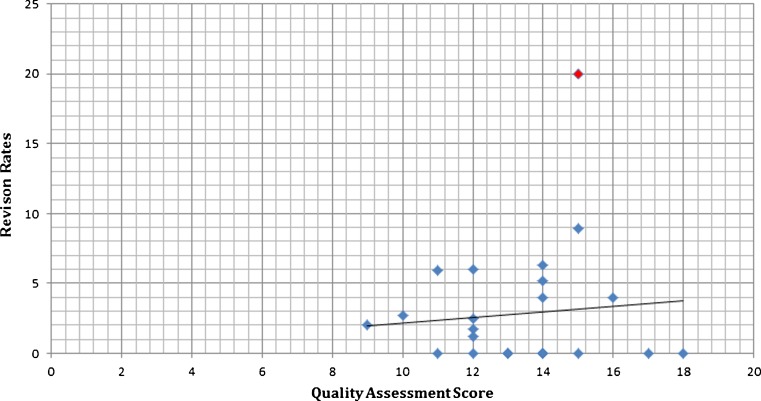
The mean score for 25 studies as per the modified Rometsch et al. and Huisstede et al. quality assessment scale was 13 points (range, 9–18 points; maximum score, 22 points) while the mean score for 23 level IV studies according to the MINORS scale was 10 points (range, 7–15 points; max score, 16 points) (Tables 2 and 3). There were 15 studies (60 %) that scored more than 60 % score on the MINORS criteria, while 11 studies (44 %) scored more than 60 % based on the modified Rometsch et al. and Huisstede et al. scale. Although not significant there was a general trend towards higher rates of component revision for any reason with studies that had higher scores on quality assessment scale when a scatter plot was analysed (p = 0.65) (Fig. 1). However, the overall correlation was found to be weak (r = 0.25).
Table 2.
Results of MINORS criteria for quality assessment of the studies
| Minors criteria | Nehme et al. (2004) [31] | Unger et al. (2005) [32] | Sporer et al. (2006) [33] | Weeden et al. (2007) [34] | Flecher et al. (2008) [35] | Sporer et al. (2008) [36] | Kim et al. (2008) [37] | Van Kleunen et al. (2009) [38] | Lakstein et al. (2009) [39] | Seigmeth et al. (2009) [41] | Malkani et al. (2009) [42] | Simon et al. (2009) [43] | Lingaraj et al. (2009) [44] | Flecher et al. (2010) [45] | Fernandez-Fairen et al. (2010) [47] | Jafari et al. (2010) [48] | Ballester-Alfaro et al. (2010) [29] | Hasart et al. (2010) [49] | Lachiewicz et al. (2010) [56] | Skytta et al. (2011) [52] | Davies et al. (2011) [30] | Del Gaizo et al.(2012) [53] | Sterheim et al.(2012) [54] | Abolghasemian et al. (2013) [55] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A clearly stated aim | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 1 |
| Prospective collection of data | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 0 |
| Endpoints appropriate to the aim of the study | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of the study endpoint | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 2 | 1 |
| Follow-up period appropriate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Loss to follow up less than 5 % | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 2 |
| Prospective calculation of the study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| An adequate control group | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Contemporary groups | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Baseline equivalence of groups | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Adequate statistical analyses | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 |
| Country of origin | USA | USA | USA | USA | USA | USA | Canada | USA | Canada | UK | USA | Australia | France and USA | Spain | USA | Spain | Germany | USA | Finland | Canada | USA | Canada | Canada | |
| Level of evidence | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV | III | IV | IV | IV | IV | IV | IV | III | IV | |
| Score | 11 | 10 | 11 | 10 | 9 | 11 | 10 | 10 | 12 | 11 | 12 | 7 | 11 | 10 | 15 | 15 | 9 | 12 | 12 | 11 | 9 | 9 | 20 | 10 |
Table 3.
