Abstract
Purpose
This study investigates the accuracy of a computed tomography (CT)-based navigation system for accurate acetabular component placement during revision total hip arthroplasty (THA).
Methods
We performed a retrospective review of 30 hips in 26 patients who underwent cementless revision THA using a CT-based navigation system; the control group consisted of 25 hips in 25 patients who underwent cementless primary THA using the same system. We analysed the deviation of anteversion and inclination angles among the pre-operative plan, intra-operative records from the navigation system and data from postoperative CT scans.
Results
There were no significant differences between groups (P < 0.05) in terms of mean deviation between pre-operative planning and postoperative measurements or between intraoperative records and postoperative measurements.
Conclusion
CT-based navigation in revision THA is a useful tool that enables the surgeon to implant the acetabular component at the precise angle determined in pre-operative planning.
Keywords: Revision total hip arthroplasty, CT-based navigation, Acetabular component
Introduction
Primary total hip arthroplasty (THA) is an extremely successful orthopaedic procedure for reducing pain and restoring mobility [3]. With prolonged life expectancy, and more hip arthroplasties being performed in younger patients, the number of revision THAs is rapidly increasing, and the number being performed annually is estimated to double between 2005 and 2030 [4, 10, 11, 20].
Malpositioning of the acetabular component in both primary and revision THA increases the risk of reduced range of movement (ROM), with an increase in dislocation, impingement, prosthesis wear and osteolysis, and effect on long-term results [2, 6, 19]. Revision THA is a technically demanding procedure, and the risk of postoperative dislocation is high [8, 17]. Moreover, bone defects of the acetabulum, which can occur after revision THA, may bias the surgeon towards a less favorable component alignment [8, 18]. Surgical navigation tools have enabled surgeons to achieve more precise placement of hip components [7, 9, 23]. Computed tomography (CT)-based navigation has demonstrated advantages over imageless systems in patients with abnormal anatomies, such as hip dysplasia or posttraumatic deformities [7], but few reports have addressed the accuracy of these systems for revision THA. This study investigated the accuracy of using a CT-based navigation system for acetabular component placement in revision THA.
Materials and methods
We performed a retrospective review of 30 hips in 29 patients who underwent cementless revision THA between May 2006 and May 2013; the control group was 25 hips in 25 patients who underwent cementless primary THA. The same navigation system was used in all operations (CT-based Hip, version 1.0; Stryker Navigation, Freiburg, Germany). Data for the control group was reported in 2012 following similar criteria [6]. Patient demographics are shown in Table 1. Diagnoses in the study group were osteolysis in seven hips, infection in six (two-stage revision), implant failure in three, aseptic loosening in nine, instability in two and other in three. There were 16 isolated acetabular component revisions and both femoral and acetabular revisions in 14. In the control group, mild developmental dysplasia (Crowe group 1) was diagnosed in 18 hips and primary osteoarthritis in seven. In the revision group the Trident acetabular cup (Stryker) was used in 11 hips, titanium (Stryker) in seven, Trilogy (Zimmer) in four, trabecular metal (Zimmer) in six and others in two. The Trident acetabular cup was used in all 25 hips in the control group.
Table 1.
