Abstract
Purpose
Latissimus dorsi and teres major transfers to the lateral side of the humerus with lengthening of the pectoralis major and subscapularis muscles for residual shoulder deformity were compared in children and skeletally mature patients.
Methods
Fifteen patients (nine children, six skeletally mature patients aged three to 30 years, follow-up one to 22 years) were treated for internal shoulder contracture after birth plexus lesions: C5–C6 (seven patients); C5–7 (five patients); C5-C8-T1 (three patients, respectively). Range of movement, Mallet shoulder function score and radiographs were assessed.
Results
Pre-operatively, shoulder function restrictions were comparable in all patients. Postoperatively, external rotation, abduction and Mallet function score improved significantly (p < 0.05) in all patients except one. There were no differences in improvement between children and skeletally mature patients (p = 0.24–1.0).
Conclusions
This technique improves external rotation and abduction of the shoulder for daily living activities in children and young, skeletally mature, patients.
Keywords: Brachial plexus birth palsy, Shoulder, Muscle transfer
Introduction
The incidence of brachial plexus birth palsy (BPBP) differs according to regional obstetric care and increases with infant birth weight. An epidemiological study from the USA refers to an incidence of 0.15 % [1], whereas a study from Finland refers to an incidence of 0.31 % [2]. Furthermore, the risk of injury of the brachial plexus is five times higher in breech delivery and may be bilateral [3]. Narakas’ categorisation is generally used according to the location and severity of the plexus lesion [4]: Group 1, with an incidence of 73 %, represents the classic lesion of the upper plexus (C5–6, Erb’s palsy), with initial absence of external rotation and abduction of the shoulder, elbow flexion and forearm supination; the prognosis for the rate of spontaneous recovery is reported to be as high as 75–95 % [5]. Group 2 comprises patients with C5–6 and C7 (extended Erb’s palsy) involvement, with the absence of wrist and digital extension; the prognosis is poorer than for group 1 patients. Groups 3 and 4, with an incidence of 25 %, with the lesion of the entire plexus (C5–T1), flail extremity, with or without Horner’s syndrome, have the worst prognosis. Multidisciplinary treatment initially includes physiotherapy with passive exercises to prevent joint contractures and strengthen the recovering muscles. In the most common upper trunk lesion, children who demonstrate significant recovery within two months usually recover normal function [6]. Incomplete recoveries in C5–C6 and C5–C7 palsies lead to shoulder muscle imbalance characterised by strong internal rotators and weak external rotators and abductors and subsequently lead to contracture of the internal rotators (pectoralis major, subscapularis, latissimus dorsi and teres major muscles). Clinical sequels are restrictions of external rotation and abduction of the extremity, with limitations to reaching the mouth and neck with the hand. Scapular winging, changes in coracoid process, humeral head flattening and glenoid deformity with possible glenohumeral joint posterior dislocation are the anatomical sequelae [7]. Active external rotation is crucial for upper-extremity function. Therefore, restoring external rotation in combination with shoulder abduction is generally recommended [6–10]. In congruent glenohumeral joints, muscle release and transfer are indicated. Latissimus dorsi and teres major muscle transfer to rotator cuff muscles is a common procedure [8]. We prefer to transfer these muscles to the lateral side of the proximal humerus to restore active external rotation, together with lengthening the pectoralis major and subscapularis muscles [9]. The aim of this study was to evaluate this method of treating residual shoulder deformities after BPBP in children and skeletally mature patients. The hypotheses were that the method can significantly improve the use of the extremity for daily living activities and that improved function following the procedure will be significantly better in children compared with in skeletally mature patients.