Quality assessment score across studies on revision total hip arthroplasty using highly porous metals
| Criteria | Nehme et al. 2004 [31] | Unger et al. 2005 [32] | Sporer et al. 2006 [33] | Weeden et al. 2007 [34] | Flecher et al. 2008 [35] | Sporer et al. 2008 [36] | Kim et al. 2008 [37] | Van Kleunen et al. 2009 [38] | Lakstein et al. 2009 [39] | Seigmeth et al. 2009 [41] | Malkani et al. 2009 [42] | Simon et al. 2009 [43] | Lingaraj et al. 2009 [44] | Flecher et al. 2010 [45] | Kosashvilli et al. 2010 [46] |
| Study population | |||||||||||||||
| Inclusion and exclusion criteria described | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Baseline characteristics descriptions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Number of hips >50 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Same STEM used in all cases | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interventions | |||||||||||||||
| Description of surgical technique | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Description of implants | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Information on cup | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Information on bearing surface | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Study design | |||||||||||||||
| Prospective | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Multi-center | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Loss to follow up <5 % | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Reason for drop out provided | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| All completed follow-up | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Outcome measurements | |||||||||||||||
| Information on cup survival | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Information on revision/explantation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Description of adverse events | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Interval between measurements identical for all patients | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Standardized or valid measurements | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Osteolysis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Measurements of peri-acetabular gaps/radiolucency | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Migration | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Analysis | |||||||||||||||
| Cumulative survival | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Score | 15 | 13 | 15 | 14 | 15 | 16 | 13 | 14 | 15 | 12 | 12 | 18 | 14 | 15 | 16 |
| Criteria | Fernandez-Fairen et al. (2010) [47] | Jafari et al. (2010) [48] | Ballester-Alfaro et al. (2010) [29] | Hasart et al. (2010) [49] | Lachiewicz et al. (2010) [56] | Skytta et al. (2011) [52] | Davies et al. (2011) [30] | Del Gaizo et al. (2012) [53] | Sterheim et al. (2012) [54] | Abologhesian et al. (2013) [55] | |||||
| Study population | |||||||||||||||
| Inclusion and exclusion criteria described | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | |||||
| Baseline characteristics descriptions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Number of hips >50 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | |||||
| Same STEM used in all cases | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Interventions | |||||||||||||||
| Description of surgical technique | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | |||||
| Description of implants | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Information on cup | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Information on bearing surface | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Study design | |||||||||||||||
| Prospective | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |||||
| Multi-center | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |||||
| Loss to follow up <5 % | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | |||||
| Reason for drop out provided | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |||||
| All completed follow-up | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | |||||
| Mean follow-up more than two years | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Outcome measurements | |||||||||||||||
| Information on cup survival | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Information on revision/explantation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Description of adverse events | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | |||||
| Interval between measurements identical for all patients | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | |||||
| Standardized or valid measurements | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Osteolysis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Measurements of peri-acetabular gaps/radiolucency | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |||||
| Migration | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | |||||
| Analysis | |||||||||||||||
| Cumulative survival | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |||||
| Score | 19 | 13 | 15 | 15 | 13 | 10 | 13 | 11 | 17 | 16 | |||||
Fig. 1.
Component re-revision rate plotted against quality assessment score
Outcome measurements
The mean overall aseptic survivorship of the highly-porous acetabular components across all types of acetabular defects was 97.2 % (range, 91.1–100 %) at a mean follow-up of 3.6 years (range, two to six years) (n = 2,083 hips) (Table 1). Revision for aseptic loosening in the major bone loss group was significantly higher at 3.4 % (n = 748 hips), compared to 0.4 % (n = 496 hips) in the minor bone loss group (OR, 8.5; 95 % CI, 2–36.3; p = 0.003).
The mean Harris hip scores improved significantly from 42 points (range, 31–75 points; n = 11 studies; 693 hips) pre-operatively to 79 points (range, 73–95 points; n = 15 studies; 834 hips) postoperatively (p < 0.0001; 95 % CI, −44.9 to −31.0) with a mean improvement of 38 points (range, 18–52 points) (Table 4).
Table 4.
Radiographic outcomes and Harris hip scores
| Author/year | Migration of cup >5 mm (%) | Acetabular osteolysis (%) | Pre op HHS/MD | Post-op Harris hip scores |
|---|---|---|---|---|
| Revision THA | ||||
| Nehme et al. (2004) [31] | 6.3 | NR | 39.3 (24–52) | 75.2 (56–92) |
| Unger et al. (2005) [32] | 1.7 | NR | 74.8 (33–95) | 94.4 (58–95) |
| Sporer et al. (2006) [33] | NR | 7.6 | 6.1 | 10.3a |
| Weeden et al. (2007) [34] | 2 | 0 | 32 (10–60) | 84 (28–100) |
| Flecher et al. (2008) [35] | 0 | 0 | 6.8 (4–9) | 10.6 (8–12) |
| Sporer et al. (2008) [36] | NR | 0 | 6.8 | 10.6 |
| Kim et al. (2008) [37] | NR | NR | NR | 40b |
| Van Kleunen et al. (2009) [38] | 1 | 1 | 55 (22–94) | 76 (25–100) |
| Lakstein et al. (2009) [39] | NR | 4 | 5.3 (1–10) | 10.6 (1–12)a |
| Seigmeth et al. (2009) [41] | 5.9 | NR | NR | 80.3 ± 16.6 |
| Malkani et al. (2009) [42] | 0 | 0 | NR | 81 (60–93) |
| Simon et al. (2009) [43] | 0 | 0 | NR | 16.1 ± 1.5c |
| Lingaraj et al. (2009) [44] | 4.3 | NR | 43 (14–86) | 75.7 (53–100) |
| Flecher et al. (2010) [45] | 0 | 0 | NR | 15.8 (9–18) |
| Kosashvilli et al. (2010) [46] | NR | NR | 31 (15–48) | 69 (56–87) |
| Fernandez-Fairen et al. (2010) [47] | 0 | 0 | 43.6 (23–62) | 80.4 ± 9.8 (43–94) |
| Jafari et al. (2010) [48] | NR | NR | NR | NR |
| Ballester-Alfaro et al. (2010) [29] | NR | NR | NR | NR |
| Hasart et al. (2010) [49] | 2.6 | 2.6 | 29 | 78 |
| Lachiewicz et al. (2010) [56] | 2.5 | 7.6 | NR | 86 (58–98) |
| Skytta et al. (2011) [52] | NR | NR | NR | NR |
| Davies et al. (2011) [30] | NR | 0 | NR | 78.2 (26–96) |
| Del Gaizo et al.(2012) [53] | 2.7 | NR | 33 (12.6- 58.7) | 81.5 (27–99.8) |
| Sterheim et al. (2012) [54] | 3.8 | NR | 38.2 (6–86.5) | 75.3 (54–94) |
| Sterheim et al. (2012) [54] | 0 | NR | 41 (10–73) | 73.0 (41.5–95) |
| Abolghasemian et al. (2013) [55] | 8.8 | NR | 15.4 (6–25)b | 37.7 (29–47)b |
NR not reported
a Merle D’Aubigne score
b Oxford hip score
c Charnley’s modification of Merle D’Aubigne score
Overall, the mean incidence of cup migration was 2.6 % (range, 0–8.8 %) and the mean prevalence of peri-acetabular radiolucency at final follow-up was 4.9 % (range, 0–42.9 %; 95 % CI, 0.03–0.06). The mean pre-operative horizontal hip centre was 21.9 mm (range, 14–39 mm) and this changed to a mean of 26.8 mm (range, 10.4–40.5 mm) postoperatively. This was however, not found to be significant (p = 0.54).
