Abstract
Interleukin 7/Interleukin 7 receptor (IL-7/IL-7R) signaling induces the upregulation of cyclin D1 to promote cell proliferation in lung cancer, but its role in preventing the apoptosis of non-small cell lung cancer (NSCLC) cell lines remains unknown. To study the role of IL-7 in lung cancer cell apoptosis, normal HBE cells as well as A549 and H1299 NSCLC cells were examined using flow cytometry. The results showed that the activation of IL-7R by its specific ligand, exogenous interleukin-7, was associated with a significant decline in apoptotic cells. Western blot and real-time PCR assays indicated that the activation of IL-7/IL-7R significantly upregulated anti-apoptotic bcl-2 and downregulated pro-apoptotic bax and p53 at both protein and mRNA levels. The knockdown of IL-7R through small interfering RNAs significantly attenuated these effects of exogenous IL-7. However, there was no significant anti-apoptotic effect in H1299 (p53-) cells. Furthermore, the inhibition of p53 significantly abolished the effects of IL-7/IL-7R on lung cancer cell apoptosis. These results strongly suggest that IL-7/IL-7R prevents apoptosis by upregulating the expression of bcl-2 and by downregulating the expression of bax, potentially via the p53 pathway in A549 and HBE cells.
Keywords: Interleukin 7, interleukin 7 receptor, p53, apoptosis, NSCLC
Introduction
Interleukin-7 (IL-7) is an essential cytokine required for the normal development of the immune system, and it maintains normal immune functions in the human body [1]. IL-7 acts as a survival factor for resting peripheral T cells via the maintenance of cellular homeostasis and by promoting the expression of anti-apoptotic proteins. In addition, IL-7 can serve as a costimulatory factor during T cell activation, a role particularly important in conditions associated with lymphopenia when IL-7 triggers homeostatic proliferation [2]. IL-7/IL-7R signaling play important role in various process of B and T cell development. Interleukin-7 receptor (IL-7R) deficiency severely impairs T-cell development due to substantial apoptosis [3-5]. Apoptosis is a tightly regulated process that plays an important role in the progression of human tumorigenesis. An important regulator of this process, tumor-suppressor p53 (TP53), is a crucial transcription factor that controls the cell cycle and apoptosis of cells under genotoxic stresses. TP53 exerts its role through activating the transcription of hundreds of genes by binding to specific sequences at their promoters [6]. Some studies have reported that the apoptosis induced by IL-7Rα deficiency is partially due to an elevated p53 activity in IL-7Rαnull mice, and that p53 inactivation permits the survival of IL-7Rαnull thymocytes, leading to exacerbated lymphomagenesis [1]. However, the relationship between IL-7/IL-7R signaling and p53 in lung cancer cell apoptosis is unclear. Recently, more studies showed that IL-7 and IL-7R played complex roles in cancer progression. Therefore, studies on the association of p53 with IL-7/IL-7R signaling will elucidate the importance of IL-7/IL-7R in tumorigenesis.
Our previous study demonstrated that IL-7R is highly expressed in human non-small cell lung cancer (NSCLC) cells [7], and that IL-7/IL-7R promotes the proliferation of A549 and LH7 NSCLC cells via the c-Fos/c-Jun pathway by upregulating cyclin D1 [8]. However, the role of IL-7/IL-7R in the apoptosis of human NSCLC cells has not been studied.
We hypothesized that IL-7/IL-7R could prevent apoptosis in NSCLC cells. The purpose of this study was to examine the effect and regulatory mechanism of the IL-7/IL-7R interaction on the apoptosis of A549, H1299, and HBE cells. IL-7/IL-7R prevented cell apoptosis by upregulating the expression of anti-apoptotic bcl-2 and by downregulating the expression of pro-apoptotic bax in A549 and HBE cells, potentially via the p53 pathway. This study provides novel evidence on the mechanisms of survival of cancer cells through IL-7/IL-7R and may aid the exploration of treatment targets for NSCLC.
