Abstract
Purpose
Extensive glenoid bone loss after failed shoulder arthroplasty represents a challenge for revision arthroplasty. Treatment options vary widely and have been a source of controversy among experts.
Methods
Between 2004 and 2010, a total of 17 patients underwent glenoid reconstruction surgery using an autologous iliac crest bone graft and secondary revision arthroplasty due to extensive glenoid bone loss after failed previous total shoulder arthroplasty. The outcomes were assessed by means of clinical examination, Constant score, and bi-plane radiography as well as pre-, postoperative and follow-up CT.
Results
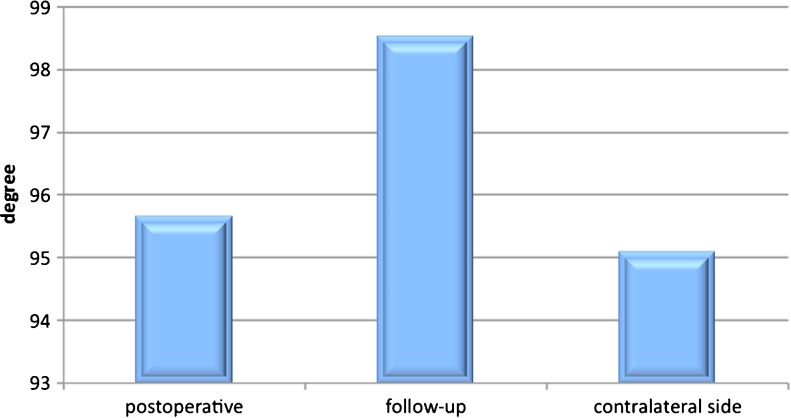
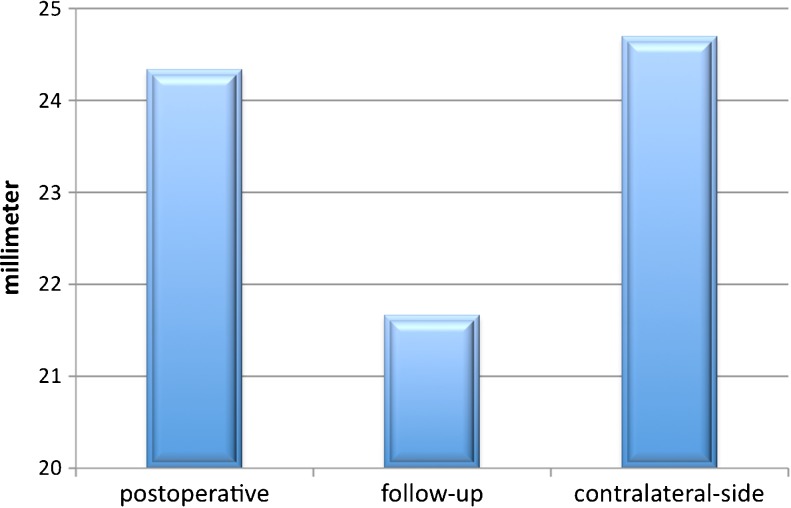
Before the revision surgery, the mean Constant score was 24 ± 17 and improved to 40 ± 13 after the glenoid rebuilding and revision arthroplasty. CT imaging revealed adequate glenoid bone stock restoration with no relevant graft resorption or loosening of the glenoid. The average postoperative antero-posterior diameter of the glenoid was 28 ± 3 mm which had decreased to 25 ± 3 mm at follow-up. The average postoperative version of the glenoid was 95.7° ± 6° and had decreased to 98.5° ± 4° at follow-up. Both the glenoid version and diameter had changed significantly (P < 0.001) comparing postoperative and follow-up CT-scans.
Conclusion
Glenoid reconstruction surgery using an iliac crest bone-block autograft prior to revision arthroplasty represents a valuable salvage procedure in cases of extensive glenoid bone loss after primary shoulder arthroplasty. Sufficient glenoid bone stock restoration is indispensable for reliable fixation of glenoid components and in turn a satisfactory clinical outcome.