The pre-operative vertical hip centre of rotation improved significantly from a mean of 39.2 mm (range, 27.6–50) to a mean of 24.1 mm (range, 7.4–47 mm) with a mean improvement of 15.1 mm (range, 7.4–47 mm; p = 0.01, 95 % CI, 3.1–26.9) (Table 5).
Table 5.
Pre and postoperative hip centre measurements
| Revision THA | Hip centre –vertical pre-operative (mm) | Hip centre—vertical postoperative (mm) | Hip centre—horizontal pre-operative (mm) | Hip centre—horizontal postoperative (mm) |
|---|---|---|---|---|
| Nehme et al. (2004) [31] | 27.6 (−16 to 52)a | 7.4(−15 to 25)a | 18.6 (−3 to 46)a | 10.4 (1–25)a |
| Unger et al. (2005) [32] | NR | NR | NR | NR |
| Sporer et al. (2006) [33] | NR | NR | NR | NR |
| Weeden et al. (2007) [34] | 38 (25–54)b | 19 (10–32)b | NR | NR |
| Flecher et al. (2008) [45] | 41 (20–66)b | 26.3 (15–47)b | 39 (14–63)b | 40.5 (23–55)b |
| Seigmeth et al. (2009) [41] | 50 (29–73)b | 28 (14–48)b | NR | NR |
| Lingaraj et al. (2009) [44] | NR | 47 (28–60)b | NR | NR |
| Flecher et al. (2010) [45] | NR | 39 (13–55)b | NR | 22 (5–41)b |
| Kosashvilli et al. (2010) [46] | NR | NR | NR | NR |
| Fernandez-Fairen et al. (2010) [47] | 34 (18–52)b | 12 (−11 to 25)b | 16 (−50 to +24)b | 31(0–49)b |
| Ballester-Alfaro et al. (2010) [29] | 35 (16–55)b | 14 (−5 to 27)b | 14 (−3 to 26)b | 30 (2–40)b |
| Abolghasemian et al. (2013) [55] | 48.5(25–98) | 24.8 (11–38) | NR | NR |
Discussion
Durable long-term fixation with low aseptic failure rates have been reported with conventional cementless acetabular components in a variety of revision scenarios [57–60]. However, less optimal outcomes have been reported in hips with severe acetabular bone loss [61, 62]. This encouraged the development of highly-porous metals with greater porosity, enhanced osteoconductivity, and higher surface friction in an attempt to achieve better initial mechanical stability and secondary biological fixation. Currently, comprehensive reports analysing the outcomes of these highly-porous metals for a variety of acetabular defects during revision hip arthroplasty are lacking. Thus, the purpose of the study was to review the clinical and radiographic outcomes of these implants in revision THA.
This study has several limitations. There were only two studies with level III evidence, so conclusions had to be drawn after combining data from reports with level IV evidence. Pooling of the data to derive conclusions on outcomes may have ignored the heterogeneity among the study population. Most studies had small sample sizes or did not report on all outcome metrics analysed in the study. Not all reports used porous metal augments for reconstruction of acetabular defects in their study population which could have been a confounding factor when analysing the outcomes. However, despite these limitations we were able to analyse the results of more than 2,000 revision hips in this review. Moreover, sub-categorisation of the acetabular defects into major and minor bone loss cohorts enabled us to compare the outcomes between these groups.