Material and methods
Cell lines and cell culture
A549, human bronchial epithelial (HBE), and H1299 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). A549 and H1299 cells were cultured in RPMI 1640 (Sigma, St. Louis, MO, USA) containing 10% HyClone fetal bovine serum (ThermoFisher Scientific, Fremont, CA, USA). HBE cells were propagated in DMEM (Sigma, St. Louis, MO, USA) with 15% FBS, 100 IU/ml penicillin (Sigma, St. Louis, MO, USA), and 100 mg/ml streptomycin (Sigma). Cells were grown on sterile tissue culture dishes and passaged every two days using 0.25% trypsin (Invitrogen). Cells were divided into six groups: NC group: cells transfected with negative control siRNA; NC+IL-7 group: cells transfected with negative control siRNA then incubated with recombinant human IL-7 (20 ng/ml) for 24 h; siIL-7R+IL-7 group: cells transfected with IL-7R siRNA then incubated with recombinant human IL-7 (20 ng/ml) for 24 h; siIL-7R group: cells transfected with IL-7R siRNA; NC+IL-7+sip53 group: cells co-transfected with negative control siRNA and p53 siRNA then incubated with recombinant human IL-7 (20 ng/ml) for 24 h; siIL-7R+IL-7+sip53 group: cells co-transfected with IL-7R siRNA and p53 siRNA then incubated with recombinant human IL-7 (20 ng/ml) for 24 h.
Antibodies and reagents
Anti-IL-7R (rabbit polyclonal, sc-662), p53 (mouse monoclonal, sc-126), bcl-2 (rabbit monoclonal, Cell Signaling Technology-2872), bax (rabbit monoclonal, Cell Signaling Technology-2774), β-Actin (mouse monoclonal, sc-47778), and GAPDH (mouse monoclonal, sc-365062), Recombinant human IL-7 was purchased from Invitrogen (Carlsbad, CA, USA), Lipofectamine 2000 was from Invitrogen (Carlsbad, CA, USA), Annexin V-FITC Apoptosis Kit (BD Pharmingen, San Jose, CA, USA).
Small interfering RNA treatment
A549, HBE, and H1299 cells were plated onto 6-well dishes and grown to 40-60% confluence before transfection with Lipofectamine 2000 and siRNAs (Genepharma, Suzhou, China). The transfection efficiency was assessed by flow cytometry. The mRNA and protein levels were assessed 48 and 72 h after transfection. Efficiencies of siIL-7R and non-specific stable negative control siRNA were tested using realtime-PCR and western blot analysis. The sequences of siRNAs targeting IL-7R, p53 (siIL-7R, sip53, respectively) and control siRNAs were: siIL-7R, 5’-GAACUCCAGAGAUCAAUAATT-3’ and 5’-UUAUUGAUCUCUGGAGUUCTT-3’; sip53, 5’-CAA UGG UUC ACU GAA GAC CTT-3’ and 5’-GGUCUUCAGUGAACCAUUGTT-3’ non-specific negative control, 5’-UUCUCCGAACGUGUCACGUTT-3’ and 5’-ACGUGACACGUUCGGAGAATT-3’.