Patient demographic data of the two groups
| Patient characteristics | Study group (n = 30) | Control group (n = 25) |
|---|---|---|
| Age | 64.7 ± 10.2 (44–82) | 64.9 ± 10.0 (51–87) |
| Sex (female/male) | 19/11 | 21/4 |
| Side (left/right) | 15/15 | 19/6 |
| Height (cm) | 156.7 ± 10.5 (141.9–170.3) | 152.1 ± 6.6 (137–165) |
| Weight (kg) | 57.4 ± 12.8 (37–89.6) | 53.8. ± 10.7 (38–77) |
| Body mass index (kg/m2) | 23.2 ± 3.5 (18.3–34.2) | 23.1 ± 3.7 (18.5–30.2) |
| Diagnosis | Osteolysis in 7 hips | Crowe 1in 18 hips |
| Infection in 6 hips | Primary osteoarthritis in 7 hips | |
| Aseptic loosening in 9 hips | ||
| Implant failure in 3 hips | ||
| Instability in 2 hips | ||
| Others in 3 hips |
All values are expressed as means ± standard deviation (range)
Pre-operative planning
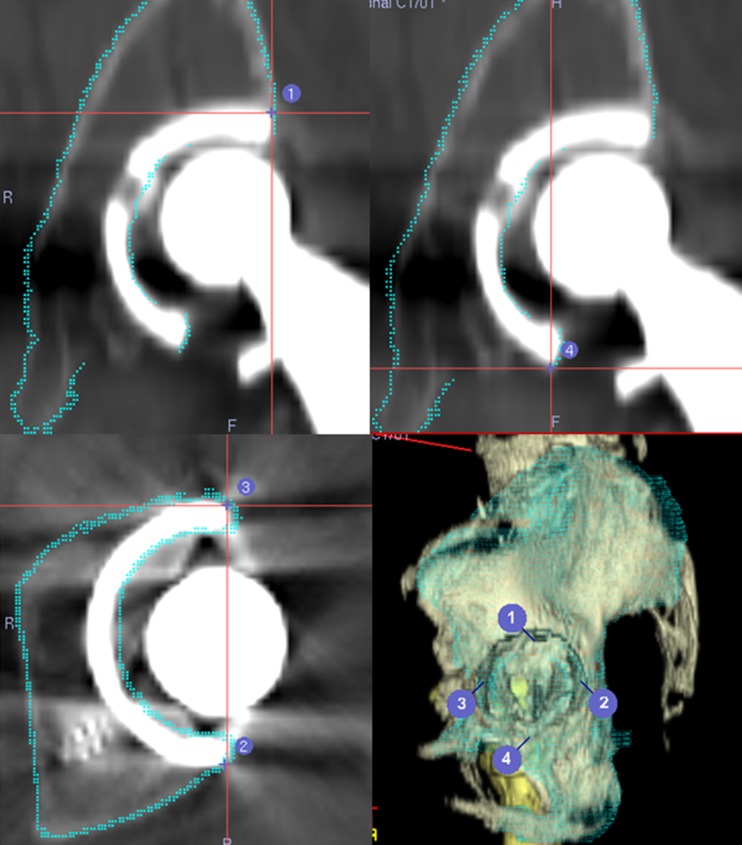
A pre-operative CT scan from the iliac wing to the femoral condyle was performed using a helical CT scanner (LightSpeed VCT; GE Medical Systems, Milwaukee, WI, USA). Slice thickness was 1 mm, and pitch was 2.5–3.0 mm (160–250 slices depending on body size). CT data were transferred to the planning module; 3D-templating software (CT-based Hip, version 1.0) was used to determine the optimal component size, angle and position. CT images from patients who have already undergone primary THA are affected by metal artifacts used in the implants [16]. So, for revision THA patients, we suppress halation of the primary implant as much as possible and perform surface registration using the implant surface, in particular, the edge of the acetabular implant, in the pre-operative planning stage (Fig. 1).
Fig. 1.
Registration points using implant surface in the pre-operative planning stage
Our goal was to implant the acetabular component at the native acetabulum with an anatomical inclination of 40° and anteversion of 20° (38.3° inclination and 12.7° anteversion in radiography), which are almost at the centre of the safe zone [12, 15]. To prevent postoperative impingement and dislocation if stem anteversion was no less than 20° nor more than 30° according to the combined anteversion theory, we used the mathematical formula [Cup anteversion + 0.7 × Stem anteversion = 37.3°] to determine optimal component position [25]. Thus, cup anteversion was based on stem anteversion. For example, based on pre-operative CT data, when anteversion of the existing femoral component that would be retained or of the newly planned femoral component was 36°, we set radiographic cup anteversion to 12°. The anterior pelvic plane defined by both the bilateral anterior superior iliac spine and pubic tubercle was used as the reference plane of the pelvis. If, due to spine and pelvic deformities, the pelvis was tilted in the sagittal plane when the patient was lying in a supine position, correction of the anterior–posterior axis was performed during pre-operative templating, as described in previous studies [6, 24]. In brief, the functional pelvic plane was used as a reference plane [14] for both groups.