The press-fit stability of hemispherical acetabular components may be compromised in the presence of bone loss as it decreases the amount of host bone contact. This may be of concern with conventional porous-coated fixation which relies on stable initial fixation for secondary bone ingrowth to occur. The newer metals have high porosity and greater surface frictional properties which potentially can improve the initial fixation strength of the cup–bone interface and enhance osseointegration and durable long-term fixation. Jafari et al. in a retrospective analysis of 295 hips at approximately two- to four-year follow-up reported that hips with major acetabular bone loss had lower aseptic failure rates with tantalum cups (12 %; three out of 26 hips) in comparison to conventional porous-coated cups (24 %; five out of 21 hips) [48]. The aseptic failure rate in the minor bone loss group using tantalum was 6 % (five out of 81) in comparison to 8 % (17 out of 214 hips) with conventional implants. Thus, there was no significant difference in survivorship between hips with tantalum and conventional implants in the minor bone loss groups. Sternheim et al., in a comparative study using highly-porous revision shells, reported higher failure rates (7.5 %; four out of 53 hips) when less than 50 % host bone contact was available compared to hips with more than 50 % host bone available (0 % failures in 49 hips) at a mean follow-up of six years (range, five to 8.5 years) [54]. However, the difference was not statistically significant. When compared to previous reports this showed that there was a statistically significant difference in aseptic loosening between hips with major and minor bone loss (2.9 % and 0.4 %; P = 0.005). At this time to the best of our knowledge, there is only one report on titanium-based highly porous metals in acetabular revisions reporting with short term follow-up [40]. Ramappa et al. reported on the outcomes of 43 acetabular component revisions in 43 patients who had AAOS type 1 to type 4 acetabular defects using titanium based highly porous metals [40]. At a mean follow-up of 18.2 months (13–24 months), the authors found that 98 % of the cups (n = 42) had osseointegration at 12 weeks follow-up. One patient with pelvic discontinuity had aseptic loosening with medial migration of more than five millimetres at final follow-up. Although initial results with titanium-based highly porous metals are encouraging, further studies with mid- to long-term data are needed.
Currently, there is controversy whether restoration of the anatomical centre of rotation optimises outcomes in revision total hip arthroplasty. Placement of the acetabular cup with a high hip centre is usually considered in the revision setting to obtain stable initial fixation for bone ingrowth to occur. Hip joint stability and correction of limb length discrepancy is achieved through compensatory lengthening by using a modular femoral component. This may not be possible during isolated acetabular revision. Kim et al. in their evaluation of 35 revision hip arthroplasties with acetabular reconstruction rings and allogeneic bone grafting reported 100 % aseptic survivorship and favourable functional and radiological outcomes with restoration of the anatomical hip centre at a mean follow-up of 3.8 years (range, 2–4.2 years) [63]. Dearborn et al., in their study of 46 hips at a mean follow-up of 10.4 years (range, 8.5 to 12.7 years), however, reported 96 % survivorship despite placement of the acetabular cup in a high hip centre [9]. Moreover, abductor function was not adversely affected in their series with a reduction in the Trendenlenburg gait from 98 % (45 out of 46 hips) to 44 % (16 out of 36 at final follow-up). Schutzer et al. similarly reported excellent survivorship (100 %) and functional outcomes after superior placement (mean 29 mm above the anatomical hip centre) of the acetabular cup in their series of 56 hips at a mean follow-up of 3.3 years (range, two to 5.3 years) [64]. In our review, there were only five studies that reported on the radiographic location of the vertical hip centre both pre and postoperatively. Overall, there was significant improvement in the postoperative vertical hip centre with a near anatomical placement to a mean of 24.1 mm (P < 0.001). Importantly, augments were used in 26 % of patients (n = 1,152) included in these five studies suggesting that augments may have contributed to improvement in the placement of acetabular components to a near normal centre of rotation.
In summary, the overall short-term results of highly-porous metals in revision hip arthroplasty are excellent with a low rate of aseptic loosening in the presence of both major and minor bone loss. Use of metal augments may aid in restoration of the hip centre in the presence of acetabular defects. Despite these advantages, long-term concerns about failure of restoration of bone stock and the generation of metal debris from shell-augment interface however, still remain. Further data is needed to determine whether these newer metals will provide durable fixation and better survivorship in the presence of significant bone loss during acetabular revisions over the more conventional methods such as structural allografts and reconstruction cages.
Appendix
Table 6 presents the pre-operative diagnosis prior to acetabular revision, and Table 7 shows the distribution of acetabular defects based on Paprosky’s classification.
Table 6.