Western blot analysis
After treatment with IL-7 (20 ng/ml) for 24 h, cells were extracted with lysis buffer (150 mM NaCl, 1% NP-40, 0.1% SDS, 2 mg/ml aprotinin and 1 mM PMSF) for 30 min at 4°C. Extracts were centrifuged at 15,000 ×g for 15 min at 4°C. Supernatants containing total protein were then harvested. Aliquots, each containing 50 mg proteins, were separated by 10% SDS-PAGE and transferred to PVDF membranes at 40 V or 100 V for 2 h at low temperature. The membranes were blocked in 5% skim milk or 5% bovine serum albumin (BSA) for 2 h and proteins were detected using monoclonal or polyclonal antibodies overnight at 4°C. Proteins were visualized using anti-mouse or anti-rabbit IgG antibodies conjugated to horse radish peroxidase (HRP) for 2 h at 28°C. Bound proteins were visualized using ECL (Thermo Fisher Scientific, Waltham, MA, USA) and detected using the BioImaging System (UVP Inc., Upland, CA, USA). The relative protein levels were calculated based on β-actin or GAPDH as the loading control.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from cells using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using SYBR Green PCR master mix (Applied Biosystems) in a total volume of 20 μl on the 7900HT Fast Real-Time PCR System (Applied Biosystems) as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 30 s. A dissociation step was performed to generate a melting curve to confirm the specificity of the amplification. β-Actin was used as the. The relative levels of gene expression were represented as ΔCt =Ct gene-Ct reference, and the fold change of gene expression was calculated by the 2-ΔΔCt method. Experiments were repeated in triplicate. The primer sequences were as follows: IL-7R forward: 5’-TGGAGACTTGGAAGATGCAG-3’, IL-7R reverse: 5’-AAGCACAGGTCAGTGAGTGC-3’; β-Actin forward: 5’-ATAGCACAGCCTGGATAGCAACGTAC-3’, β-Actin reverse: 5’-CACCTTCTACAATGAGCTGCGTGTG-3’; bcl-2 forward: 5’-TGGCCAGGGTCAGAGTTAAA-3’, bcl-2 reverse: 5’-TGGCCTCTCTTGCGGAGTA-3’; bax forward: 5’-TTGCTTCAGGGTTTCATCCA-3’, bax reverse: 5’-AGACACTCGCTCAGCTTCTTG-3’; p53 forward: 5’-TCAGTCTACCTCCCGCCATA-3’, p53 reverse: 5’-TTACATCTCCCAAACATCCCT-3’.
Annexin V staining
After treatment with IL-7 (20 ng/ml) for 24 h, cells were harvested and washed twice with cold PBS by gentle shaking. Resuspended cells were added to Binding buffer (1×) and adjusted to a cell density of 2-5×105/ml. In the dark, 5 μl Annexin V-FITC (50 mM TRIS, 100 mM NaCl, 1% BSA, 0.02% sodium azide, pH 7.4) was added to the cell suspension mix of 195 ml and incubated for 10 min at room temperature before adding 190 ml binding buffer (1×) and 10 μl PI. Ten thousand events per sample were acquired using a FACS-scan flow cytometer (Becton-Dickinson, San Jose, CA, USA) and the percentage of cell apoptosis analyzed using CellQuest analysis software (Becton-Dickinson).
Statistical analysis
Data were analyzed using SPSS 16.0 software. One-way analysis of variance (ANOVA) was used to evaluate the differences between groups with various treatments, and the least significant difference (LSD) test or Dunnett’s T3 test was used for post hoc subgroup analysis. The p-value was based on two-sided statistical analysis, and *p<0.05 or **p<0.01 was considered to indicate statistical significance.
Results
IL-7/IL-7R prevents apoptosis of A549 and HBE cells
Western blot analysis (Figure 1A) and real-time PCR (Figure 1B) revealed that IL-7R expression was significantly higher in lung cancer cell lines (A549 and H1299) compared to a normal bronchial epithelial cell line (HBE). To determine whether activation of IL-7R also influences apoptosis of A549, H1299 and HBE cells, IL-7R activation and inhibition were induced with exogenous IL-7 (20 ng/ml) and with IL-7R small interfering RNA (siRNA), respectively. According to our published data, IL-7 at a concentration of 20 ng/ml significantly promoted cell proliferation [7,8]. Therefore, IL-7 at 20 ng/ml was used in the following experiment. The effectiveness of IL-7R-specific siRNAs was tested and shown to efficiently block IL-7R expression in A549, H1299, and HBE cell lines (Figure 1C, 1D). Annexin V staining was performed along with flow cytometry to determine the effect of IL-7/IL-7R on apoptosis in A549 and HBE cells. As shown in Figure 2, the proportion of A549 and HBE pre-apoptotic cells in the IL-7 stimulated group was significantly reduced compared to the negative control groups (**p<0.01), but the inhibition of IL-7R by siRNA transfection enhanced apoptosis when compared to the negative control groups (**p<0.01). Taken together, these results indicated that the activation of IL-7R can inhibit the apoptosis of A549 and HBE cells, an effect abolished by the inhibition of IL-7R.
Figure 1.

Expression of IL-7R in NSCLC cell lines. A, B: The protein and mRNA expression level of IL-7R was analyzed by western blotting and real-time PCR in HBE, A549, and H1299 cell lines. C, D: Western blot analysis and real-time PCR analysis of IL-7R depletion efficiency in HBE, A549, and H1299 cells at the protein and transcriptional level.