Patients and methods
Fifteen patients aged three to 30 years (nine children and six skeletally mature patients) were surgically treated between 1990 and 2012 for sequelae of BPBP (Table 1) and grouped according to the following lesion type: upper plexus, seven patients; extended upper plexus, five patients; complete plexus, three patients. All patients/parents gave informed consent prior to inclusion in the study. The follow-up period ranged from one to 22 years. Indications for treatment were age greater than three years; limited active external rotation; radiologically nondislocated glenohumeral joint; supple passive shoulder range of movement (ROM) except external rotation. Pre-operative and postoperative clinical evaluation comprised subjective and objective assessments: A subjective postoperative assessment was made by patients or their parents: 1= significant improvement (ability to easily reach mouth and neck with the hand); 2 = partial improvement (ability to reach mouth and neck but with difficultly); 3 = no improvement; 4 = deterioration to pre-operative status. For objective assessment, the global passive and active ROM (both scapulothoracic and glenohumeral) were measured using a goniometer, and active shoulder function was assessed with the use of a modified Mallet score [11]. In this classification, five different shoulder movements are evaluated: abduction, external rotation, placing the hand behind the neck, placing the hand as high as possible on the spine and placing the hand to the mouth. Each shoulder movement was subsequently graded on a scale of 1 (no movement) to 5 (normal function). The sum of the values determines the aggregate Mallet score. Single movements and aggregate Mallet scores of children and skeletally mature patients were statistically compared. Comparisons of means for continuous variables were performed using paired Student’s t test with StatSoft, Inc. 2011 STATISTICA (data analysis software system), version 10. www.statsoft.com. P values were two-tailed, and the level of significance was set at p < 0.05. Plain radiographs, and computed tomography (CT) scans beginning in 1998, of both shoulders were used pre-operatively to categorise glenohumeral joint morphology according to Waters et al. [7] (Table 1). Shoulder muscles were pre-operatively assessed electromyographically in 11 patients using concentric needle electrodes.
Table 1.
Patient characteristics preopreatively
| Patient no. | Birth year | Paresis | Gender | Delivery | Age at surgery (years) | Side | Follow-up | ROM before surgery | Mallet classification preoperatively | Mallet score(average) | Radiological deformity grade | Upper limb shortening (cm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABD | FLEX | EXT | ER | IR | ABD | ER | Hand on neck | Hand on spine | Hand to mouth | Sum | |||||||||||
| Children | |||||||||||||||||||||
| 1 | 1987 | C5–C7 | M | Breech position, clavicle fxt. | 6 | L | 19 | 90 | 80 | 30 | −10 | 10 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 3 |
| 2 | 2000 | C5–T1 | M | BW 4,680 g | 3 | R | 9 | 80 | 80 | 30 | −30 | 30 | 3 | 2 | 2 | 3 | 2 | 12 | 2.4 | 2 | 2 |
| 3 | 1994 | C5–C6 | F | BW 4,500 g, clavicle fxt. | 10 | L | 7 | 80 | 80 | 30 | −10 | 30 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 1 |
