Abstract
Purpose
The increase in methicillin-resistant Staphylococcus aureus (MRSA) infections is currently a major health care problem. Vancomycin is still often the first-line anti-microbiological agent for treating such infections; however, a recent decline in efficacy of vancomycin in MRSA infections has raised concerns and accelerated the search for new antibiotics. The aim of this study was to establish a MRSA peri-implant osteomyelitis animal model for future testing of new anti-microbiological agents under typical MRSA infection conditions.
Methods
Eighteen randomised NZW-rabbits underwent a standardised surgical procedure with the insertion of a femoral bone implant. Animals were then divided into group 1 (MRSA inoculation, no antibiotics; M/N), group 2 (MRSA inoculation, Vancomyin; M/V), and group 3 (no MRSA inoculation, no antibiotics; N/N). The primary study outcome parameters were animal leucocyte count, animal weight, and animal body temperature at one, seven, and 42 days after surgery. Additionally, a histo-morphometrical score was established and adjusted to a modified histological Smeltzer score.
Results
Macroscopic and histo-morphometrical findings showed a peri-implant osteomyelitis in group 1 with both increased acute and chronic infection parameters in M/N, as compared to M/V and N/N, indicating that vancomycin treatment prevented typical morphological changes of MRSA peri-implant osteomyelitis. Similarly, there was a reduction in animal weight and increase in leucocyte count and body temperature in group 1 (each p < 0.005). Vancomycin treatment again resulted in significantly reduced leucocyte count and body temperature, and increased animal body weight.
Conclusions
Here we have established a peri-implant MRSA osteomyelitis model that successfully combined clinical and laboratory outcome parameters of infection with histo-morphometrical results; this model appears to be valuable for future experimental use and therapeutic monitoring of new anti-microbiological MRSA drugs.
Keywords: Animal model, MRSA, Osteomyelitis, Bone substitute
Introduction
The annual prevalence of nosocomial infections in Germany is currently between 500,000 and 800,000 patients and over two million in the United States [1]. Typically these infections are induced by bacterial resistance to at least one antimicrobial agent used for treatment. With regard to periprosthetic implant infection, Staphyloccus aureus remains the most prevalent pathogen. The current gold standard therapy includes a surgical debridement supported by long-term administration of anti-microbiological agents [2]. However, the recent increase in resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA) is a significant global healthcare problem particularly within hospitals [3]. Vancomycin is still the first-line antimicrobial drug for such MRSA infections in both soft tissue and bone. However, the widespread use of vancomycin leads to a reduced susceptibility in S. aureus, and is one reason for the increase in resistance of other bacteria such as enterococci (VRE). Therefore, a sustained and more intensive search for new anti-microbiological agents has begun [4].
However, limited clinical data is available on the efficacy of new antibiotics such as linezolid or daptomycin [5] for treating bone and joint infections, and so far there are no comparative experimental studies on the efficacy and bioavailability of new antibiotics versus vancomycin in MRSA bone infections.
Thus, the purpose of our study was to establish a new MRSA peri-implant osteomyelitis animal model, and to determine clinical parameters to monitor the infection after MRSA inoculation, and under anti-microbiological therapy with vancomycin. Data on histo-morphological changes and animal leucocyte numbers, weight, and body temperature were collected over time, and the results showed that these parameters may be useful indeed to monitor osteomyelitis.
Materials and methods
Definition of a peri-implant infection
We defined a peri-implant infection in accordance with clinical parameters (body weight, body temperature), changes in the histomorphological score, laboratory findings (leucocyte numbers) and bacterial detection [6–9].
Implants
Custom-made cylindrical titanium implants (4.1 mm diameter; 5 mm length) were manufactured by Aesculap AG & Co. KG (Tuttlingen / Germany). All implants were coated with pure titanium powder at 0.35 mm thickness applied via a plasma spray process under vacuum conditions. This approved surface technique (Plasmapore®) has been in clinical use since 1986.