Figure 2.

Effect of IL-7/IL-7R on apoptosis in A549 and HBE cells. A, B: A549 and HBE cells were treated with IL-7 (20 ng/ml) for 24 h after transfection with IL-7R siRNA (siIL-7R). C: After treatment, apoptosis was estimated using Annexin V staining. Each bar represents the mean±SD of three independent experiments. *p<0.05 or **p<0.01, compared with negative control cells.
IL-7/IL-7R regulates apoptosis-related proteins to inhibit apoptosis in lung cancer cells
B-cell lymphoma-2 (bcl-2) is an antiapoptotic protein important in the regulation of apoptosis in various cell types [9]. Bax is a death inducer expressed in thymocytes [10]. The ratio between antiapoptotic bcl-2 and proapoptotic bax, as well as between other bcl-2 homologues, is critical in determining the survival of many cell types [11]. While this ratio is inherent to stages of cell development, it can be reset by either endogenous or external stimuli [12-14].
In our previous studies, the inhibition of apoptosis in A549 and HBE cells was associated with the regulation of anti-apoptotic bcl-2 and pro-apoptotic bax. Other work has also established that apoptosis involves the expression of bcl-2 and bax [15,16]. To determine the relationship of bcl-2 and bax expression with the IL-7/IL-7R-mediated inhibition of apoptosis in A549 and HBE cells, western blot analysis and real-time PCR assays were performed. The expression of anti-apoptotic bcl-2 and pro-apoptotic bax were upregulated and downregulated, respectively, in the IL-7 group at both protein (Figure 3A) and mRNA (Figure 3B) levels compared to the negative control group, while blocking IL-7R reversed these effects. These results indicated that the inhibitory function of IL-7/IL-7R on apoptosis in NSCLC cells possibly involves bcl-2 and bax.
Figure 3.

Effect of IL-7/IL-7R on the expression of bcl-2 and bax in A549 and HBE cells. A, B: A549 and HBE cells were treated with IL-7 (20 ng/ml) for 24 h after transfection with IL-7R siRNA (siIL-7R). After treatment, the expression of bcl-2 and bax at both protein and mRNA was estimated using western blotting and real-time PCR. Each bar represents the mean±SD of three independent experiments. *p<0.05 or **p<0.01, compared with negative control cells.
IL-7/IL-7R inhibits apoptosis in a p53-dependent manner
The p53 pathway normally inhibits cell proliferation and induces apoptosis, and inactivation of the p53 pathway can promote tumorigenesis. To confirm whether the inhibition of apoptosis by IL-7/IL-7R is associated with p53, the levels of p53 protein and mRNA were assessed through western blotting analysis and real-time PCR, respectively. As shown in Figure 4, p53 protein and mRNA in A549 and HBE cells significantly decreased in the IL-7 group (**p<0.01), but increased with IL-7R siRNA transfection (*p<0.05).
Figure 4.

Effect of IL-7/IL-7R on the expression of p53 in A549 and HBE cells. A, B: A549 and HBE cells were treated with IL-7 (20 ng/ml) for 24 h after transfection with IL-7R siRNA (siIL-7R). After treatment, the expression of p53 at both protein and mRNA was estimated using western blotting and real-time PCR. Each bar represents the mean±SD of three independent experiments. *p<0.05 or **p<0.01, compared with negative control cells.