| 4 | 2000 | C5–C7 | M | Transversal position, BW 3,300 g | 6 | L | 6 | 60 | 70 | 30 | −10 | 20 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 4 | 2 |
| 5 | 1998 | C5–C6 | M | Difficult delivery | 12 | L | 2 | 60 | 70 | 30 | −45 | 40 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 1 |
| 6 | 2003 | C5–T1 | M | Narrow birth canal | 7 | R | 2 | 20 | 70 | 30 | −20 | 30 | 2 | 2 | 2 | 2 | 2 | 10 | 2 | 2 | 3 |
| 7 | 2006 | C5–C6 | M | Difficult delivery | 4 | L | 2 | 70 | 80 | 30 | −5 | 30 | 3 | 2 | 2 | 3 | 2 | 12 | 2.4 | 2 | 1 |
| 8 | 2003 | C5–6 | F | BW 3,940 g, difficult delivery | 7 | R | 2 | 140 | 110 | 30 | 0 | 30 | 3 | 2 | 2 | 2 | 3 | 12 | 2.4 | 3 | 1 |
| 9 | 2004 | C5–C7 | M | BW 3,700 g, difficult delivery | 8 | R | 1.5 | 120 | 130 | 30 | 0 | 20 | 4 | 2 | 2 | 2 | 2 | 12 | 2.4 | 2 | 1 |
| Average | 80 | 85.55 | 30 | −14.44 | 26.66 | 3 | 2 | 2 | 2.22 | 2.11 | 11.33 | 2.26 | 1.66 | ||||||||
| Standard deviation | 35 | 20.684 | 0 | 14.88 | 8.66 | 0.5 | 0 | 0 | 0.44 | 0.33 | 0.70 | 0.14 | 0.86 | ||||||||
| Skeletally mature | |||||||||||||||||||||
| 10 | 1975 | C5–C6 | F | BW 4,000 g, forceps, clavicle fxt. | 15 | R | 22 | 170 | 170 | 40 | −40 | 40 | 4 | 2 | 2 | 3 | 3 | 14 | 2.8 | 2 | 2 |
| 11 | 1978 | C5–C6 | F | Difficult delivery | 17 | L | 17 | 40 | 55 | 30 | −20 | 30 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 3 |
| 12 | 1975 | C5–C6 | M | Clavicle fxt. | 22 | L | 15 | 20 | 170 | 30 | −20 | 40 | 3 | 2 | 2 | 3 | 2 | 12 | 2.4 | 2 | 2 |
| 13 | 1983 | C5–C7 | F | BW 4,500 g | 20 | R | 9 | 45 | 60 | 30 | −30 | 30 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 2 |
| 14 | 1978 | C5–T1 | F | Unknown | 30 | R | 5 | 40 | 40 | 30 | 0 | 20 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 2 | 2 |
| 15 | 1995 | C5–C6 | M | Unknown | 17 | R | 1 | 160 | 160 | 30 | 0 | 30 | 4 | 2 | 2 | 3 | 3 | 14 | 2.8 | 2 | 1 |
| Average | 79.16 | 109.16 | 31.66 | −18.33 | 31.66 | 3.33 | 2 | 2 | 2.5 | 2.33 | 12.16 | 2.43 | 2 | ||||||||
| Standard deviation | 67.11 | 63.43 | 4.08 | 16.02 | 7.52 | 0.51 | 0 | 0 | 0.54 | 0.51 | 1.47 | 0.29 | 0.632 | ||||||||
Grade 2-retroversion of the glenoid<5 deg.; grade 3-posterior humeral subluxation; grade 4 –subluxation with presence of false glenoid
ABD abduction, FLEX flexion, EXT extension, ER external rotation, IR internal rotation, fxt. fracture, BW birth weight
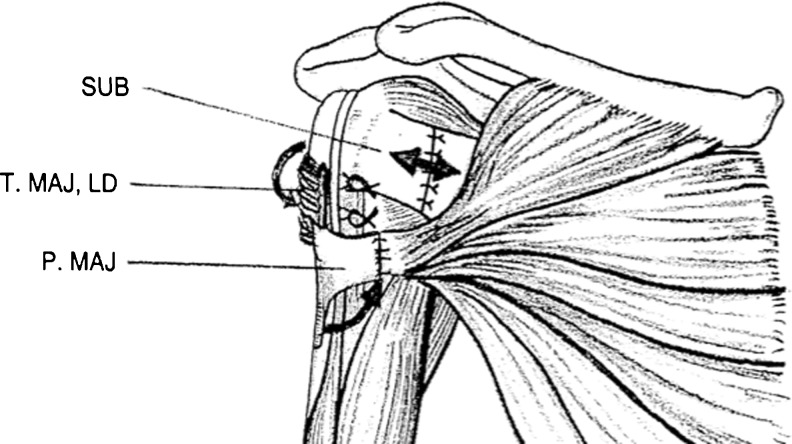
In a surgical procedure (Fig. 1), tendons of the pectoralis major and subscapularis muscles were lengthened in Z-form; the joint capsule remained intact. In the second step, tendons of the latissimus dorsi and teres major were released from the crista tuberculi minoris and transferred between the periosteum and deltoid muscle to the lateral side of the humerus and fixed to the crista tuberculi majoris using bone suture or anchors. The extremity was then fixed in the shoulder spica at 45° of abduction and maximum external rotation for six weeks. Physiotherapy then followed at an in-patient department for one week and three months in an outpatient clinic. Standard protocol included passive and active ROM exercises and voluntary activation of the latissimus dorsi and teres major muscles. Patients were regularly followed clinically and radiologically in six-month intervals until two years after surgery.
Fig. 1.