Pre-operative diagnosis prior to acetabular revision
| Author/year | Number of hips | Osteolysis | Aseptic loosening | Infection | Trauma | Dislocation | Misc |
|---|---|---|---|---|---|---|---|
| Revision THA | |||||||
| Nehme et al. (2004) [31] | 16 | 0 | 15 | 1 | 0 | 0 | 0 |
| Unger et al. (2005) [32] | 60 | 50 | 10 | 0 | 0 | 0 | 0 |
| Sporer et al. (2006) [33] | 13 | NR | NR | NR | NR | NR | NR |
| Weeden et al. (2007) [34] | 43 | 0 | 37 | 4 | 0 | 0 | 2 |
| Flecher et al. (2008) [35] | 23 | NR | 17 | NR | NR | NR | NR |
| Sporer et al. (2008) [36] | 28 | 0 | 23 | 4 | 1 | 0 | 0 |
| Kim et al.(2008) [37] | 46 | NR | 39 A+O | 0 | 0 | 0 | 7 |
| Van Kleunen et al. (2009) [38] | 97 | 0 | 73 | 17 | 2 | 0 | 5 |
| Lakstein et al. (2009) [39] | 53 | 0 | 48 | 2 | 0 | 3 | 0 |
| Seigmeth et al. (2009) [41] | 34 | 0 | 29 | 2 | 0 | 1 | 2 |
| Malkani et al. (2009) [42] | 22 | NR | NR | NR | NR | NR | NR |
| Simon et al. (2009) [43] | 53 | NR | 45 | NR | NR | 8 | NR |
| Lingaraj et al. (2009) [44] | 23 | 0 | 21 | 1 | 1 | 0 | 0 |
| Flecher et al. (2010) [45] | 72 | NR | NR | NR | NR | NR | NR |
| Kosashvilli et al. (2010) [46] | NR | NR | NR | NR | NR | NR | NR |
| Fernandez-Fairen et al.(2010) [47] | 263 | 62 | 186 | 0 | 0 | 15 | 0 |
| Jafari et al. (2010) [48] | 81 | 2 | 73 | 0 | 0 | 6 | 0 |
| Ballester-Alfaro et al. (2010) [29] | NR | NR | NR | NR | NR | NR | |
| Hasart et al. (2010) [49] | NR | NR | NR | NR | NR | NR | |
| Lachiewicz et al. (2010) [56] | 39 | NR | NR | NR | NR | NR | NR |
| Skytta et al. (2011) [52] | 827 | 0 | 330 | 39 | 19 | 88 | 112a; 46b; 197c |
| Davies et al. (2011) [30] | 0 | 9 | 27 | 4 | 0 | 0 | 0 |
| Del Gaizo et al. (2012) [53] | 37 | 0 | 31 | 5 | 0 | 1 | 0 |
| Sterheim et al. (2012) [54] | 49 | 0 | 43 | 3 | 0 | 3 | 0 |
| Sterheim et al. (2012) [54] | 53 | 0 | 50 | 1 | 0 | 2 | 0 |
| Abolghasemian (2013) [55] | 34 | 0 | 29 | 2 | 0 | 0 | 3 |
NR not reported, A+O aseptic loosening and osteolysis
aLiner exchange
bGirdlestone Arthroplasty
cUnknown
Table 7.
Distribution of acetabular defects based on Paprosky’s classification
| Author/year | Grade 1 (n) | Grade 2a (n) | Grade 2b (n) | Grade 2c | Grade 3a | Grade 3b |
|---|---|---|---|---|---|---|
| Revision THA | ||||||
| Nehme et al. (2004) [31] | 0 | 1 | 3 | 1 | 5 | 6 |
| Unger et al. (2005) [32] | 2 | 16 | 25 | 10 | 7 | 2 |
| Sporer et al. (2006) [33] | 0 | 0 | 0 | 0 | 0 | 13 |
| Weeden et al. (2007) [34] | 0 | 0 | 0 | 0 | 33 | 10 |
| Flecher et al. (2008) [35] | 0 | 0 | 0 | 0 | 17 | 6 |
| Sporer et al. (2008) [36] | 0 | 0 | 0 | 0 | 28 | 0 |
| Kim et al.(2008) [37] | 0; 3c | 20 | 4 | 9 | 6 | 4 |
| Van Kleunen et al. (2009) [38] | 0 | 24 | 19 | 19 | 19 | 16 |
| Lakstein et al. (2009) [39] | NR | NR | NR | NR | NR | NR |
| Seigmeth et al. (2009) [41] | 0 | 4 | 2 | 1 | 19 | 8 |
| Malkani et al. (2009) [42] | NR | 19b | NR | NR | 6d | NR |
| Simon et al. (2009) [43] | 15 | 15 | 14 | 5 | 4 | 0 |
| Lingaraj et al. (2009) [44] | 0 | 0 | 0 | 0 | 16 | 7 |
| Flecher et al. (2010) [45] | 13 | 14 | 14 | 0 | 23 | 8 |
| Kosashvilli et al. (2010) [46] | NR | NR | NR | NR | NR | NR |
| Fernandez-Fairen et al.(2010) [47] | 20 | 73 | 82 | 39 | 40 | 9 |
| Jafari et al. (2010) [48] | 55 (1 to 2b) | – | – | 26 (2c to 3b) | – | – |
| Ballester-Alfaro et al. (2010) [29] | 0 | 0 | 0 | 0 | 13 | 6 |
| Hasart et al. (2010) [49] | 0 | 0 | 0 | 0 | 38 (3 a and b) | 0 |
| Lachiewicz et al. (2010) [56] | 2 | 11b | – | – | 8 | 18 |
| Skytta et al. (2011) [52] | NR | NR | NR | NR | NR | NR |
| Davies et al. (2011) [30] | 0 | 0 | 0 | 10 | 21 | 11 |
| Del Gaizo et al.(2012) [53] | 0 | 0 | 0 | 0 | 37 | 0 |
| Sterheim et al. (2012) [54] >50 % | NR | NR | NR | NR | NR | NR |
| Sterheim et al. (2012) [54] <50 % | NR | NR | NR | NR | NR | NR |
| Abolghasemian (2013) [55] | 18a | NR | NR | NR | 14a | NR |
NR not reported
aGross Classification
bPaprosky Grade 2
ccannot be classified
dPaprosky Grade 3
References
- 1.Ng TP, Chiu KY. Acetabular revision without cement. J Arthroplasty. 2003;18(4):435–441. doi: 10.1016/s0883-5403(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 2.Lie SA, Havelin LI, Furnes ON, Engesaeter LB, Vollset SE. Failure rates for 4762 revision total hip arthroplasties in the Norwegian Arthroplasty Register. J Bone Joint Surg Br. 2004;86(4):504–509. [PubMed] [Google Scholar]