Keywords: Glenoid loosening, Glenoid bone loss, Glenoid reconstruction, Iliac crest bone-block autograft, Shoulder arthroplasty, Revision surgery
Introduction
Significant glenoid bone loss can occur because of defect arthropathy, chronic shoulder dislocation, post traumatic and revision surgery of hemi or total shoulder arthroplasty (TSA). Revision arthroplasty for glenoid loosening is one of the most common reasons for revision surgery [1, 2] and can be a challenge, especially in cases of extensive glenoid bone loss [3]. According to Churchill et al., non-infectious glenoid-component failure may occur because of a lack of quality and quantity of bone support [4]. Antuna et al. introduced a classification for the different types of glenoid bone deficiencies after the loosening of the glenoid component. It distinguishes central from peripheral and combined osteolysis subdivided into mild, moderate and severe degrees of bone loss [1]. In cases of loosening of the glenoid component combined with significant glenoid bone loss, sufficient glenoid component fixation may necessitate glenoid bone grafting. In such cases, only glenoid bone grafting allows for stable and accurate positioning of the glenoid component which, together with soft-tissue balancing, is the prerequisite for adequate postoperative function and long-term durability of the implant [5–7]. According to Bell et al., bone grafting should be considered if a glenoid defect exceeds 30 % of the articular surface and the glenoid retroversion exceeds 15° [5, 8, 9]. Despite improvement in terms of pain relief, the simple removal of the loose glenoid component, without bone grafting, generates poor functional results. Advanced erosion of the native glenoid can preclude future glenoid-revision arthroplasty [1, 2]. As a salvage procedure, depending on the extent of the glenoid bone defect, Scalise et al. suggest that bone grafting without concomitant glenoid component reimplantation may be a viable surgical option [10]. However, there is no data on whether prosthetic glenoid resurfacing results in greater pain relief than graft reconstruction of the glenoid alone.
In the early years of glenoid revision surgery, concentric glenoid reaming was introduced and became an accepted standard. Over the decades, new methods involving different types of bone grafting have become popular [4]. For salvage reconstruction of the glenoid, some authors prefer structural autografts, while others prefer particulate compressed allografts [8]. According to Scalise et al., bone grafting of large glenoid defects with allograft bone results in high rates of graft subsidence, particularly when there is no supportive native glenoid vault [10].
The purpose of this study was to evaluate the mid-term clinical and radiological results of revision TSA after iliac crest bone-block autografting as a treatment option for failed shoulder arthroplasty with extensive secondary glenoid bone loss. To our knowledge this is the first paper describing a two-step approach of removal of the old prosthesis for extensive glenoid bone loss after failed previous shoulder arthroplasty followed by glenoid reconstruction surgery using an autologous iliac crest bone graft and secondary revision arthroplasty.
Material and methods
Of the 17 patients treated at the authors’ institution between 2004 and 2010, 11 were examined at follow-up. Two patients were lost to follow-up, three patients underwent revision surgery with a reverse design because of rotator cuff insufficiency and were therefore excluded from study, and one patient died from unrelated causes. The indication for revision surgery was a failed total shoulder arthroplasty with an aseptic glenoid component loosening causing extensive bone loss in all cases. Exclusion criteria were a previous reversed shoulder arthroplasty (RSAs), a rotator cuff tear revealed at revision surgery, a former postoperative septic complication, and neurological or musculoskeletal disorders. Before surgery sepsis was excluded by blood analysis. If the blood analysis was positive a labelled scan was performed, and additionally an arthrocentesis for bacteriological examination if needed. The patients required revision surgery because of moderate to severe pain, and each case involved extensive destruction of the glenoid bone stock either from the loosening of the glenoid component or from secondary erosion. Grafting was chosen by the surgeon when the remnant glenoid bone stock was insufficient to maintain the adequate version or fixation of the revision prosthesis. All of the deficiencies were classified intraoperatively according to the classification of Antuna in severe combined defects [1]. The indication for initial shoulder arthroplasty (SA) was primary and post-traumatic osteoarthritis. The mean time from primary SA to removal of the loose glenoid and autograft reconstruction was 80 ± 47 months, and the average time for glenoid reimplantation was 117 ± 25 days after the bone grafting. In this time frame the patients were immobilised with a sling. The timing was determined by the senior consultant of our department according to the principles of fracture healing and his vast experience in shoulder surgery. All of the patients underwent a preoperative clinical examination regarding range of motion, daily restrictions and pain, as well as the Constant score, examined by the same resident using a goniometer. In addition to routine postoperative check-ups and continuous X-rays (postoperative, one month, three months, six months and yearly), the patients were re-examined at a mean follow-up of 45 ± 18 months, which included a clinical examination and a measure of their Constant scores.
Statistics
For comparison of the preoperative, postoperative and follow-up glenoid diameter and version, two-sided, paired Student t-tests were used. Statistical difference was defined as a p value of 0.05. Descriptive statistics were accomplished by means of Microsoft office excel (Microsoft, Redmond, Washington, USA).