Intra-operative procedures
All surgeries were performed by one surgeon (TK) using a posterolateral approach. Intra-operative surface registration was performed using the method reported by Sugano et al. [24]. Briefly, a reference tracker was mounted on the acetabulum wing, and surface matching was performed by touching >30 points around the acetabulum with a pointer after resectioning the femoral head. In the study group, the digitizing area was made as wide as possible to include the native acetabulum, the ala of the ilium and the posterior wall and the rim of the primary acetabular component. Additional time needed for setup and registration of the navigation system was five to ten minutes in both groups. After registration, the surgeon removed the primary acetabular component. Next, he reamed the acetabulum and implanted the acetabular component with real-time confirmation of both component angles on the navigation monitor. The rim of the primary acetabular component was not used for digitising points in the six hips that required revision THA for infection; in those cases, we performed a two-stage revision THA. After implantation of the acetabular component, final cup orientation was recorded (intra-operative record).
Postoperative procedures
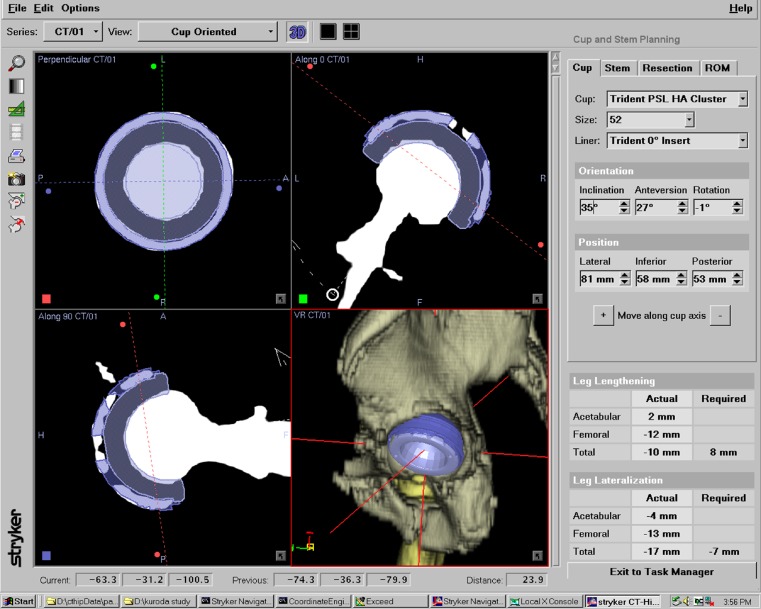
In all cases, a postoperative CT scan was performed about ten days after the operation; 3D pelvic bone surface models were reconstructed from the data. As reported previously, we used the functional pelvic zero position to measure cup orientation (inclination and anteversion): the pelvis, with the patient in a supine position on the CT scan table, was axially rotated until the bilateral anterior iliac spines touched the same horizontal plane; then, the interteardrop line was used as the mediolateral axis [6, 23]. We manually established the same coordinate plane as that determined in pre-operative planning and measured various parameters so that virtual computer-aided-design models of the acetabular component could be superimposed on the images of the actual implanted component (Fig. 2) [6]. We evaluated the deviation of radiographic anteversion and inclination angle among the pre-operative plan, intraoperative records from the navigation system and data from postoperative CT scans. We also investigated whether the pre-operatively planned size of the component was the same as that actually implanted. Error was evaluated by root mean square (RMS) analysis to compare the accuracy of the registration process between the two groups [6, 22].
Fig. 2.
Component position and angle were measured by superimposing the computer-aided-design model of the acetabular component on the image of the actual implanted component
Measurements were performed by the author (KK) independent of the operating surgeon . To reduce error, each measurement was performed three times, and the mean value was used. The variability of postoperative measurement with this method has been assessed and reported by Kajino et al. [6].
Statistical analysis
A mean difference of 3° in cup-placement-navigation accuracy was identified as significant, as discussed previously [7]. A sample size power analysis showed that 24 patients in each group would be sufficient to determine whether there was a significant difference with power of 0.8 and p < .05. Statistical analyses were performed using SPSS ver.19.0 (SPSS, Inc, Chicago, IL, USA). The unpaired t test was used to compare RMS and accuracy of cup positioning. We used a Χ2 test to compare the concordance rate of planned and implanted cup size. In all analyses, P <0.05 indicated statistical significance.