Z lengthening pectoralis major muscle tendon in the sagittal plane (PMAJ, thin arrow), subscapularis muscle in frontal plane (SUB, double arrow), lateral transfer of teres major muscle (T.MAJ) and latissimus dorsi muscle (LD) around the humerus to the crista tuberculi majoris
Results
Demographic and clinical preoperative data are shown in Table 1. All patients were unable to place the hand on the neck and were limited when bringing the hand to the mouth (Figs. 2a and 3a). From shoulder movements, mainly external rotation was restricted to −14° on average in children [range 0–45°, standard deviation (SD) 15°] and −18° in skeletally mature patients (range 0–40°, SD 16°). Abduction varied widely and averaged >80° in children (range 20–160°, SD 35°) and 79° in skeletally mature patients (range 20–170, SD 67°). Pre-operative aggregate Mallet score ranged from 10 to 14 points, and all patients were enrolled in Mallet classification system group 2 or 3. The main restrictions of functions in the Mallet score were noted in both the external rotation and placement of the hand behind the neck (average 2 points), and placing the hand at the mouth (average 2.1 points in children and 2.3 in skeletally mature patients). There were no significant pre-operative differences in scored functions between groups (p = 0.23–1.00). On radiological examination, glenoid retroversion (grade 2) was present in 13 patients and glenohumeral joint subluxation in two (grades 3 and 4). Pre-operative electromyographic examinations showed mostly reduced activity to grade 3–3.5 in the deltoid, supraspinatus and infraspinatus muscles. Activity of remaining muscles was reduced mostly to grade 4. In all cases, changes after denervation and reinnervation were described. Shortening of the upper extremity was noted in all patients and ranged from 1 to 3 cm. Results after treatment are noted in Table 2 and in Figs. 2b, 3b and 4. In the subjective assessment, eight patients improved significantly (reached the mouth and neck easily), and six improved partially (reached the mouth and the neck but with difficulty); one did not improve (could not reach the mouth and neck). In the objective assessment, the main observed function—namely, active external rotation—improved to 21° (SD 14°) in children and to 15° (SD 10°) in skeletally mature patients. Aggregate Mallet score ranged from 11 to 19 (average 17) points. All patients except one improved from 2 to 8 (average 5.1) points and moved to group 3 or 4 of this classification. One skeletally mature patient (no. 11) did not improve, and gain in Mallet score was only 0.2 points.
Fig. 2.
Patient 2, a 3-year-old boy, after C5–T1 lesion of brachial plexus on the right side before surgical treatment: a limited abduction and internal rotation contracture 30°; b 1 year after surgery, active external rotation enables bringing the hand to the mouth with abduction >40° (partial trumpet sign)
Fig. 3.
Patient 13, a 20-year-old woman, after C5–C7 brachial plexus lesion on the right side: a before surgical treatment; very limited elevation, and internal contracture 30°; b 1 year after surgery, improvement in shoulder elevation to 170° and external rotation to 20°
Table 2.
Patient characteristics postoperatively
| Patient no. | ROM after surgery | Mallet classification | Mallet score gain | Subjective assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABD | FLEX | EXT | ER | IR | ABD | ER | Hand on neck | Hand on spine | Hand to mouth | Sum | Average | |||
| Children | ||||||||||||||
| 1 | 75 | 100 | 40 | 35 | 30 | 3 | 4 | 3 | 4 | 3 | 17 | 3.4 | 6 | 2 |
| 2 | 130 | 130 | 40 | −5 | 60 | 4 | 2 | 2 | 3 | 3 | 14 | 3.2 | 2 | 2.5 |
| 3 | 150 | 150 | 40 | 25 | 60 | 4 | 4 | 3 | 3 | 4 | 18 | 3.6 | 7 | 2 |
| 4 | 90 | 90 | 20 | 20 | 0 | 3 | 3 | 3 | 3 | 4 | 16 | 3.2 | 5 | 2 |
| 5 | 80 | 80 | 30 | 30 | 20 | 3 | 4 | 4 | 3 | 4 | 18 | 3.6 | 7 | 1 |
| 6 | 80 | 80 | 20 | 10 | 30 | 3 | 3 | 3 | 3 | 3 | 15 | 3 | 5 | 2 |
| 7 | 90 | 80 | 30 | 5 | 20 | 4 | 3 | 4 | 3 | 3 | 17 | 3.4 | 4 | 1 |
| 8 | 110 | 90 | 30 | 35 | 20 | 4 | 4 | 4 | 4 | 3 | 19 | 3.2 | 3 | 1 |
| 9 | 170 | 130 | 30 | 30 | 30 | 4 | 4 | 4 | 4 | 3 | 19 | 3.2 | 7 | 1 |
| Average | 108.33 | 103.3 | 31.1 | 20.6 | 30 | 3.55 | 3.44 | 3.33 | 3.33 | 3.33 | 17 | 3.31 | 5.11 | 1.61 |
| Standard deviation | 34.27 | 26.46 | 7.82 | 14.2 | 19 | 0.52 | 0.72 | 1.40 | 0.5 | 0.5 | 1.73 | 0.20 | 1.83 | 0.60 |
| Skeletally mature | ||||||||||||||
| 10 | 170 | 170 | 60 | 15 | 40 | 4 | 3 | 4 | 4 | 4 | 19 | 3.8 | 5 | 1 |
| 11 | 45 | 30 | 10 | −5 | 30 | 3 | 2 | 2 | 2 | 2 | 11 | 2.2 | 0.2 | 3 |
| 12 | 170 | 175 | 35 | 15 | 60 | 4 | 3 | 4 | 4 | 4 | 19 | 3.8 | 8 | 1 |
| 13 | 170 | 170 | 40 | 20 | 60 | 4 | 3 | 4 | 4 | 4 | 19 | 3.8 | 8 | 1 |
| 14 | 60 | 50 | 20 | 20 | 20 | 3 | 3 | 3 | 3 | 3 | 15 | 3 | 4 | 2 |
| 15 | 170 | 170 | 30 | 25 | 30 | 4 | 4 | 4 | 4 | 3 | 19 | 3.8 | 6 | 1 |
| Average | 130.833 | 127.5 | 32.5 | 15 | 40 | 3.66 | 3 | 3.5 | 3.5 | 3.33 | 17 | 3.4 | 5.2 | |
| Standard deviation | 60.8619 | 68.1 | 17.2 | 10.5 | 17 | 0.51 | 0.63 | 0.83 | 0.83 | 0.81 | 3.34 | 0.66 | 2.92 | |
ABD abduction, FLEX flexion, EXT extension, ER external rotation, IR internal rotation
Fig. 4.