- 3.Neumann D, Dueckelmann L, Thaler C, Dorn U. Revision total hip arthroplasty using a cementless tapered revision stem in patients with a mean age of 82 years. Int Orthop. 2012;36(5):961–965. doi: 10.1007/s00264-011-1379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattyn C, Mulliez A, Verdonk R, Audenaert E. Revision hip arthroplasty using a cementless modular tapered stem. Int Orthop. 2012;36(1):35–41. doi: 10.1007/s00264-011-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuh R, Neumann D, Rauf R, Hofstaetter J, Boehler N, Labek G. Revision rate of Birmingham Hip Resurfacing arthroplasty: comparison of published literature and arthroplasty register data. Int Orthop. 2012;36(7):1349–1354. doi: 10.1007/s00264-012-1502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton JE, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen-year follow-up study. J Bone Joint Surg Am. 2001;83-A(11):1706–1711. doi: 10.2106/00004623-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Dearborn JT, Harris WH. Acetabular revision arthroplasty using so-called jumbo cementless components: an average 7-year follow-up study. J Arthroplasty. 2000;15(1):8–15. doi: 10.1016/s0883-5403(00)90999-9. [DOI] [PubMed] [Google Scholar]
- 8.Whaley AL, Berry DJ, Harmsen WS. Extra-large uncemented hemispherical acetabular components for revision total hip arthroplasty. J Bone Joint Surg Am. 2001;83-A(9):1352–1357. doi: 10.2106/00004623-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Dearborn JT, Harris WH. High placement of an acetabular component inserted without cement in a revision total hip arthroplasty. Results after a mean of ten years. J Bone Joint Surg Am. 1999;81(4):469–480. doi: 10.2106/00004623-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chen WM, Engh CA, Jr, Hopper RH, Jr, McAuley JP, Engh CA. Acetabular revision with use of a bilobed component inserted without cement in patients who have acetabular bone-stock deficiency. J Bone Joint Surg Am. 2000;82(2):197–206. doi: 10.2106/00004623-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Rosson J, Schatzker J. The use of reinforcement rings to reconstruct deficient acetabula. J Bone Joint Surg Br. 1992;74(5):716–720. doi: 10.1302/0301-620X.74B5.1527120. [DOI] [PubMed] [Google Scholar]
- 12.Harris WH. Reconstruction at a high hip center in acetabular revision surgery using a cementless acetabular component. Orthopedics. 1998;21(9):991–992. doi: 10.3928/0147-7447-19980901-17. [DOI] [PubMed] [Google Scholar]
- 13.Choi HR, Anderson D, Foster S, Beal M, Lee JA, Barr C, Malchau H, McCarthy J, Kwon YM. Acetabular cup positioning in revision total hip arthroplasty with Paprosky type III acetabular defects: Martell radiographic analysis. Int Orthop. 2013 doi: 10.1007/s00264-013-2008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai T, Ohzono K, Nishii T, Takao M, Miki H, Nakamura N, Sugano N. Modular acetabular reconstructive cup in acetabular revision total hip arthroplasty at a minimum ten year follow-up. Int Orthop. 2013;37(4):605–610. doi: 10.1007/s00264-013-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalczewski JB, Rutkowska-Sak L, Marczak D, Slowinska I, Slowinski R, Sibinski M. Bone graft incorporation after revision hip arthroplasty in patients with rheumatoid arthritis, seventy eight revisions using bone allografts with or without metal reinforcements. Int Orthop. 2013;37(4):595–598. doi: 10.1007/s00264-013-1794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop. 2011;35(2):289–298. doi: 10.1007/s00264-010-1198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paprosky WG, Magnus RE. Principles of bone grafting in revision total hip arthroplasty. Acetabular technique. Clin Orthop Relat Res. 1994;298:147–155. [PubMed] [Google Scholar]
- 18.Paprosky W, Sporer S, O'Rourke MR. The treatment of pelvic discontinuity with acetabular cages. Clin Orthop Relat Res. 2006;453:183–187. doi: 10.1097/01.blo.0000246530.52253.7b. [DOI] [PubMed] [Google Scholar]
- 19.Perka C, Ludwig R. Reconstruction of segmental defects during revision procedures of the acetabulum with the Burch-Schneider anti-protrusio cage. J Arthroplasty. 2001;16(5):568–574. doi: 10.1054/arth.2001.23919. [DOI] [PubMed] [Google Scholar]
- 20.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81(5):907–914. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 21.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Khan M, Adamich J, Simunovic N, Philippon MJ, Bhandari M, Ayeni OR. Surgical management of internal snapping hip syndrome: a systematic review evaluating open and arthroscopic approaches. Arthroscopy. 2013 doi: 10.1016/j.arthro.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Huisstede B, Miedema HS, van Opstal T, de Ronde MT, Verhaar JA, Koes BW. Interventions for treating the radial tunnel syndrome: a systematic review of observational studies. J Hand Surg [Am] 2008;33(1):72–78. doi: 10.1016/j.jhsa.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Rometsch E, Bos PK, Koes BW. Survival of short hip stems with a "modern", trochanter-sparing design - a systematic literature review. Hip Int. 2012;22(4):344–354. doi: 10.5301/HIP.2012.9472. [DOI] [PubMed] [Google Scholar]