Results
Table 2 presents detailed results of component-angle analysis. In summary, mean deviations between pre-operative planning and postoperative measurement were, respectively, 2.6° ± 1.8° inclination and 2.2° ± 2.2° anteversion in the study group and 2.0° ± 1.6° and 2.2° ± 1.3° n the control group. The mean deviations between intra-operative records and postoperative measurements were, respectively, 2.2° ± 2.1° inclination and 1.6° ± 1.2° anteversion in the study group and 1.5° ± 1.1° and 1.6° ± 1.1° in the control group. There were no significant differences between groups (P = 0.12, 0.89, 0.14,and 0.98, respectively).
Table 2.
Results of component-angle measurements
| Parameters | Inclination | Anteversion | ||
|---|---|---|---|---|
| Study group | Control group | Study group | Control group | |
| Pre-op. planning | 37.7 ± 1.1 (36.0–39.5) | 38.3 ± 0.0 (38.3) | 23.9 ± 6.1 (15–42) | 12.7 ± 0.0 (12.7) |
| Intra-op. record | 35.9 ± 2.6 (29.8–40.7) | 37.3 ± 1.5 (35.7–41.2) | 25.2 ± 6.1 (15–42) | 12.0 ± 1.2 (10.1–14.5) |
| Postmeasurement | 35.7 ± 3.1 (31.3–43.6) | 36.7 ± 2.0 (34.3–39.3) | 24.3 ± 8.0 (12.7–44.0) | 10.8 ± 1.6 (8.5–14.0) |
| Postop–pre-op | 2.6 ± 1.8 (0–6.9) | 2.0 ± 1.6 (0.2–5.4) | 2.2 ± 2.2 (0.1–5.4) | 2.2 ± 1.3 (0–4.2) |
| P value | 0.12 | 0.89 | ||
| Postop.–Intra-op. | 2.2 ± 2.1 (0–6.1) | 1.5 ± 1.1 (0–3.9) | 1.6 ± 1.2 (0.2–4.8) | 1.6 ± 1.1 (0–4.1) |
| P value | 0.14 | 0.98 | ||
All values expressed as means ± standard deviation (range)
Unpaired t test
Pre-op. pre-operative, Intra-op. intra-operative, Postop. postoperative
Table 3 details results of component-size planning. Accuracy was 53.3 % (13/30) in the study group and 88.0 % (22/25) in the control group (P < 0.05). Implanted cup size was within one size difference in 93.3 % (28/30) of the study group and in 88.0 % (23/25) of the control group (P = 0.470). RMS registration error was 0.87 ± 0.21 mm in the study group and 0.86 ± 0.21 mm in the control group. Again, there was no significant difference between groups (P = 0.964).
Table 3.
Results of component size and root mean square (RMS) analysis
| Parameters | Study group (n = 30) | Control group (n = 25) | P value |
|---|---|---|---|
| Accuracy of component size | |||
| Same | 16/30 (53.3 %) | 22/25 (88.0 %) | <0.05 |
| Within 1 size difference | 28/30 (93.3 %) | 22/25 (88.0 %) | 0.470 |
| Error of RMS analysis (mm) | 0.87 ± 0.21 (0.52–1.37) | 0.86 ± 0.21 (0.51–1.29) | 0.964 |
All values expressed as means ± standard deviation (range)
Unpaired t test
There were no complications related to use of the navigation system. Intraclass correlation coefficients of the intraobserver measurement in inclination and anteversion were 0.915 and 0.974, respectively.
Discussion
Some reports suggest that a more precise placement of all hip components can be achieved with surgical navigation than with conventional methods [21, 23]. Particularly in revision THA, where it is often difficult to get a picture of the pelvic surface because of primary implant halation and bone defects, using navigation is thought to be disadvantageous. To solve this problem, in pre-operative planning, we suppress halation of the primary implant as much as possible and perform surface registration using the implant surface—in particular, the edge of the acetabular implant if the acetabular socket is well fixed or fibrous ingrowth is stable. During surgery, we perform surface registration using the surface of the primary acetabular implant before we remove the primary implant. RMS analysis, which showed a registration error of 0.865 mm, indicates the accuracy of this registration process.