Patient 10, a 37-year-old woman, after C5–C6 brachial plexus lesion on the right side: long-term result 22 years after surgery; active external rotation enables bringing the hand to the mouth, with only mild abduction (no trumpet sign)
From the particular analysis and comparison of functions in children and skeletally mature patients using the Mallet classification system (Table 3), all function improved significantly (p < 0.05) except abduction in skeletally mature patients (average 0.33 points, p = 0.17). In the children’s group, external rotation improved most significantly (average 1.44 points, p = 0.0003), whereas in the skeletally mature group, placing the hand on the neck improved most significantly (average 1.5 points, p = 0.007). Improved Mallet scores for all functions were not significant between groups (p = 0.24–1).
Table 3.
Improvement in Mallet scores
| Function | Group | Preoperative (average) | Postperative (average) | Difference | P value | |
|---|---|---|---|---|---|---|
| ABD | Children | 3 | 3.56 | 0.56 | 0.013* | |
| Skeletally mature | 3.33 | 3.66 | 0.33 | 0.17 | ||
| ER | Children | 2 | 3.44 | 1.44 | 0.0003* | |
| Skeletally mature | 2 | 3 | 1 | 0.011* | ||
| Hand on neck | Children | 2 | 3.33 | 1.33 | 0.0004* | |
| Skeletally mature | 2 | 3.5 | 1.5 | 0.007* | ||
| Hand on spine | Children | 2.22 | 3.33 | 1.11 | 0.002* | |
| Skeletally mature | 2.5 | 3.5 | 1 | 0.011* | ||
| Hand to mouth | Children | 2.11 | 3.33 | 1.11 | 0.0005* | |
| Skeletally mature | 2.33 | 3.33 | 1 | 0.04* | ||
ABD abduction, ER external rotation, IR internal rotation
*Statistically significant
The method failed in two patients in the children’s group, and subsequent surgical procedures were necessary: transferred muscles had to be fixed to the humerus in patient 11; external rotation osteotomy was added due to posterior glenohumeral subluxation in patient 2, two years after initial treatment; except for in this patient, all glenohumeral joints remained radiologically centred. There were no other peri-operative or postoperative complications.
Discussion
This retrospective study evaluated the results of a unified surgical procedure for treating residual changes in the shoulder after BPBP in children and skeletally mature patients. Our first hypothesis was confirmed because the majority of patients improved significantly in daily living activities. The second hypothesis was not confirmed, because there were no significant differences in improvement between groups.
Despite a low incidence of BPBP in regions with good obstetric care and the fact that spontaneous complete recovery is reported in the majority of cases in the first two months of life, 20–30 % of cases do not recover spontaneously [5]. Incomplete recovery of muscle function in the upper-trunk lesion leads to internal rotation contracture of the shoulder joint and to early dysplastic changes of the humeral head and glenoid, beginning at the age of six months [2, 7, 12]. Preventing these sequences includes conservative treatment such as targeted physiotherapy and botulin toxinum application [13]. Surgical procedures encompass muscle release and are often done endoscopically [14–16]; however, the combination of tendon releases and muscle transfers are mostly used to reinforce external rotation or abduction, and eventually, rotation osteotomy and glenohumeral joint reduction are added. Lengthening pectoralis major and subscapularis muscles and transferring latissimus dorsi and teres major muscles, as described by Green and Tachdjian [9] as the combination of methods of Severe-L’Episcopo-Zachary, is preferred in our institution. Long-term satisfactory results have been published in larger series of patients [17], but surgical techniques vary significantly. Furthermore, these techniques were described mainly in children, especially in adolescents [18], whereas our cohort comprised six skeletally mature patients. Except for two failures in our study, the method improved shoulder function in both subjective and objective evaluations (Mallet score).