Surgical technique
The revision surgery consisted of a two-stage procedure:
-
First step
The revision surgery was performed through a delto-pectoral approach. In seven shoulders, only the head of the humeral component was removed to allow for improved glenoid exposure, and the stem was retained. A new head was then placed on the stem. In four cases, the humeral component had to be removed with the stem. In three cases, a Girdlestone situation was created, which means that both the humeral component and the glenoid component were removed, and in one case, a humeral spacer was implanted (Fig. 1). The humeral spacer was used in this case because an infection could not be excluded pre- or intraoperatively. Loose glenoid components were removed, and the remnant glenoid bone stock was thoroughly debrided. The final decision to use bone grafting was made intraoperatively. In all of the cases the deficiencies could be classified according to Antuna [1] in severe combined defects. Careful preparation of the remnant glenoid bone was performed to match the contour of the back of the bone block. The bone graft was obtained from the ipsilateral iliac crest. Depending on the dimension of the defect, the tricortical bone graft varied in size, but the bone graft was generally smaller than four centimetres long and three centimetres wide. In all cases, the graft was shaped to be slightly larger than the defect. The preparation of the graft aimed to maximise the contact area with the native glenoid. The graft was fixed by two to four cannulated 2.7-mm titanium screws (Stryker Leibinger Micro Implants, Freiburg, Germany). To avoid damaging of the humeral component, the screw heads were countersunk. To allow for a secondary glenoid component implantation, the screws were not placed in the center of the graft (Fig. 2).
Postoperatively, the shoulder was immobilised in a sling for four weeks. Under a physiotherapist’s guidance, passive motion was started the day after surgery. Active motion and progressive strengthening below the shoulder level were initiated four weeks after the surgery.
-
Second step
Three months after the surgery and after the graft incorporation was verified by CT scan, the second step consisted of the removal of the head of the humeral component (in seven patients) followed by the removal of the screws in the glenoid bone graft. In some cases, the humeral component shaped a new vault into the graft. The rebuilt glenoid bone stock was reamed to create a convex base for the new glenoid component. In all cases, a cemented keeled polyethylene glenoid component (Stryker Howmedica, Michigan, USA; and Universe PE Glenoid, Arthrex, Naples, FL) was used (Fig. 3). A new humeral head was subsequently attached to the humeral stem. In four patients, a new humeral component was implanted (Howmedica anatomical shoulder prosthesis, Stryker Howmedica, Michigan, USA). Postoperatively, the patients were immobilised in a sling for four weeks; only passive motion and a maximum elevation of 60° were allowed. After four weeks, active motion up to 90° and slight strength exercises were started. Depending on the patients pain level, full activity was allowed three months after the operation.
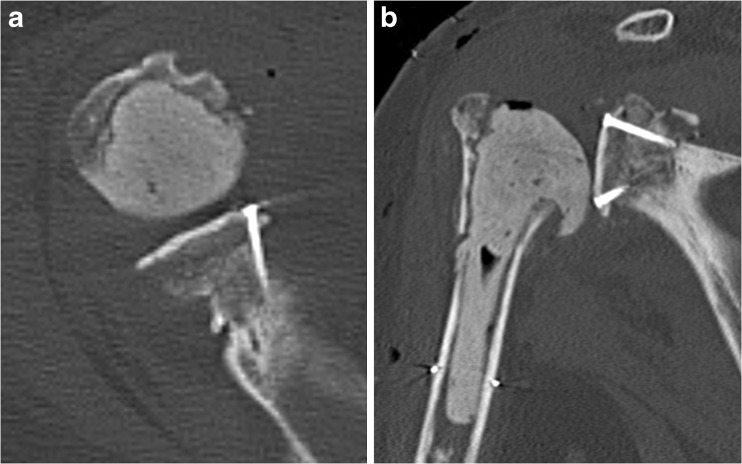
Fig. 1.
Cemented humeral spacer after autologous bone grafting. a Postoperative axillary radiograph. b Postoperative AP radiograph
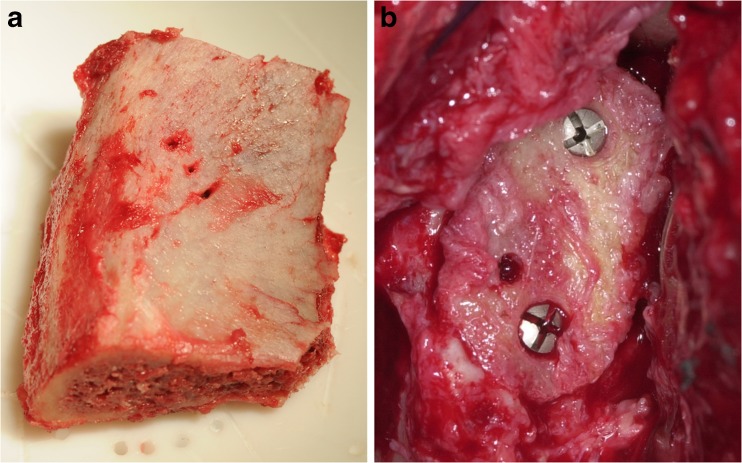
Fig. 2.