As a transcriptional promoter, p53 protein regulates the transcription and expression of a variety of target genes required for cell cycle arrest and apoptosis, including Bcl-2 and Bax [17]. In addition, p53 is known to directly activate proapoptotic Bax to permeabilize mitochondria and engage the apoptotic program [18]. To further determine the role of the p53 pathway in the anti-apoptotic action of IL-7/IL-7R, siRNAs targeting p53 were applied to block wild-type p53. A549 and HBE cells were co-transfected with IL-7R siRNAs and p53 siRNAs. Blocking wild-type p53 significantly abolished siIL-7R-mediated apoptotic effects in A549 and HBE cells and the alterations in bcl-2 and bax expression (Figures 5, 6). Flow cytometry analysis further demonstrated that the apoptotic rate was lower in cells co-transfected with IL-7R siRNAs and p53 siRNAs compared to cells transfected with IL-7R siRNAs without p53 siRNAs (**p<0.01, Figure 5). Western blotting analysis showed that co-transfection of both siRNAs led to the upregulation of bcl-2 and the downregulation of bax compared to the transfection of IL-7R siRNAs without p53 siRNAs Figure 4. Effect of IL-7/IL-7R on the expression of p53 in A549 and HBE cells. A, B: A549 and HBE cells were treated with IL-7 (20 ng/ml) for 24 h after transfection with IL-7R siRNA (siIL-7R). After treatment, the expression of p53 at both protein and mRNA was estimated using western blotting and real-time PCR. Each bar represents the mean±SD of three independent experiments. *p<0.05 or **p<0.01, compared with negative control cells. (**p<0.01, Figure 6). These results indicated that the regulation of apoptosis by IL-7/IL-7R was associated with bcl-2 and bax via the p53 pathway.
Figure 5.
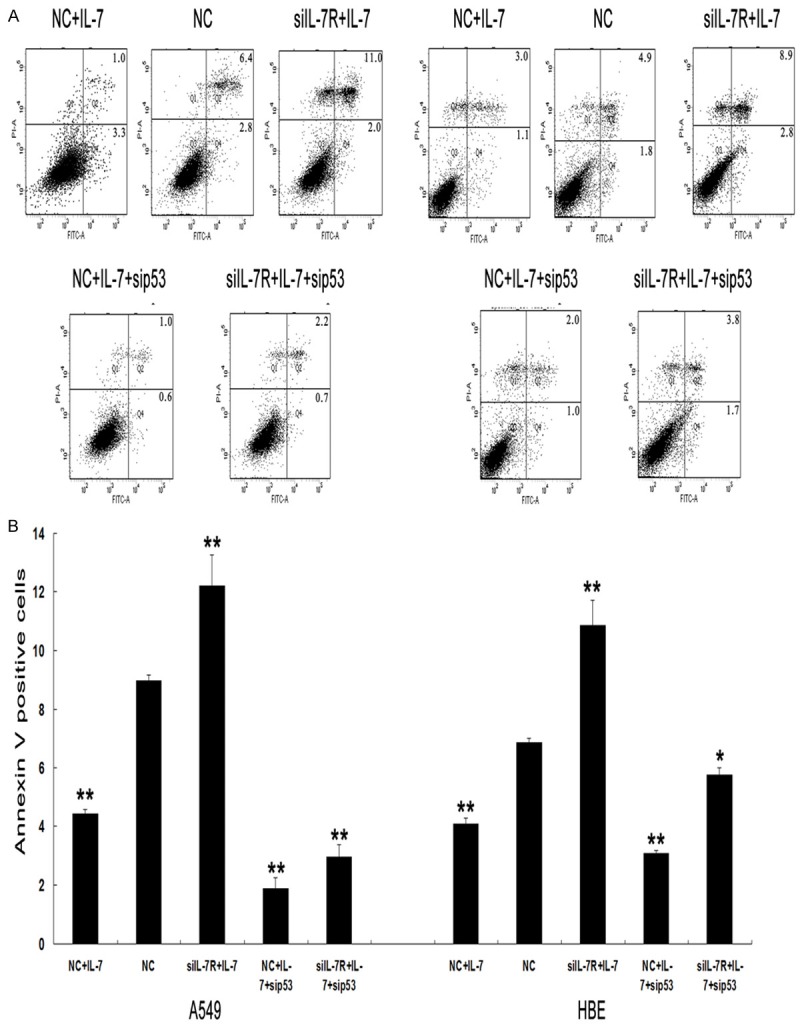
Effect of IL-7/IL-7R on apoptosis in A549 and HBE cells after inhibiting p53 activation. A: A549 and HBE cells were divided into five groups as described in Methods section. B: Apoptosis was estimated using the Annexin V staining. Each bar represents the percentage of Annexin positive cells of three independent experiments. *p<0.05, or **p<0.01, compared with negative control cells.
Figure 6.