The critical outcome of the surgical procedure is improvement in global external rotation, which improved by an average of 1.4 points in children and 1 point in skeletally mature patients; placing the hand on the neck improved most significantly (1.33 and 1.5 points, respectively) as the result of combined external rotation and abduction. Shoulder abduction improved by only 0.33–0.56 points, which can be explained as follows: (1) This procedure is aimed at reinforcing external rotation, with improved abduction as a secondary effect. (2) Patients had relatively good pre-operative global abduction. Radiological changes did not deteriorate after this procedure, but we did not particularly follow glenohumeral-joint remodelling. Most authors did not observe joint remodeling after tendon transfers, even when surgical procedures were done at an early age [17, 19]; however, some authors describe glenohumeral realignment and glenoid retroversion remodelling after arthroscopic release of the anterior capsule and subscapularis tendon with tendon transfers [14]. In significant glenohumeral dysplasia and humeral head dislocation, glenoplasty or external rotation osteotomy of the proximal humerus is recommended [17, 20–22]. In our study, humeral osteotomy was additionally used in one patient due to external rotation failure. Some authors report deterioration of external rotation and abduction in long-term follow-up [23–25]. We did not see this complication in the follow-up, which ranged from 1 to 22 years, except for one failure. Furthermore, we did not see a significant loss in active internal rotation; such loss may impair the ability to reach the midline in front of the body [17].
Most authors recommend repairing muscle imbalance early, in the second year of life, to prevent dysplastic changes [7, 10, 14, 17]. We recommend this method after the age of three, when cooperation of the child in the subsequent rehabilitation can be expected. According to our results, this method can also improve upper-extremity function for daily living activities and sports in older children and young adults when the glenohumeral joint is centred and passive ROM is not significantly restricted (except for external rotation) and latissimus dorsi and teres major activity is adequate. It is in concordance with the conclusions of Pagnotta et al. [23] and Ozturk et al. [18] that the clinical result is related with the type of paralysis and pre-operative shoulder function and not with age. However, those authors did not use reconstruction in skeletally mature patients. Most of our patients participated in some type of sport, which is in concordance with Bae et al. [26].
Needle electromyography was used in 73 % patients in our study and offered useful information concerning muscle function. Reduced electrical activity was found in the most severely affected muscles (deltoid, supraspinatus, infraspinatus) and correlated with function. Because of incomplete data, these data are not shown. Electromyographic studies as part of surgical treatment evaluation are reported only exceptionally [27]. The infraspinatus muscle is described as the most important muscle for external rotation, and its electrical activity correlates with external rotation power and muscle volume on magnetic resonance imaging (MRI) [27, 28]. In our opinion, the teres major and latissimus dorsi muscles should be examined before transfer. MRI offers a universal method for comparing shoulder morphology and functional potential of muscles [17], but the necessity of using general anesthesia in preschool-age children represents a significant disadvantage.
This study has the following limitations and the strengths: The first limitation is that it is a retrospective study of a relatively small group of patients; however, that can be explained by the small incidence of residual changes in the shoulder in the relatively small geographic study area with a population of ten million inhabitants. This is probably due to the high standard of obstetric care in the region. Van Kooten et al. [29] published results of nine patients only, for similar reasons. The second limitation is that surgical procedures were performed by two surgeons from one institution, so the effects of institutional bias may be possible. Study strengths are that all patients were treated using the same surgical method; and we also treated six skeletally mature patients. We found only one paper dealing with skeletally mature patients; however, that study used different operative techniques [30].
In conclusion, transferring latissimus dorsi and teres major muscles to the lateral part of the proximal humerus combined with lengthening pectoralis major and subscapularis muscles represents a useful method for improving shoulder function for daily living activities after brachial plexus birth palsy. The method is useful in patients with nondislocated, relatively congruent, glenohumeral joints; functional results are comparable between children and skeletally mature patients.