a Autologous iliac crest bone graft. b Autologous bone graft fixed with two cannulated 2.7-mm titanium screws on the glenoid
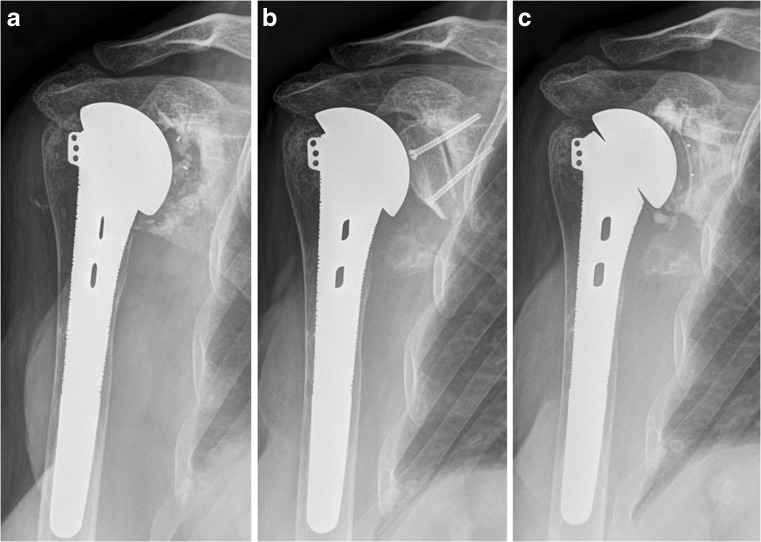
Fig. 3.
Chronological sequence of the two-step process in a patient with a structural autologous iliac crest bone graft. a Preoperative AP radiograph eight years after a TSA. b Postoperative radiograph showing an iliac crest bone autograft fixed with two screws. c Three-year follow-up AP radiograph showing good graft incorporation and no radiolucent lines (RLLs) around the glenoid component
Radiological evaluation
Preoperative, postoperative and follow-up radiography was obtained for all patients, which included AP, trans-scapular, and axillary views (if motion was not impeded). Preoperative CT scans were not always available, but all patients underwent immediate postoperative and follow-up CT after a mean of three months. Special attention was given to the morphology and version of the rebuilt glenoid and the graft’s incorporation and remodelling over time. Glenohumeral subluxation was assessed by follow-up CT while considering the humeral head’s direction and amount of translation relative to the centre of the glenoid. Following Iannotti, bone loss of the reconstructed glenoid (graft resorption) was measured on antero-posterior radiographs [11]. Radiolucent lines (RLLs) around the glenoid component, glenoid bone spurs, glenoid component loosening, humeral subsidence and humeral RLL (according to Cofield’s classification [12]) were also analysed at follow-up. Using the available CT scans, the glenoid diameter and version were measured preoperatively, postoperatively, and at follow-up (Fig. 4). The glenoid version was measured using the Friedman method [13]. The measurements were compared to the patient’s healthy contralateral side (Figs. 5 and 6).
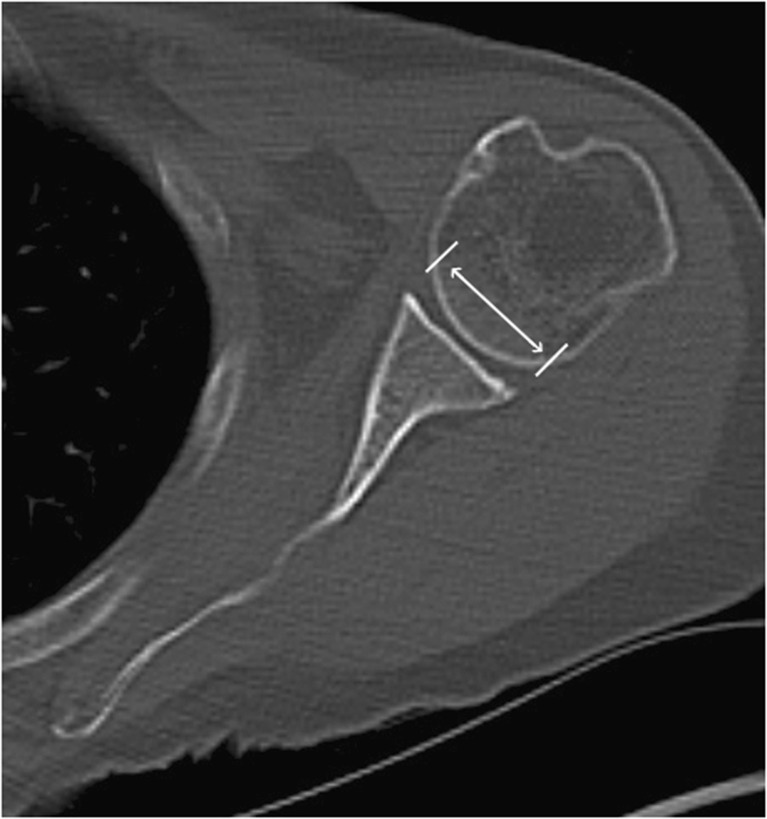
Fig. 4.