Effect of IL-7/IL-7R on the expression of bcl-2 and bax in A549 and HBE cells after inhibiting p53 activation. A: A549 and HBE cells were divided into five groups. The expression of IL-7R, p53, bcl-2 and bax protein level was estimated using western blotting. B: Densitometry analysis of western blot experiments. Data were normalized against GAPDH and each bar represents the mean±SD of three independent experiments. *p<0.05 or **p<0.01, compared with negative control cells.
Effects of IL-7/IL-7R on the apoptotic capabilities of H1299 cells
To further validate the regulation of p53 by IL-7/IL-7R signaling, the p53-deficient cell line H1299 [19] was treated with IL-7R siRNAs (Figure 1C, 1D) then apoptosis detected using Annexin V staining. Flow cytometry analysis demonstrated that the apoptosis rate only slightly decreased in cells treated with IL-7, and slightly increased in cells transfected with IL-7R siRNAs (Figure 7A, 7B) compared to the negative control group. Western blot assays showed that the expression of bcl-2 and bax did not significantly differ between the IL-7 group and the negative control group (Figure 7C); however, the group treated with IL-7R siRNAs experienced a slight decrease in bcl-2 and increase in bax (Figure 7C, 7D). These results indicated that the regulation of apoptosis by IL-7/IL-7R in p53-present cells was probably associated with the p53 pathway, but that the regulation of apoptosis in p53-deficient cells was probably associated with other pathways.
Figure 7.

Effect of IL-7/IL-7R on apoptosis in H1299 cells. A: H1299 cells were treated with IL-7 (20 ng/ml) for 24 h after transfection with IL-7R siRNA (siIL-7R). After treatment, apoptosis was estimated using Annexin V staining. B: Each bar represents the percentage of Annexin-positive cells from three independent experiments. C: The expression of bcl-2 and bax protein was estimated using western blotting. D: Densitometry analysis of western blot experiments. Data were normalized against GAPDH and each bar represents the mean±SD of three independent experiments.
Discussion
Apoptosis is a tightly regulated process that plays an important role in tumorigenesis and immune response [20]. IL-7/IL-7R signaling is tightly associated with survival and apoptosis in T and B cells [21,22]. Many studies on IL-7/IL-7R and apoptosis have focused on T and B cellular homeostasis and HIV infection [23,24], whereas few studies have examined their role in human tumors. In a previous study, we found that IL-7 and IL-7R were overexpressed in human NSCLC and that its expression correlated with clinical stage, lymph node metastasis, and the reduced survival of NSCLC patients [7]. IL-7/IL-7R induced cyclin D1 upregulation and promoted cell proliferation in lung cancer cells [8], indicating their role in lung cancer tumorigenesis.
In the present study, the expression level of IL-7R was higher in A549 and H1299 lung cancer cell lines than in the HBE cell line, consistent with results observed in NSCLC tissues. IL-7/IL-7R prevented the apoptosis of A549 cells, and blocking IL-7R abolished this effect. Similar results were also achieved in HBE cells. In addition, IL-7 stimulation increased bcl-2 expression and decreased bax and p53 mRNA and protein levels, effects reversed by blocking IL-7R. Moreover, blocking wild-type p53 attenuated the apoptotic effects from blocking IL-7R. In p53-deficient H1299 cells, IL-7 stimulation did not obviously reduce the apoptosis rate, and blocking IL-7R only slightly increased the apoptotic rate. These findings suggest that IL-7/IL-7R potentially regulates the apoptosis of lung cancer cells via the p53 pathway, but is not the sole pathway in this process.
The regulatory mechanisms of IL-7 and IL-7R on cell apoptosis and survival are complex. IL-7 has been reported to increase the expression of bcl-2 or bcl-xL in human and murine naive T cells as well as in murine IL-7-dependent T-cell lines [25-27]. IL-7 also reportedly suppresses precursor B cell apoptosis and produces low Bax and high Bcl-2 levels [28]. The antiapoptotic effect of IL-7 has been shown to be partially due to its upregulation of the antiapoptotic protein bcl-2 in CD4+, CD45RA+ naive T cells [29,30]. In agreement with results from these different cell models, the present study showed that the activation of IL-7R prevented apoptosis, resulting in an increase in the expression of anti-apoptotic bcl-2 and a decrease in the expression of pro-apoptotic bax in lung cancer cells.