Animal model, surgical procedure and study randomisation
This study was conducted after approval by the local Institutional Animal Welfare Review Board (No 35–9185.81/G-99/03). Female New Zealand White rabbits used in pre-experiments showed that the biocompatibility of drug usage was similar to humans. Eighteen animals (weight 3.0–3.5 kg; age average seven months) were pre-medicated using an intramuscular injection of 10 mg benzodiazepine (Valium® 10; Roche) and 0.5 mg atropine (Atropin 0.5; Braun), and anaesthetised using ketamine (50 mg/kg; Hostaket®; Hoechst Roussel Vet) in combination with xylazine (5 mg/kg; Rompun®; Bayer).
The surgical procedure for implant insertion was done using a direct lateral approach to the distal femur as described previously [10]. The surgery was performed under strict aseptic conditions. The lateral femoral condyle was surgically exposed and a central hole was drilled about five millimetres proximal to the articular cartilage using a diamond shaper with an outer diameter of four millimetres. The implants were inserted into the cancellous bone using the press-fit technique. The animals were randomly assigned to one of the experimental groups:
Inoculation of 106 colony forming units (CFU) of MRSA; no anti-microbiological treatment (M/N; six animals); bacteriology: 6/6 detection of MRSA
Inoculation of 106 CFU of MRSA; treatment with vancomycin (VANCO-cell®; Cell pharm GmbH, Hannover, Germany) at 25 mg/kg subcutaneous neck soft tissue twice daily for ten days (M/V; six animals); bacteriology: 1/6 detection of MRSA
No inoculation of MRSA; no anti-microbiological treatment (control group; N/N, six animals); bacteriology: 0/6 detection of MRSA
Titanium cylinders were coated intra-operatively with 25 μl of the bacterial suspension containing 106 CFU of MRSA and implanted into the right femoral condyle [11]. The wound was rinsed, closed in layers, and sprayed with antiseptic plastic film. In pre-experiments, the correct insertion and positioning of the implant was verified by X-rays (Fig. 1).
Fig. 1.
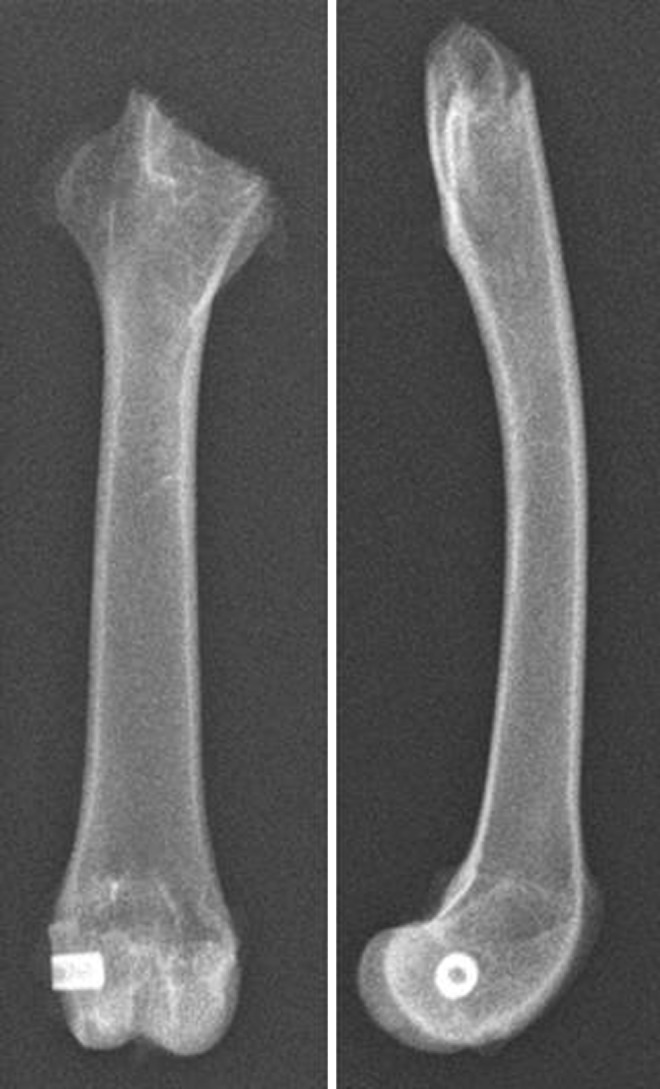
Correct insertion and positioning of the implant verified by X-ray
The first dose of vancomycin in group 2 was administered eight hours postoperatively. For pain suppression, all animals received carprofene (4 mg/kg; Rimadyl®; Pfizer). The animals were unrestricted in movement and food consumption in their cages. Six weeks postoperatively, the animals were euthanised with an overdose of pentobarbital sodium (Narcoren®; Rhone Merieux) irrespective of the treatment group. The veterinarian was blinded to the treatment group.