Axial CT showing the measurement technique applied measuring the antero-posterior diameter of the glenoid preoperative, postoperative and at follow-up
Fig. 5.
Comparison of postoperative and follow-up averages of the version of the graft-augmented glenoid in relation to the contralateral side
Fig. 6.
Comparison of postoperative and follow-up averages of the antero-posterior diameter of the graft-augmented glenoid in relation to the contralateral side
Results
Clinical outcome
Eleven patients were included in this retrospective investigation, and the mean age was 67 ± 11 years at the time of follow-up. Six patients were female, and five were male. The dominant extremity was involved in five cases. The average Constant score increased from 24 to 40 points, and the average active anterior elevation increased from 48° to 100°. In spite of a low Constant score in general the patients were satisfied with their clinical results and the pain significantly improved, by a mean of 6 ± 3 points, on the VAS (Table 1).
Table 1.
Patient characteristics
| Sex, age at surgery (years) | Reason for revision surgery | Survival of old prosthesis (months) | Time from glenoid remodelling to TSA (days) | Definite prosthesis | Constant score (preop) | Constant score (postop) | Pain (preop) | Pain (postop) |
|---|---|---|---|---|---|---|---|---|
| F, 48 | Glenoid loosening | 120 | 98 | TSA | 7 | 42 | 2 | 8 |
| F, 80 | Glenoid loosening | 96 | 111 | TSA | 18 | 44 | 2 | 9 |
| F, 79 | Glenoid loosening | 60 | 95 | TSA | 26 | 47 | 4 | 11 |
| M, 65 | Glenoid loosening | 180 | 97 | TSA | 29 | 50 | 5 | 12 |
| M, 74 | Glenoid loosening | 72 | 102 | TSA | 28 | 71 | 4 | 15 |
| M, 74 | Glenoid loosening | 168 | 150 | TSA | 53 | 75 | 8 | 5 |
| F, 53 | Glenoid loosening | 36 | 126 | TSA | 24 | 50 | 2 | 8 |
| F, 80 | Glenoid loosening | 24 | 170 | TSA | 14 | 38 | 8 | 11 |
| M, 70 | Glenoid loosening | 48 | 136 | TSA | 18 | 40 | 5 | 10 |
| M, 51 | Glenoid loosening | 72 | 101 | TSA | 22 | 38 | 3 | 8 |
| F, 61 | Glenoid loosening | 63 | 115 | TSA | 23 | 40 | 5 | 13 |
To date, postoperative complications after the second stage needed further treatment in two patients (18 %) because of ongoing pain and moderate restriction in daily life.
Radiological results
In 82.5 % of the patients primary osteoarthritis and in 17.5 % post-traumatic osteoarthritis was the reason for initial SA. After revision with glenoid reconstruction and bone grafting, all of the CT scans showed graft incorporation at follow-up. Partial graft resorption was observed in all patients. Graft resorption was less than 25 % in one patient, 25 % in three patients, 50 % in five patients and 75 % in two patients. Although the resorption of the graft seemed to be quite high the graft implantation remained stable. The average postoperative antero-posterior diameter of the rebuilt glenoid immediately after stage 1 was 28 ± 3 mm; the minimum diameter was 22 mm and the maximum was 32 mm. At three-months follow-up, there was a significant decrease (p = 0.0056) in the glenoid diameter, compared to the initial size of the autograft, to an average of 25 ± 3 mm, with a minimum of 20 mm and a maximum of 29 mm. The average postoperative version of the glenoid after stage 1 was 95.7°, with a minimum of 91° and a maximum of +102°. At follow-up, there was a significant decrease (p = 0.134) in the glenoid version to an average of 98.5°, with a minimum of 90° and a maximum of 104°. Between the postoperative and follow-up CTs, the average version and diameter decreased significantly (Figs. 5 and 6). Screw-related problems were present in five patients (45 %) (Table 2). Following Cofield’s classification, on conventional radiographs humeral radiolucency was present in four cases (36 %). No humeral subsidence or subluxation was detected.