Kibe and his colleagues reported that in IL-7Rαnull mice, IL-7R deficiency-induced apoptosis is partially due to an elevated p53 activity and that the expression of bcl-2 decreased [1]. Our p53 inhibition studies demonstrated that IL-7/IL-7R protect A549 and HBE cells from apoptosis and regulate the expression of bcl-2 and bax by activating the p53 pathway. While one study showed that withdrawal of IL-7 from D1 cells (IL-7-dependent cells established from CD4-CD8- cells sorted from p53-/- mouse thymocytes) induced apoptosis and bcl-2, bcl-xl mRNA expression rapidly declined with no relationship to p53 [31]. Another research study reported IL-7 could protect pro-T1, -T2, and -T3 cells from rapid apoptotic death, which correlated with bcl-2 and bax levels and was independent of Fas and p53 pathways [32]. Our results in H1299 cells deficient in p53 showed that there were probably other pathways involved in the apoptotic process mediated by IL-7/IL-7R. In this regard, the interaction of IL-7/IL-7R with the p53 pathway or other pathways may be quite complex. In native and memory CD4+ T cells, IL-7 together with IL-2 could regulate the balance between proliferation and Fas-mediated apoptosis [33]. In addition, IL-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating bcl-2 proteins and activating the JAK/STAT signaling pathway [34]. Therefore, the regulatory mechanisms of IL-7/IL-7R signaling are more various and extensive than previously thought. Despite the necessity of further research on these matters, our study further elucidates the function of IL-7/IL-7R in the regulation apoptosis and survival in lung cancer cells.
In conclusion, this study demonstrates that the activation of IL-7/IL-7R signaling can significantly prevent the apoptosis of lung cancer cells, a process potentially mediated via the p53 pathway. Although there may be other pathways involved in this reaction process, these results may help clarify the mechanisms of cancer cell survival and provide new insight into the roles of IL-7/IL-7R in lung cancer.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30972967) and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20092104110018) and Program for Liaoning Excellent Talents in University.
Disclosure of conflict of interest
None.
References
- 1.Kibe R, Zhang S, Guo D, Marrero L, Tsien F, Rodriguez P, Khan S, Zieske A, Huang J, Li W, Durum SK, Iwakuma T, Cui Y. IL-7Ra deficiency in p53null mice exacerbates thymocyte telomere erosion and lymphomagenesis. Cell Death Differ. 2012;19:1139–51. doi: 10.1038/cdd.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sammicheli S, Dang VP, Ruffin N, Pham HT, Lantto R, Vivar N, Chiodi F, Rethi B. IL-7 Promotes CD95-Induced Apoptosis in B Cells via the IFN-γ/STAT1 Pathway. PLoS One. 2011;12:e28629. doi: 10.1371/journal.pone.0028629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 4.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 5.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;10:749–58. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming J, Zhang Q, Qiu X, Wang E. Interleukin 7/Interleukin 7 Receptor Induce c-Fos/c-Jun-Dependent Vascular Endothelial Growth Factor-D Up-Regulation: A Mechanism of Lymphangiogenesis in Lung Cancer. Eur J Cancer. 2009;45:866–73. doi: 10.1016/j.ejca.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ming J, Guo C, Zhang Q, Qiu X, Wang E. Interleukin 7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer. Cancer Immunol Immunother. 2012;61:79–88. doi: 10.1007/s00262-011-1078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 10.Reed JC. Double identity for protections of the Bcl-2 family. Nature. 1997;387:773–6. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 11.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the Bcl-2 family and cell death. Blood. 1996;2:386–401. [PubMed] [Google Scholar]
- 12.Gibson LF, Piktel D, Narayanan R, Nuñez G, Landreth KS. Stromal cells regulate bcl-2 and bax expression in pro-B cells. Exp Hematol. 1996;24:628–37. [PubMed] [Google Scholar]
- 13.Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–10. [PubMed] [Google Scholar]