For each animal a fresh inoculum of a MRSA reference strain (ATCC 33591) was prepared by an overnight culture in brain-heart infusion (BHI) broth. Cells were pelleted and washed twice in phosphate-buffered saline (PBS). CFU counts were determined by serial dilution plating on blood agar. The final bacterial suspension in PBS consisted of 106 CFU / 25 μl.
Laboratory and clinical examinations
Blood and serum samples (0.5 mL) were taken preoperatively and six weeks postoperatively from the ear vein from all animals in the study. The blood samples were analysed for routine laboratory parameters (blood count, leucocyte number). An analysis of CrP levels was not feasible for technical reasons. On days 0, 7, 14, 21 and 42, rectal body temperature was measured and body weight determined. The parameters were sequentially depicted over time.
Histo-morphological evaluation
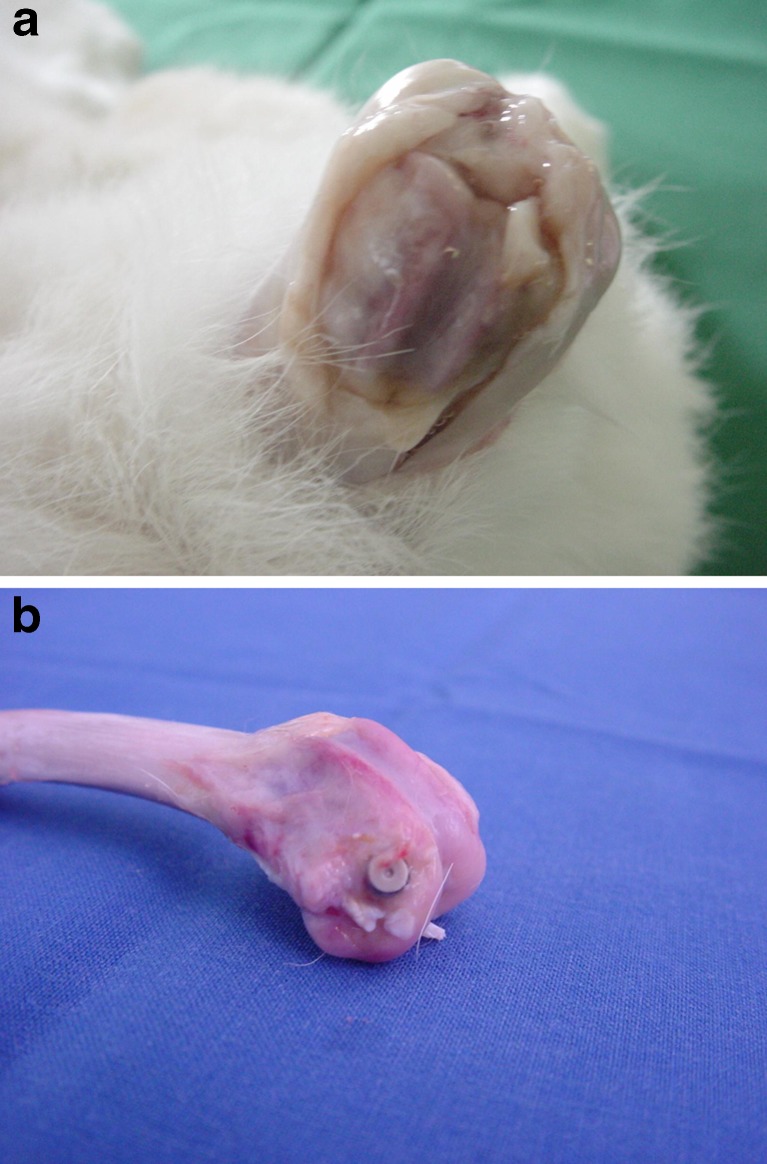
After animal sacrifice, macroscopic evaluation and picture documentation were done (Fig. 2). A well-accepted, standardised, histological evaluation of osteomyelitis was achieved according to the scoring system of Smeltzer et al. [12]. In our peri-implant osteomyelitis models, however, we were particularly interested in the interface area between bone and implant. Therefore, a correlation of histo-morphometrical results with histological parameters was necessary. The semi-quantitative histo-morphometrical analysis was performed by a blinded investigator in accordance with a recent report [10]. Specimens were briefly ethanol-fixed (70 %), embedded in methylmethacrylate, and cut into 100–150 mm slices. Then slides were ground using grinding paper and stained with Masson–Goldner tri-chrome and with haematoxylin and eosin.