Table 2.
Resultsᅟ
| Patient number | Graft fixation (screws) | Incorporation (%) | Graft resorption (%) | Version of the glenoid (postop) | Version of the glenoid (at FU) | Diameter of the glenoid (postop, mm) | Diameter of the glenoid at (FU, mm) | Screw-related complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 100 | 50 | −7° | −13° | 26 | 21 | Damage to the old humeral component |
| 2 | 2 | 100 | 25 | −8° | −9° | 25 | 24 | Periarticular calcinosis |
| 3 | 2 | 100 | <25 | −4° | −8° | 28 | 25 | Broken screw |
| 4 | 3 | 100 | 50 | 5° | −3° | 27 | 20 | No |
| 5 | 2 | 100 | 50 | −6° | −10° | 30 | 24 | No |
| 6 | 3 | 100 | 75 | −3° | −14° | 30 | 26 | Damage to the old humeral component |
| 7 | 3 | 100 | 25 | −1° | −10° | 31 | 29 | No |
| 8 | 2 | 100 | 50 | −7° | −11° | 22 | 20 | No |
| 9 | 3 | 100 | 75 | 3° | −10° | 32 | 25 | Abrasion of one screw |
| 10 | 2 | 100 | 25 | −7° | −10° | 31 | 29 | No |
| 11 | 4 | 100 | 50 | 12° | 7° | 29 | 28 | No |
FU follow-up
Discussion
After failed TSA, glenoid reconstruction is a technically demanding procedure. Several treatment options have been described with varying outcomes [14, 15]. Glenoid restoration using autologous bone graft should be considered if there is massive destruction of the glenoid and insufficient support for the new glenoid component [14, 15] or if the version of the glenoid is inadequate [5]. If the final decision is to proceed with bone grafting, several aspects should be considered as outlined below.
This study describes a two-stage procedure for reconstruction of the glenoid in patients with failed arthroplasty. There are two possibilities for time management in glenoid reconstruction. Some surgeons prefer a one-stage procedure that involves the removal of the loosened glenoid, bone grafting and the direct reimplantation of a new glenoid component [8]. Following Cofield et al. [16] we favour a two-stage procedure. One rationale is the possible presence of a clinically non-apparent low-grade infection, which has been described by Cheung in prosthetic replacement failure [17]. But principally respecting the standards of traumatic surgery guidelines, bone healing takes place after four to six weeks, and after three months loading of the implant should be possible. In a low-grade infection, the removal of all components and antibiotic therapy are needed. Melis et al. noted that low-grade infection should be suspected in cases of glenoid loosening or failure. To prevent recurrent postoperative infections, their strategy involved a two-stage procedure (i.e., prosthetic removal with an antibiotic spacer followed by a reimplantation six to eight weeks later) [18]. If an infection can be excluded, the humeral component can be left in situ after the graft augmentation. In contrast, the early contact forces on the bone graft are useful for graft integration and for the prevention of excessive graft resorption, which has been explained by Frost et al. [19]. As Moroder et al. observed, bone grafts that are used for the reconstruction of the glenoid are subject to an anatomical remodelling process [20]. Accordingly, we observed that the compression of the humeral component on the graft, which was exerted by the musculature, formed a new concavity that facilitated the subsequent glenoid-component implantation (Fig. 7). However, there was a recurrent increase in retroversion in all of our patients. One possible explanation for this phenomenon is the chronic soft-tissue imbalance of the shoulder.
Fig. 7.
Axillary CT at a follow-up examination showing a patient with autologous iliac crest autograft incorporation and an implanted polyethylene glenoid. A new glenoid vault and version were created
In addition to the extent of the glenoid surface, the version of the glenoid is important. Compared to patients with an adequate glenoid version and volume, Hill observed a tenfold higher rate of glenoid component failure in patients who required glenoid bone grafting at the time of a primary total shoulder replacement [14]. Correction of the version by reaming has been shown to have a limit of approximately 15° [5, 13, 20]. In a biomechanical study, Shapiro et al. found that a glenoid component retroversion of 15° significantly affected the glenohumeral contact area and led to increased contact pressure. As a consequence, retroversion may result in the eccentric loading of the glenoid component, which is associated with glenoid wear and loosening [9]. In a biomechanical study, Nyffeler et al. observed that with every degree of increasing retroversion, posterior subluxation increases by 0.5 mm, which leads to a posteriorly directed force vector. For every 4° change in the version, there was a 2° average force vector change away from the center of the glenoid [21]. This leads to an eccentric loading of the glenoid component and increased contact pressure at the posterior aspect of the glenoid, which has been described as the antero-posterior “rocking horse” phenomenon [9]. Kircher et al. validated the improved accuracy of the proper insertion of the glenoid component positioning using navigation, which signifies the importance of ensuring correct anatomical placement during total shoulder arthroplasty [7]. In our series, the initially corrected version of the glenoid increased postoperatively, but all patients displayed a slight retroversion increase at the final follow-up.