- 14.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 15.Wörnle M, Schmid H, Merkle M, Banas B. Effects of chemokines on proliferation and apoptosis of human mesangial cells. BMC Nephrol. 2004;5:8. doi: 10.1186/1471-2369-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei P, Li W, Wang M, Zhu H, Huang B, Huang L, Shen G. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocytederived dendritic cells. Immunobiology. 2008;213:417–26. doi: 10.1016/j.imbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Burmakin M, Shi Y, Hedstrom E, Kogner P, Selivanova G. Dual targeting of wild type and mutant p53 by small molecule RITA results in the inhibition of N-Myc and key survival oncogenes and kills neuroblastoma cells in vivo and in vitro. Clin Cancer Res. 2013;19:5092–103. doi: 10.1158/1078-0432.CCR-12-2211. [DOI] [PubMed] [Google Scholar]
- 18.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;30:1010–14. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 19.Gwinn MR, Leonard SS, Sargent LM, Lowry DT, McKinstry K, Meighan T, Reynolds SH, Kashon M, Castranova V, Vallyathan V. The role of p53 in silica-induced cellular and molecular responses associated with carcinogenesis. J Toxicol Environ Health. 2009;72:1509–19. doi: 10.1080/15287390903129291. [DOI] [PubMed] [Google Scholar]
- 20.Chetoui N, Boisvert M, Gendron S, Aoudjit F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology. 2010;3:418–26. doi: 10.1111/j.1365-2567.2009.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; Mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Shalapour S, Deiser K, Kühl AA, Glauben R, Krug SM, Fischer A, Sercan O, Chappaz S, Bereswill S, Heimesaat MM, Loddenkemper C, Fromm M, Finke D, Hämmerling GJ, Arnold B, Siegmund B, Schüler T. Interleukin-7 links T lymphocyte and intestinal epithelial cell homeostasis. PLoS One. 2012;2:e31939. doi: 10.1371/journal.pone.0031939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ElKassar N, Gress RE. An Overview of IL-7 Biology and Its Use in Immunotherapy. J Immunotoxicol. 2010;1:1–7. doi: 10.3109/15476910903453296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusconi S, Vitiello P, Adorni F, Colella E, Focà E, Capetti A, Meraviglia P, Abeli C, Bonora S, D’Annunzio M, Di Biagio A, Di Pietro M, Butini L, Orofino G, Colafigli M, d'Ettorre G, Francisci D, Parruti G, Soria A, Buonomini AR, Tommasi C, Mosti S, Bai F, Di Nardo Stuppino S, Morosi M, Montano M, Tau P, Merlini E, Marchetti G. Maraviroc as Intensification Strategy in HIV-1 Positive Patients with Deficient Immunological Response: an Italian Randomized Clinical Trial. PLoS One. 2013;11:e80157. doi: 10.1371/journal.pone.0080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82:645–56. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–13. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Chaudhury P, Osmond DG. Regulation of Cell Survival During B Lymphopoiesis: Apoptosis and Bcl-2/Bax Content of Precursor B Cells in Bone Marrow of Mice with Altered Expression of IL-7 and Recombinase-Activating Gene-2. J Immunol. 1999;162:1931–40. [PubMed] [Google Scholar]
- 29.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 30.Maraskovsky E, O’Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. BCL2 can rescue T lymphocyte development in IL-7 receptor deficient mice but not in mutant RAG-/- mice. Cell. 1997;89:1011. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Khaled AR, Reynolds D, Young HA, Lee CK, Durum SK. Characterization of an interleukin-7-dependent thymic cell line derived from a p53-/- mouse. J Immunol Methods. 2003;274:177–84. doi: 10.1016/s0022-1759(02)00513-6. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The Trophic Action of IL-7 on Pro-T Cells: Inhibition of Apoptosis of Pro-T1, -T2, and -T3 Cells Correlates with Bcl-2 and Bax Levels and Is Independent of Fas and p53 Pathways. J Immunol. 1998;160:5735–41. [PubMed] [Google Scholar]
- 33.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of Naive and Memory CD4+ T Cells: IL-2 and IL-7 Differentially Regulate the Balance Between Proliferation and Fas-Mediated Apoptosis. J Immunol. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 34.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]