- 25.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9(1):33–44. doi: 10.1016/0883-5403(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 26.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 27.Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD, Jr, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982. A two to five-year follow-up. J Bone Joint Surg Am. 1985;67(7):1074–1085. [PubMed] [Google Scholar]
- 28.Ranawat CS, Dorr LD, Inglis AE. Total hip arthroplasty in protrusio acetabuli of rheumatoid arthritis. J Bone Joint Surg Am. 1980;62(7):1059–1065. [PubMed] [Google Scholar]
- 29.Ballester Alfaro JJ, Sueiro Fernandez J. Trabecular metal buttress augment and the Trabecular metal cup-cage construct in revision hip arthroplasty for severe acetabular bone loss and pelvic discontinuity. Hip Int. 2010;20(Suppl 7):119–127. doi: 10.1177/11207000100200s720. [DOI] [PubMed] [Google Scholar]
- 30.Davies JH, Laflamme GY, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty. 2011;26(8):1245–1250. doi: 10.1016/j.arth.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–208. doi: 10.1097/01.blo.0000150133.88271.80. [DOI] [PubMed] [Google Scholar]
- 32.Unger AS, Lewis RJ, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthroplasty. 2005;20(8):1002–1009. doi: 10.1016/j.arth.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(6 Suppl 2):87–90. doi: 10.1016/j.arth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Weeden SH, Schmidt RH. The use of tantalum porous metal implants for Paprosky 3A and 3B defects. J Arthroplasty. 2007;22(6 Suppl 2):151–155. doi: 10.1016/j.arth.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Flecher X, Sporer S, Paprosky W. Management of severe bone loss in acetabular revision using a trabecular metal shell. J Arthroplasty. 2008;23(7):949–955. doi: 10.1016/j.arth.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty. 2006;21(6 Suppl 2):83–86. doi: 10.1016/j.arth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Kim WY, Greidanus NV, Duncan CP, Masri BA, Garbuz DS. Porous tantalum uncemented acetabular shells in revision total hip replacement: two to four year clinical and radiographic results. Hip Int. 2008;18(1):17–22. doi: 10.1177/112070000801800104. [DOI] [PubMed] [Google Scholar]
- 38.Van Kleunen JP, Lee GC, Lementowski PW, Nelson CL, Garino JP. Acetabular revisions using trabecular metal cups and augments. J Arthroplasty. 2009;24(6 Suppl):64–68. doi: 10.1016/j.arth.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Trabecular metal cups for acetabular defects with 50% or less host bone contact. Clin Orthop Relat Res. 2009;467(9):2318–2324. doi: 10.1007/s11999-009-0772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramappa M, Bajwa A, Kulkarni A, McMurtry I, Port A. Early results of a new highly porous modular acetabular cup in revision arthroplasty. Hip Int. 2009;19(3):239–244. doi: 10.1177/112070000901900309. [DOI] [PubMed] [Google Scholar]
- 41.Siegmeth A, Duncan CP, Masri BA, Kim WY, Garbuz DS. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res. 2009;467(1):199–205. doi: 10.1007/s11999-008-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malkani AL, Price MR, Crawford CH, 3rd, Baker DL. Acetabular component revision using a porous tantalum biomaterial: a case series. J Arthroplasty. 2009;24(7):1068–1073. doi: 10.1016/j.arth.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Simon JP, Bellemans J. Clinical and radiological evaluation of modular trabecular metal acetabular cups. Short-term results in 64 hips. Acta Orthop Belg. 2009;75(5):623–630. [PubMed] [Google Scholar]
- 44.Lingaraj K, Teo YH, Bergman N. The management of severe acetabular bone defects in revision hip arthroplasty using modular porous metal components. J Bone Joint Surg Br. 2009;91(12):1555–1560. doi: 10.1302/0301-620X.91B12.22517. [DOI] [PubMed] [Google Scholar]