Fig. 2.
Picture documentation after sacrifice
The Smeltzer score was used to evaluate four different parameters: intraosseous acute (IAI) and chronic inflammation (ICI), periosteal inflammation, and bone necrosis. For practical reasons, we limited the analysis of acute and chronic inflammation within the standardised peri-implant area, which correlated to new soft tissue and bone formation. More specifically, new soft tissue formation was scored from 0–4 (0 = 0–10 %; 1 = 10–20 %; 2 = 20–30 %; 3 = 30–40 %; 4 = 40–50 %). Accordingly, new bone formation was also evaluated from 0–4 (0 = 50–40 %; 1 = 40–30 %; 2 = 30–20 %, 3 = 20–10 %, 4 = 10–0 %).
Statistical analysis
Primary outcome was measured according to the leucocyte count. Secondary outcome measures were animal body temperature and body weight, as well as the histo-morphological score. Complete data sets were available for 18 animals. Mean and standard deviation (SD) were calculated for continuous, median, and interquartile ranges for ordinal variables. Association between continuous and discrete variables was tested by Student’s t test. Data of the outcome variables and confounders were tested in a one-way analysis. In the case of abnormal distribution of LAR-values in this study, median, interquartile range, and Wilcoxon signed-rank test results were calculated. All tests were two-sided and a P value ≤ 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, California, USA.
Results
Study population and macroscopic results
Two animals had to be euthanised due to systemic infections (one animal in each group 1 and 2). No other surgical or non-surgical related complications occurred.
All animals in group 1 (M/N) had severe bone inflammation with new bone and soft tissue formation around the implant (Fig. 2a). Animals from both group 2 (M/V) and 3 (N/N) showed only minimal to moderate signs of peri-implant osteomyelitis (Fig. 2b).
Laboratory and clinical findings
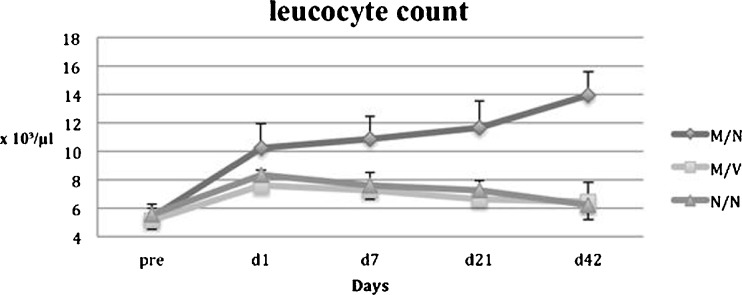
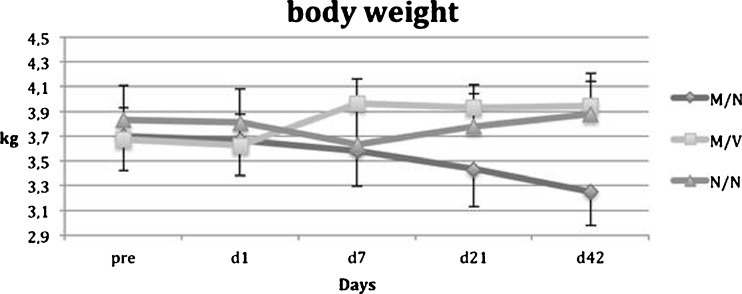
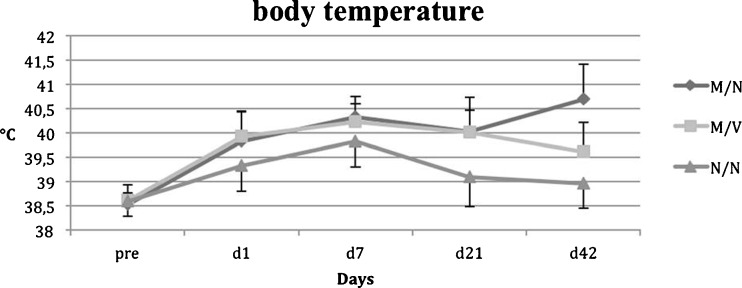