A report from Kajino et al. on the usefulness of CT-based hip navigation suggests that its accuracy does not depend on the degree of pelvic deformity [6]. Their accuracy was 1.5° ± 1.2° inclination and 2.5° ± 1.7° anteversion between intra-operative records and postoperative measurement in the pelvic deformity group. Our study suggests that CT-based navigation is, in fact, a useful tool in revision THA, enabling the surgeon to implant the acetabular component at the precise angle determined in pre-operative planning. Nakamura et al. were the first to report on the usefulness of navigation systems for revision THA [16]. Their accuracy was 2.0° ± 2.0° inclination and 2.5° ± 2.0° anteversion between intra-operative records and postoperative measurement in the revision THA group. To overcome the issue of metal artifacts, they took bone-surface registration points from the ilium and body of the ischium to avoid using points around the hip that included metal components [16]. We achieved equivalent precision using the surface of the implant.
Instability is a common complication after revision THA [4]. The incidence of hip dislocation, reported to range from 1 % to 3 % following primary THA, is as high as 7–25 % in revision THA [5]. The risk of dislocation is greatest in the first 12 weeks after arthroplasty, with approximately 60–70 % occurring during the first six weeks [13]. Therefore, precisely implanting the acetabular component to decrease the incidence of postoperative hip dislocations is more important in revision THA. We found only two cases of posterior dislocation (6.67 %) in early postoperative follow-up, and these were due to patient noncompliance, not to malposition of the acetabular component. In addition, they were single events and did not repeat. The incidence of hip dislocation in the revision THA group tended to be relatively lower than that reported in previous studies. However, we cannot appropriately make that comparison because our postoperative follow-up period was short and dislocation aetiology following THA is multifactorial, including not only component malposition but also impingement, soft issue laxity, femoral head size, offset, etc. [1, 5, 13].
There are limitations to our study: Firstly, it was retrospective, with a relatively small number of patients, because we included only cases in which reconstruction was accomplished with a cementless cup. Secondly, there were notable differences between groups in preplanning, intra-operative and postoperative anteversion measurement data. This is why the range of femoral anteversion data was so wide in the study group. As previously mentioned, in both groups we had a target angle for implanting the acetabular component according to the combined anteversion theory, so differences in femoral anteversion of both groups led to difference in anteversion preplanning and intra-operative and postoperative data. We think these notable differences were unlikely to affect results because we evaluated the accuracy of the navigation system for revision THA using a deviation between preplanning, intra-operative and postoperative data.
Conclusion
CT-based navigation in revision THA is a useful tool that enables the surgeon to implant the acetabular component at the precise angle determined in pre-operative planning.