Further reaming of a glenoid with extensive bone loss compromises the central fixation of the glenoid component so that adequate stability cannot be obtained. Haines et al. claimed that the reaming of the subchondral bone can cause structural weaknesses and increase the risk of component loosening [22]. Walch also identified excessive reaming of the subchondral bone as a risk factor in midterm glenoid component subsidence [23]. This finding was confirmed by Sutton et al., they found that with every millimetre of bone removal, the amount of total available bone area for implant fixation decreases by 5 %. Consequently, minimal reaming is recommended to create a flat surface for the baseplate [24]. According to our measurements we were able to improve the amount of bone stock available. According to Collins et al., careful preparation of the glenoid bone to match the contour of the back of the glenoid component stabilises the component against eccentric loads during the daily use of the prosthesis [25]. This is considered when revision glenoid component implantation is performed in the second step of the procedure.
While bone grafting for glenoid defects is known to be effective, the best graft type is unknown. Some authors prefer femoral allografts to reconstruct glenoid defects [1, 6, 10, 17, 26], but others suggest an autogenous iliac crest bone graft [5, 14, 27]. Neer and Morrison described the outcome for 20 shoulders that were treated for eccentric glenoid wear with a large segmental graft [15]. The authors decided that loosening of the bone stock is not a contraindication for an unconstrained total shoulder prosthesis. They used corticocancellous grafts from the autologous humeral head to fill out the glenoid defects in their study. This type of salvage procedure is commonly used by surgeons and produces reasonable results [2, 6, 14, 16].
Reverse shoulder arthroplasty is nowadays another popular treatment option in revision of failed shoulder arthroplasty in case of an irreparable rotator cuff deficiency [28]. The indication must be very clear, as the complication rate of this procedure is relatively high [29, 30]. Therefore patient selection and other treatment options should be carefully considered.
This study does have some limitations, beginning with the small number of patients. However, the infrequency of complex shoulder pathologies makes large case series of these patients difficult to obtain. Also to be mentioned is that the technique described needs two more disruptions of the subscapularis after the index operation. Another limitation relates to the difficulty to assess the glenoid-diameter on CT scan because of the metallic artifacts created by the humeral component. Additionally, graft incorporation was measured according to Iannotti’s technique [11], but the reliability of this technique has not been verified. Finally, no preoperative CT evaluation was available for 45 % of the patients followed-up, which significantly restricted the ability to compare the preoperative and postoperative data.
Summary and conclusions
Our study showed that the two-stage approach has the potential to reconstruct and maintain glenoid bone stock volume during total shoulder revision arthroplasty. Therefore, glenoid reconstruction using an iliac crest bone-block autograft in two-step revision arthroplasty is a valuable solution for glenoid loosening associated with extensive glenoid defects. However, the salvage character of this procedure must be considered.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
415-EP/73/171-2012
Footnotes
Work was performed at the Paracelsus Medical University Salzburg, Austria.
Contributor Information
Thomas Hoffelner, Phone: +43-662-448255089, FAX: +43-662-448255018, Email: t.hoffelner@salk.at.
Philipp Moroder, Email: p.moroder@salk.at.
Alexander Auffarth, Email: a.auffarth@salk.at.
Mark Tauber, Email: tauber@atos-muenchen.de.
Herbert Resch, Email: h.resch@salk.at.