Results from group 1 (M/N) showed a continuous increase in both the leucocyte count (Fig. 3) and body temperature (Fig. 4) (p = 0.0002 / 0.0012 vs. preoperatively), while animal weight (Fig. 5) was significantly decreased throughout a six-week period postoperatively. However, in group 2 (M/V) anti-microbiological treatment with vancomycin resulted in significantly reduced body temperature and normalised leucocyte counts as was similarly observed in the control group 3 (N/N).
Fig. 3.
Leucocyte count
Fig. 4.
Body weight
Fig. 5.
Body temperature
Group 3 (N/N) showed initially a slight trend (p = 0.54 ANOVA) towards decreased body weight but was followed by a continuous increase in body weight during the follow-up period. Body temperature of control animals showed no differences over the experimental time period. None of the control animals showed evidence of local or systemic infections throughout the experimental periods. The leucocyte count in controls showed no significant difference within the treatment period, though a slight increase from 5.4 ± 0.63 to 6.2 ±0.81 (p = 0.15) should be noted. No significant differences in whole blood count were detectable throughout the entire experimental period, irrespective of treatment group.
Modified histological scoring system
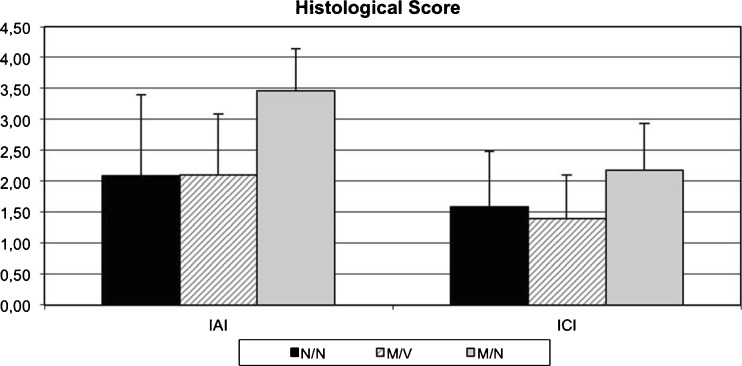
The results of the modified histo-morphological score supported the above shown clinical data. The IAI and ICI scores in group 1 (M/N) were significantly higher than in group 3 (N/N) and group 2 (M/V). More specifically, the average IAI score in group 1 (M/N) was significantly higher (3.45±0.69) than in group 2 (M/V 2.10±0.99; p = 0.018) and in group 3 (N/N; 2.08±1.33; p = 0.022) (Fig. 6). Similarly, the ICI score ranged between 1.40±0.7 in group 2 (M/V) and 1.58±0.9 in group 3 (N/N), and 2.18±0.75 in group 1 (M/N) respectively. These results demonstrate that this modified histological scoring system effectively assessed appropriate parameters associated with peri-implant MRSA osteomyelitis.
Fig. 6.