References
- 1.Bourne RB, Hehin R. The dislocating hip; what to do, what to do. J Arthroplasty. 2004;19(4 Suppl1):111–114. doi: 10.1016/j.arth.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Choi HR, Anderson D, Foster S, Beal M, Lee JA, Barr C, Malchau H, McCarthy J, Kwon YM. Acetabular cup positioning in revision total hip arthroplasty with Paprosky type III acetabular defects: Martell radiographic analysis. Int Orthop. 2013;37:1905–1910. doi: 10.1007/s00264-013-2008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devane PA, Wraighte PJ, Ong DCG, Horne JG. Do joint registeries report true rates of hip dislocation? Clin Orthop Relat Res. 2012;470:3003–3006. doi: 10.1007/s11999-012-2323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbuz DS, Masri BA, Duncan CP, Greidanus NV, Bohm ER, Petrak MJ, Della Valle CJ, Gross AE. The Frank Stinchfield Award: dislocation in revision HA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop Relat Res. 2012;470:351–356. doi: 10.1007/s11999-011-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel MT, Malkani AL, Yallanti MR, Baker DL. Decreased dislocation after revision total hip arthroplasty using larger femoral head size and posterior capsular repair. J Arthroplasty. 2009;24(6 Suppl):73–76. doi: 10.1016/j.arth.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Kajino Y, Kabata T, Maeda T, Iwai S, Kuroda K, Tsuchiya H. Dose degree of the pelvic deformity affect the accuracy of computed tomography-based hip navigation? J Arthroplasty. 2012;27:1651–1657. doi: 10.1016/j.arth.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Kalteis T, Handel M, Bäthis PL, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty. J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 8.Kosashvili Y, Backstein D, Safir O, Lakstein D, Gross AE. Dislocation and infection after revision total hip arthroplasty: comparison between the first and multiply revised total hip arthroplasty. J Arthroplasty. 2011;26(8):1170–1175. doi: 10.1016/j.arth.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Kumar MA, Shetty MS, Kiran KG, Kini AR. Validation of navigation assisted cup placement in total hip arthroplasty. Int Orthop. 2012;36:17–22. doi: 10.1007/s00264-011-1268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurts S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 11.Labek G, Janda W, Agreiter M, Schuh R, Bohler N. Organisation, data evaluation, interpretation and effect of arthroplasty register data on the outcome in terms of revision rate in total hip arthroplasty. Int Orthop. 2011;35:157–163. doi: 10.1007/s00264-010-1131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 13.Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip dislocation. Clin Orthop Relat Res. 2002;405:46–53. doi: 10.1097/00003086-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Miki H, Yamanashi W, Nishii T, Sato Y, Yoshikawa H, Sugano N. Anatomic hip range of Moion after implantation during total hip arthroplasty as measured by a navigation system. J Arthroplasty. 2007;7:946–952. doi: 10.1016/j.arth.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura N, Nishii T, Kitada M, Iwana D, Sugano N (2013) Application of computed tomography-based navigation for revision total hip arthroplasty. J Arthropasty Mar 21, in press [DOI] [PubMed]
- 17.Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop. 2011;35:289–298. doi: 10.1007/s00264-010-1198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Sotelo J, Berry DJ. Epidemiology of instability after total hip replacement. Orthop Clin North Am. 2001;32:543–552. doi: 10.1016/S0030-5898(05)70225-X. [DOI] [PubMed] [Google Scholar]
- 19.Soong M, Rubash HE, Macaulay W. Dislocation after total hip arthroplasty. J Am Acad Orthop Surg. 2004;12:314–321. doi: 10.5435/00124635-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Splinger BD, Fehring TK, Griffin WL, Odum SM, Masonis JL. Why total hip arthroplasty fails. Clin Orthop Relat Res. 2009;467:166–173. doi: 10.1007/s11999-008-0566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugano N, Nishii T, Miki H, Yoshikawa H, Sato Y, Tamura S. Mid-term results of cementless total hip replacement using a ceramic-on-ceramic bearing with and without computer navigation. J Bone Joint Surg Br. 2007;89:455–460. doi: 10.1302/0301-620X.89B4.18458. [DOI] [PubMed] [Google Scholar]
- 22.Sugano N, Sasama T, Sato Y, Nakajima Y, Nishii T, Yonenobu K, Tamura S, Ochi T. Accuracy evaluation of surface-based registration methods in a computer navigation system for hip surgery performed through a posterolateral approach. Comput Aided Surg. 2001;6(4):195–203. doi: 10.3109/10929080109146083. [DOI] [PubMed] [Google Scholar]
- 23.Sugano N, Takao M, Sakai T, Nishi T, Miki H. Does CT-based navigation improve the long-term survival in ceramic-on-ceramic THA? Clin Orthop Relat Res. 2012;470:3054–3059. doi: 10.1007/s11999-012-2378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugano N, Takao M, Sakai T, Nishii T, Miki H, Nakamura N. Comparison of mini-incision total hip arthroplasty through an anterior approach and a posterior approach using navigation. Orthop Clin North Am. 2009;40:365–370. doi: 10.1016/j.ocl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Widmer KH, Zurfluh Complaint positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815–821. doi: 10.1016/j.orthres.2003.11.001. [DOI] [PubMed] [Google Scholar]