Acknowledgments
Conflict of interest
None.
References
- 1.Foad SL, Mehlman CT, Ying J. The epidemiology of neonatal brachial plexus palsy in the United States. J Bone Joint Surg (Am) 2008;90:1258–1264. doi: 10.2106/JBJS.G.00853. [DOI] [PubMed] [Google Scholar]
- 2.Poyhia TH, Lamminen AE, Peltonen JI, Kirjavainen MO, Willamo PJ, Neitosvaara Y. Brachial plexus birth injury: US screening for glenohural joint instability. Radiology. 2010;254:253–260. doi: 10.1148/radiol.09090570. [DOI] [PubMed] [Google Scholar]
- 3.Geutjens G, Gilbert A, Helsen K. Obstetric brachial plexus palsy associated with breech delivery. A different pattern of injury. J Bone Joint Surg (Br) 1996;78:303–306. [PubMed] [Google Scholar]
- 4.Narakas AO. Obstetrical brachial plexus injuries. In: Lamb DW, editor. The paralysed hand. Edinburgh: Churchil Livingstone; 1987. pp. 116–135. [Google Scholar]
- 5.Foad SL, Mehlman CT, Foad MB, Lippert WC. Prognosis following neonatal brachial plexus palsy: an evidence-based review. J Child Orthop. 2009;3:459–463. doi: 10.1007/s11832-009-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedowitz DI, Gibson B, Williams GR, Kozin SH. Arthoscopic treatment of posterior glenohumeral joint subluxation. J Shoulder Elbow Surg. 2007;16:6–13. doi: 10.1016/j.jse.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Waters PM, Smith GR, Jaramillo D. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg (Am) 1998;80:668–677. doi: 10.2106/00004623-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hoffer MM, Phipps GJ. Closed reduction and tendon transfer for treatment of dislocation of the glenohumeral joint secondary to brachial plexus palsy. J Bone Joint Surg (Am) 1998;80:997–1001. doi: 10.2106/00004623-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Green WT, Tachdjian MO. Correction of residual deformities of the shoulder in obstetrical palsy. J Bone Joint Surg (Am) 1963;45:1544–1545. [Google Scholar]
- 10.Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg (Am) 2010;35:322–331. doi: 10.1016/j.jhsa.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Bea DS, Waters PM, Zurakowski D. Reliability of three classification systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg (Am) 2003;85:1753–1738. doi: 10.2106/00004623-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Sibinski M, Wozniakovski B, Drobniewski M, Synder M. Secondary gleno-humeral joint dysaplasia in children with persistent obstetric brachial plexus palsy. Int Orthop. 2010;34:863–867. doi: 10.1007/s00264-010-0965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezaki M, Malungpaisrope K, Harrison RJ, Kills JK, Oishi SN, Delgado M. Onabotulinumtoxin A injection as an adjunct in the teratment of posterior shoulder subluxation in neonatal brachial plexus palsy. J Bone Joint Surg (Am) 2010;92(12):2171–2177. doi: 10.2106/JBJS.I.00499. [DOI] [PubMed] [Google Scholar]
- 14.Pearl ML, Edgerton BW, Kazimiroff PA, Burchette RJ, Wong K. Arthroscopic release and latissimus dorsi transfer for shoulder internal rotation contarctures and glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg (Am) 2006;88:564–574. doi: 10.2106/JBJS.D.02872. [DOI] [PubMed] [Google Scholar]
- 15.Kozin SH, Boardman MJ, Chafetz RS, Williams GR, Janlon A. Arthroscopic treatment of internal rotation contacture and glenohumeral dysplasia in childern with brachial plexus birth palsy. J Shoulder Elbow Surg. 2010;19:101–110. doi: 10.1016/j.jse.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Mehlman CT, DeVoe WB, Lippert WC, Michaud LJ, Allgier AJ, Foad SL. Arthroscopically assisted Severe-L’Episcopo procedure improves clinical and radiological outcomes in neonatal brachial plexus palsy patients. J Child Orthop. 2011;31:341–351. doi: 10.1097/BPO.0b013e31820cada8. [DOI] [PubMed] [Google Scholar]