Histological score
Discussion
Here we have established an animal model of peri-implant MRSA osteomyelitis by characterising both histo-morphological and the detailed sequential development of clinical infection parameters. To our knowledge this is the first animal model of osteomyelitis that closely monitors the course of peri-implant osteomyelitis according to clinical factors used in humans, such as the leucocyte count, body weight, and temperature. This study demonstrated that a close monitoring of infection can be achieved in rabbits by observing leucocyte count, body temperature, and body weight, in combination with the histo-morphological and adjusted histological results. Together with the previously shown biomechanical testing [10] this model will be appropriate for future studies assessing the efficacy of new anti-microbiological agents such as linezolid in MRSA infections [10]. Importantly, this may be extended to any new anti-microbiological agent introduced for use in humans. Moreover, the current data confirms the efficacy of intravenous vancomycin in early stage experimental MRSA infections of metallic implants, in that there was a decrease in infectious activity according to the tested parameters (Figs. 2–6).
There are serious concerns about the increase of MRSA infections [4], particularly in older patients, and hospital or ICU-acquired infections [13], which is possibly intensified by the presence of an ever increasing zoonotic reservoir [14].
Historically speaking, anti-microbiological usage of penicillin was recognised in 1942 for its success in treating staphylococcal infections by eradicating bacteria from blood and improving survival rates overall survival [15]. However, in 2008, 66 years later, a patient with bacterial sepsis caused by multi-resistant bacteria did not survive the infection despite the enormous increase in medical knowledge within the last six decades [16]. The current threats of resistant bacteria has become larger by the problem of increased MRSA resistance to vancomycin and linezolid, and even increased resistance to the salvage antibiotic daptomycin [17].
Similarly, a significant increase of MRSA soft tissue and bone infections has been recorded, as well as MRSA periprosthetic infections [18]. This leads to enormous socio-economic costs which in turn leads to an increasing need for research and new antibiotics to fight MRSA infections [19]. The optimal treatment for orthopaedic MRSA infections involves both surgery and medical treatment [2]. A radical surgical debridement should be followed by an extended period of appropriate antibiotic treatment. Among antimicrobial agents used for infections with multi-resistant bacteria, vancomycin is still the fist-line drug against gram-positive microorganisms including MRSA [20]. However, the need for new antimicrobiological agents is urgent due to increased vancomycin resistance [4].
Many alternative models with both their advantages and disadvantages have been described in the literature. Generally speaking, models of impaired fracture healing can be divided into models of delayed union or nonunion (atrophic and hypertrophic), segmental defects, and fracture-related osteomyelitis [21].
According to differences in the clinical presentation of osteomyelitis, different models have been created with variations in animal type and bacterial inoculation type.
As the incidence of MRSA infections has risen dramatically, and this rise is particularly noticeable in MRSA periprosthetic joint infections, the need for appropriate periprosthetic MRSA animal models is obvious. In the literature, one model of periprosthetic infection and systemic MRSA infection has been described [22]. Other models use a local MRSA inoculation, and many efforts have been made to develop new MRSA animal models, including the MRSA inoculation of rabbit knees [23] and rat bones [24].
As for periprosthetic infections, recent studies have included an assessment of the efficacy of teicoplanin in experimental Staphylococcus aureus infections of joint prostheses [25], a rabbit model of knee joint infection [26], a rat experimental foreign-body infection by MRSA [27], and more recently an assessment of the efficacy of salvage antibiotic daptomycin [28]. A comprehensive clinical follow-up examination has also been done by Lucke et al. in a peri-implant rat model of tibial osteomyelitis by inoculation of Staphylococcus aureus into the tibial cavity in combination with Kirschner wires [29].
However, unlike our model, Lucke et al. found no changes in blood results and body weight, indicating that our model may be more sensitive for evaluating osteomyelitis in humans. Here, bone histology was evaluated according to Petty et al. [30] and included parameters such as abscess formation, sequestrum formation, enlargement, and destruction of cancellous bone. In contrast to a standard histological scoring we chose to combine histo-morphometrical parameters and the histological score as obtained by Smeltzer et al. In this way we obtained semi-quantitative numbers, enabling an appropriated evaluation of the area between implant and surrounding bone and soft tissue. It should be noted, however, that the final evaluation of implant anchoring requires a biomechanical analysis with implant withdrawal under mechanical stress [10].