- 45.Flecher X, Paprosky W, Grillo JC, Aubaniac JM, Argenson JN. Do tantalum components provide adequate primary fixation in all acetabular revisions? Orthop Traumatol Surg Res. 2010;96(3):235–241. doi: 10.1016/j.otsr.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Kosashvili Y, Safir O, Backstein D, Lakstein D, Gross AE. Salvage of failed acetabular cages by nonbuttressed trabecular metal cups. Clin Orthop Relat Res. 2010;468(2):466–471. doi: 10.1007/s11999-009-0935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Fairen M, Murcia A, Blanco A, Merono A, Murcia A, Jr, Ballester J. Revision of failed total hip arthroplasty acetabular cups to porous tantalum components: a 5-year follow-up study. J Arthroplasty. 2010;25(6):865–872. doi: 10.1016/j.arth.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 48.Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, Hozack WJ. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res. 2010;468(2):459–465. doi: 10.1007/s11999-009-1090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasart O, Perka C, Lehnigk R, Tohtz S. Reconstruction of large acetabular defects using trabecular metal augments. Oper Orthop Traumatol. 2010;22(3):268–277. doi: 10.1007/s00064-010-8026-9. [DOI] [PubMed] [Google Scholar]
- 50.Markuszewski J, Wierusz-Kozlowska M, Wozniak W, Lapaj L, Kokoszka P. Porous tantalum modular cups in revision hip arthroplasty. Chir Narzadow Ruchu Ortop Pol. 2011;76(4):197–200. [PubMed] [Google Scholar]
- 51.Pierannunzii L, Mambretti A, D'Imporzano M. Trabecular metal cup without augments for acetabular revision in case of extensive bone loss and low bone-prosthesis contact. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):133–137. doi: 10.1177/03946320110241S225. [DOI] [PubMed] [Google Scholar]
- 52.Skytta ET, Eskelinen A, Paavolainen PO, Remes VM. Early results of 827 trabecular metal revision shells in acetabular revision. J Arthroplasty. 2011;26(3):342–345. doi: 10.1016/j.arth.2010.01.106. [DOI] [PubMed] [Google Scholar]
- 53.Del Gaizo DJ, Kancherla V, Sporer SM, Paprosky WG. Tantalum augments for Paprosky IIIA defects remain stable at midterm followup. Clin Orthop Relat Res. 2012;470(2):395–401. doi: 10.1007/s11999-011-2170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sternheim A, Backstein D, Kuzyk PR, Goshua G, Berkovich Y, Safir O, Gross AE. Porous metal revision shells for management of contained acetabular bone defects at a mean follow-up of six years: a comparison between up to 50% bleeding host bone contact and more than 50% contact. J Bone Joint Surg Br. 2012;94(2):158–162. doi: 10.1302/0301-620X.94B2.27871. [DOI] [PubMed] [Google Scholar]
- 55.Abolghasemian M, Tangsataporn S, Sternheim A, Backstein D, Safir O, Gross AE. Combined trabecular metal acetabular shell and augment for acetabular revision with substantial bone loss: a mid-term review. Bone Joint J. 2013;95-B(2):166–172. doi: 10.1302/0301-620X.95B2.30608. [DOI] [PubMed] [Google Scholar]
- 56.Lachiewicz PF, Soileau ES. Tantalum components in difficult acetabular revisions. Clin Orthop Relat Res. 2010;468(2):454–458. doi: 10.1007/s11999-009-0940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trumm BN, Callaghan JJ, Liu SS, Goetz DD, Johnston RC. Revision with cementless acetabular components: a concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2012;94(21):2001–2004. doi: 10.2106/JBJS.L.00058. [DOI] [PubMed] [Google Scholar]
- 58.Park DK, Della Valle CJ, Quigley L, Moric M, Rosenberg AG, Galante JO. Revision of the acetabular component without cement. A concise follow-up, at twenty to twenty-four years, of a previous report. J Bone Joint Surg Am. 2009;91(2):350–355. doi: 10.2106/JBJS.H.00302. [DOI] [PubMed] [Google Scholar]
- 59.Della Valle CJ, Shuaipaj T, Berger RA, Rosenberg AG, Shott S, Jacobs JJ, Galante JO. Revision of the acetabular component without cement after total hip arthroplasty. A concise follow-up, at fifteen to nineteen years, of a previous report. J Bone Joint Surg Am. 2005;87(8):1795–1800. doi: 10.2106/JBJS.D.01818. [DOI] [PubMed] [Google Scholar]
- 60.Hallstrom BR, Golladay GJ, Vittetoe DA, Harris WH. Cementless acetabular revision with the Harris-Galante porous prosthesis. Results after a minimum of ten years of follow-up. J Bone Joint Surg Am. 2004;86-A(5):1007–1011. doi: 10.2106/00004623-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Cimbrelo E, Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro J. The survival and fate of acetabular reconstruction with impaction grafting for large defects. Clin Orthop Relat Res. 2010;468(12):3304–3313. doi: 10.1007/s11999-010-1395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berry DJ. Identification and management of pelvic discontinuity. Orthopedics. 2001;24(9):881–882. doi: 10.3928/0147-7447-20010901-25. [DOI] [PubMed] [Google Scholar]
- 63.Kim DH, Cho SH, Jeong ST, Park HB, Hwang SC, Park JS. Restoration of the center of rotation in revision total hip arthroplasty. J Arthroplasty. 2010;25(7):1041–1046. doi: 10.1016/j.arth.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Schutzer SF, Harris WH. High placement of porous-coated acetabular components in complex total hip arthroplasty. J Arthroplasty. 1994;9(4):359–367. doi: 10.1016/0883-5403(94)90045-0. [DOI] [PubMed] [Google Scholar]