References
- 1.Antuna SA, Sperling JW, Cofield RH, et al. Glenoid revision surgery after total shoulder arthroplasty. J Should Elb Surg. 2001;10:217–224. doi: 10.1067/mse.2001.113961. [DOI] [PubMed] [Google Scholar]
- 2.Neyton L, Boileau P, Nove-Josserand L, et al. Glenoid bone grafting with a reverse design prosthesis. J Should Elb Surg. 2007;16:71–78. doi: 10.1016/j.jse.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008) J Bone Joint Surg Br. 2011;93:1513–1517. doi: 10.1302/0301-620X.93B11.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchill RS. Post-treatment glenoid classification system for total shoulder arthroplasty. J Should Elb Surg. 2012;21:537–544. doi: 10.1016/j.jse.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Bell RH, Noble JS. The management of significant glenoid deficiency in total shoulder arthroplasty. J Should Elb Surg. 2000;9:248–256. doi: 10.1067/mse.2000.103656. [DOI] [PubMed] [Google Scholar]
- 6.Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85:251–258. doi: 10.2106/00004623-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kircher J, Wiedemann M, Magosch P, et al. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Should Elb Surg. 2009;18:515–520. doi: 10.1016/j.jse.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Should Elb Surg. 2000;9:361–367. doi: 10.1067/mse.2000.106921. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro TA, Gupta A, McGarry MH, et al. Biomechanical effects of arthroscopic capsulorrhaphy in line with the fibers of the anterior band of the inferior glenohumeral ligament. Am J Sports Med. 2012;40:672–680. doi: 10.1177/0363546511430307. [DOI] [PubMed] [Google Scholar]
- 10.Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:139–145. doi: 10.1007/s11999-007-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Should Elb Surg. 2012;21:765–771. doi: 10.1016/j.jse.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 12.Sperling JW, Cofield RH, O’Driscoll SW, et al. Radiographic assessment of ingrowth total shoulder arthroplasty. J Should Elb Surg. 2000;9:507–513. doi: 10.1067/mse.2000.109384. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RJ, Hawthorne KB, Genez BM. The use of computerized-tomography in the measurement of glenoid version. J Bone Joint Surg Am . 1992;74:1032–1037. [PubMed] [Google Scholar]
- 14.Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. doi: 10.1302/0301-620X.83B8.11709. [DOI] [PubMed] [Google Scholar]
- 15.Neer CS, 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70:1154–1162. [PubMed] [Google Scholar]
- 16.Cofield RH. Bone grafting for glenoid bone deficiencies in shoulder arthritis: a review. J Shoulder Elbow Surg. 2007;16:273–281. doi: 10.1016/j.jse.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Cheung EV, Sperling JW, Cofield RH. Reimplantation of a glenoid component following component removal and allogenic bone-grafting. J Bone Joint Surg Am. 2007;89:1777–1783. doi: 10.2106/JBJS.F.00711. [DOI] [PubMed] [Google Scholar]
- 18.Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Should Elb Surg. 2012;21:342–349. doi: 10.1016/j.jse.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18:305–316. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 20.Moroder P, Hirzinger C, Lederer S, et al. Restoration of anterior glenoid bone defects in posttraumatic recurrent anterior shoulder instability using the J-bone graft shows anatomic graft remodeling. Am J Sports Med. 2012;40:1544–1550. doi: 10.1177/0363546512446681. [DOI] [PubMed] [Google Scholar]
- 21.Nyffeler RW, Sheikh R, Atkinson TS, et al. Effects of glenoid component version on humeral head displacement and joint reaction forces: an experimental study. J Should Elb Surg. 2006;15:625–629. doi: 10.1016/j.jse.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Sperling JW, Cofield RH, Rowland CM. Heterotopic ossification after total shoulder arthroplasty. J Arthroplasty. 2000;15:179–182. doi: 10.1016/S0883-5403(00)90154-2. [DOI] [PubMed] [Google Scholar]
- 23.Walch G, Young AA, Melis B, et al. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Should Elb Surg. 2011;20:385–394. doi: 10.1016/j.jse.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Sutton LG, Werner FW, Jones AK, et al. Optimization of glenoid fixation in reverse shoulder arthroplasty using 3-dimensional modeling. J Should Elb Surg. 2010;19:664–669. doi: 10.1016/j.jse.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Collins D, Tencer A, Sidles J, et al. Edge displacement and deformation of glenoid components in response to eccentric loading. The effect of preparation of the glenoid bone. J Bone Joint Surg Am. 1992;74:501–507. [PubMed] [Google Scholar]
- 26.Williams GR, Jr, Iannotti JP. Options for glenoid bone loss: composites of prosthetics and biologics. J Should Elb Surg. 2007;16:267–272. doi: 10.1016/j.jse.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449–462. [PubMed] [Google Scholar]
- 28.Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty-outcome and follow-up. Int Orthop. 2013;37:67–75. doi: 10.1007/s00264-012-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34:1075–1082. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarlat MM. Complications with reverse total shoulder arthroplasty and recent evolutions. Int Orthop. 2013;37:843–851. doi: 10.1007/s00264-013-1832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]