As a study limitation we were unable to perform CrP analysis due to irregular antibodies and cross-reactions with rabbit proteins. In the statistical analysis we did not include bacteriological results to avoid multivariate analysis which would be difficult with the present sample size.
Conclusions
We have established a peri-implant MRSA osteomyelitis model that successfully combines clinical and laboratory outcome infection parameters with histo-morphometric results, allowing the development of a model which appears valuable for future experimental use for the assessment of new drugs. Future studies using this or similar reproducible animal MRSA peri-implant infection models could test such new antimicrobial drugs for their efficacy against multi-resistant Staphylococcus aureus infections of orthopaedic implants and their osseous bed. By this means, the efficacy of such new antimicrobial drugs can be compared with current standard treatments of MRSA infections.
References
- 1.Geffers C, Gastmeier P. Nosocomial infections and multidrug-resistant organisms in Germany: epidemiological data from KISS (the hospital infection surveillance system) Deutsches Arzteblatt Int. 2011;108:87–93. doi: 10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment algorithms for chronic osteomyelitis. Deutsches Arzteblatt Int. 2012;109:257–264. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira DC, Tomasz A, de Lencastre H. Secrets of success of a human pathogen: molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect Dis. 2002;2:180–189. doi: 10.1016/S1473-3099(02)00227-X. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez P, Fernandez-Barat L, Torres A. New therapy options for MRSA with respiratory infection/pneumonia. Curr Opin Infect Dis. 2012;25:159–165. doi: 10.1097/QCO.0b013e3283509cfa. [DOI] [PubMed] [Google Scholar]
- 5.Rice DA, Mendez-Vigo L. Daptomycin in bone and joint infections: a review of the literature. Arch Orthop Trauma Surg. 2009;129:1495–1504. doi: 10.1007/s00402-008-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito S, Leone S. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents. 2008;32:287–293. doi: 10.1016/j.ijantimicag.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35:1621–1626. doi: 10.1007/s00264-010-1175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wimmer MD, Randau TM, Petersdorf S, Pagenstert GI, Weisskopf M, Wirtz DC, Gravius S. Evaluation of an interdisciplinary therapy algorithm in patients with prosthetic joint infections. Int Orthop. 2013 doi: 10.1007/s00264-013-1995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder K, Simank HG, Lorenz H, Swoboda S, Geiss HK, Helbig L. Implant stability in the treatment of MRSA bone implant infections with linezolid versus vancomycin in a rabbit model. J Orthop Res Off Publ Orthop Res Soc. 2012;30:190–195. doi: 10.1002/jor.21516. [DOI] [PubMed] [Google Scholar]
- 11.Simank HG, Stuber M, Frahm R, Helbig L, van Lenthe H, Muller R. The influence of surface coatings of dicalcium phosphate (DCPD) and growth and differentiation factor-5 (GDF-5) on the stability of titanium implants in vivo. Biomaterials. 2006;27:3988–3994. doi: 10.1016/j.biomaterials.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Smeltzer MS, Thomas JR, Hickmon SG, Skinner RA, Nelson CL, Griffith D, Parr TR, Jr, Evans RP. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res Off Publ Orthop Res Soc. 1997;15:414–421. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]
- 13.Rieg S, Peyerl-Hoffmann G, de With K, Theilacker C, Wagner D, Hubner J, Dettenkofer M, Kaasch A, Seifert H, Schneider C, Kern WV. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect. 2009;59:232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Kock R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, Becker K. The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Deutsches Arzteblatt Int. 2011;108:761–767. doi: 10.3238/arztebl.2011.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lax E (2004) The mold in Dr. Florey’s coat: the story of the penicillin miracle. Holt Paperbacks, NY