- 17.Kozin SH. The evaluation and treatment of children with brachial plexus birth palsy. J Hand Surg. 2011;36-A:1360–1369. doi: 10.1016/j.jhsa.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Ozturk K, Bulbul M, Demir BL, Buyukkurt CD, Ayanoglu S, Esenyel CZ. Reconstruction of shoulder abduction and external rotation with latissimus dorsi and teres major tarnsfer in obstetric brachial plexus palsy. Acta Orthop Traumatol Turc. 2010;44(3):186–193. doi: 10.3944/AOTT.2010.2332. [DOI] [PubMed] [Google Scholar]
- 19.Waters PM, Bae DS. The early effects of tendon transfers and open capsulorhaphy on glenohumeral deformity in brachial plexus birth palsy. J Bone Joint Surg (Am) 2008;90:2171–2179. doi: 10.2106/JBJS.G.01517. [DOI] [PubMed] [Google Scholar]
- 20.Di Mascio L, Chin K-F, Fox M, Sinisi M. Glenoplasty for complex shoulder subluxation and dislocation in children with obstetric brachial plexus palsy. J Bone Joint Surg (Br) 2011;93:102–107. doi: 10.1302/0301-620X.93B1.25051. [DOI] [PubMed] [Google Scholar]
- 21.Abzug JM, Chafetz RS, Gaughan JP, Ashworth S, Kozin SH. Shoulder function after medial approach and derotational humeral osteotomy in patients with brachial plexus birth palsy. J Pediatr Orthop (Am) 2010;30(5):469–474. doi: 10.1097/BPO.0b013e3181df8604. [DOI] [PubMed] [Google Scholar]
- 22.Kammbhampati SBS, Birch R, Cobiella C, Chen L. Posterior subluxation and dislocation of the shoulder in obstetric brachial plexus palsy. J Bone Joint Surg (Br) 2006;88(2):213–219. doi: 10.1302/0301-620X.88B2.17185. [DOI] [PubMed] [Google Scholar]
- 23.Pagnotta A, Haerle M, Gilbert A. Long term resultrs on abduction and external rotation of the shoulder after latissimus dorsi transfer for sequelae of obstetric palsy. Clin Orthop Rel Res. 2004;429:199–205. doi: 10.1097/01.blo.0000138957.11939.70. [DOI] [PubMed] [Google Scholar]
- 24.Bertelli JA. Lengthening of subscapularis and transefr of lower trapezius in the correction of recurrent internal contracture following obstetric brachial plexus palsy. J Bone Joint Surg (Br) 2009;91:943–948. doi: 10.1302/0301-620X.91B7.21795. [DOI] [PubMed] [Google Scholar]
- 25.Kirkos JM, Kyrkos MJ, Kapetanos GA, Haritidis JH. Brachial plexus palsy secondary to birth injuries. Long.-erm results of anterior release and tendon transfers around the shoulder. J Bone Joint Surg (Br) 2005;87:3231–3235. doi: 10.1302/0301-620x.87b2.14739. [DOI] [PubMed] [Google Scholar]
- 26.Bae DS, Zurakowski A, Avallone N, Yu R, Waters PM. Sports participation in selected children with brachial plexus palsy. J Pediatr Orthop (Am) 2009;29:496–503. doi: 10.1097/BPO.0b013e3181aa9583. [DOI] [PubMed] [Google Scholar]
- 27.Talbert RJ, Michaud LJ, Kinnett DG, Laor T, Foad SL, Schnell B, Salisbury S. EMG and MRI are independently related to the shoulder external rotation function in neonatal brachial plexus palsy. J Pediatr Orthop (Am) 2011;31(2):194–204. doi: 10.1097/BPO.0b013e3182092892. [DOI] [PubMed] [Google Scholar]
- 28.Hogendoorn S, Van Overvest KL, Wat I, Duisens AW, Nelissen R. Structural changes in muscle and glenohumeral joint deformity in neonatal brachial plexus palsy. J Bone Joint Surg (Am) 2010;92:935–942. doi: 10.2106/JBJS.I.00193. [DOI] [PubMed] [Google Scholar]
- 29.Van Kooten EO, Fortuin S, Winters HA, Ritt MJ, Van der Sluijs HA. Results of latissimus dorsi transfer in obstetrical brachial plexus palsy. Tech Hand Up Extrem Surg. 2008;12:195–199. doi: 10.1097/BTH.0b013e318176b32f. [DOI] [PubMed] [Google Scholar]
- 30.Terzis JK, Kostopoulos E. Our experience with secondary reconstruction of external rotation in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2010;126(3):951–963. doi: 10.1097/PRS.0b013e3181e603d3. [DOI] [PubMed] [Google Scholar]