- 16.Schwartz BS, Ngo PD, Guglielmo BJ. Daptomycin treatment failure for vancomycin-resistant Enterococcus faecium infective endocarditis: impact of protein binding? Ann Pharmacother. 2008;42:289–290. doi: 10.1345/aph.1K548. [DOI] [PubMed] [Google Scholar]
- 17.van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naive patient–a review of the literature. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2011;30:603–610. doi: 10.1007/s10096-010-1128-3. [DOI] [PubMed] [Google Scholar]
- 18.Giannoudis PV, Townsend R, Homer-Vanniasinkam S, Wilcox MH. Soft tissue and bone MRSA infections. Injury. 2011;42(Suppl 5):S1–S2. doi: 10.1016/S0020-1383(11)70124-3. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplast. 2010;25:103–107. doi: 10.1016/j.arth.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Marschall J, Lane MA, Beekmann SE, Polgreen PM, Babcock HM. Current management of prosthetic joint infections in adults: results of an emerging infections network survey. Int J Antimicrob Agents. 2013;41:272–277. doi: 10.1016/j.ijantimicag.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills LA, Simpson AH. In vivo models of bone repair. J Bone Joint Surg Br Vol. 2012;94:865–874. doi: 10.1302/0301-620X.94B7.27370. [DOI] [PubMed] [Google Scholar]
- 22.Poultsides LA, Papatheodorou LK, Karachalios TS, Khaldi L, Maniatis A, Petinaki E, Malizos KN. Novel model for studying hematogenous infection in an experimental setting of implant-related infection by a community-acquired methicillin-resistant S. aureus strain. J Orthop Res Off Publ Orthop Res Soc. 2008;26:1355–1362. doi: 10.1002/jor.20608. [DOI] [PubMed] [Google Scholar]
- 23.Gaudin A, Amador Del Valle G, Hamel A, Le Mabecque V, Miegeville AF, Potel G, Caillon J, Jacqueline C. A new experimental model of acute osteomyelitis due to methicillin-resistant Staphylococcus aureus in rabbit. Lett Appl Microbiol. 2011;52:253–257. doi: 10.1111/j.1472-765X.2010.02992.x. [DOI] [PubMed] [Google Scholar]
- 24.Oguz E, Ekinci S, Eroglu M, Bilgic S, Koca K, Durusu M, Kaldirim U, Sadir S, Yurttas Y, Cakmak G, Kilic A, Purtuloglu T, Ozyurek S, Cekli Y, Ozkan H, Sehirlioglu A. Evaluation and comparison of the effects of hyperbaric oxygen and ozonized oxygen as adjuvant treatments in an experimental osteomyelitis model. J Surg Res. 2011;171:e61–e68. doi: 10.1016/j.jss.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Saleh Mghir A, Cremieux AC, Bleton R, Ismael F, Manteau M, Dautrey S, Massias L, Garry L, Sales N, Maziere B, Carbon C. Efficacy of teicoplanin and autoradiographic diffusion pattern of [14C]teicoplanin in experimental Staphylococcus aureus infection of joint prostheses. Antimicrob Agents Chemother. 1998;42:2830–2835. doi: 10.1128/aac.42.11.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res Off Publ Orthop Res Soc. 2005;23:1100–1104. doi: 10.1016/j.orthres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Garrigos C, Murillo O, Euba G, Verdaguer R, Tubau F, Cabellos C, Cabo J, Ariza J. Efficacy of usual and high doses of daptomycin in combination with rifampin versus alternative therapies in experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5251–5256. doi: 10.1128/AAC.00226-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niska JA, Shahbazian JH, Ramos RI, Pribaz JR, Billi F, Francis KP, Miller LS. Daptomycin and tigecycline have broader effective dose ranges than vancomycin as prophylaxis against a Staphylococcus aureus surgical implant infection in mice. Antimicrob Agents Chemother. 2012;56:2590–2597. doi: 10.1128/AAC.06291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Stemberger A, Haas NP, Raschke M. A new model of implant-related osteomyelitis in rats. J Biomed Mater Res Part B Appl Biomater. 2003;67:593–602. doi: 10.1002/jbm.b.10051. [DOI] [PubMed] [Google Scholar]
- 30.Petty W, Spanier S, Shuster JJ. Prevention of infection after total joint replacement. Experiments with a canine model. J Bone Joint Surg Am Vol. 1988;70:536–539. [PubMed] [Google Scholar]