Abstract
The Notch signaling pathway is a cell program that is active during early development of multicellular organisms and is required for the formation of basic structures in the growing embryo. Scientific evidence which has accumulated during the last years clearly indicates that aberrant pathway activation may also be critical for the pathogenesis of malignant disease. Despite some limited information the exact role of the Notch signaling pathway in acute myeloid leukemia (AML) remains poorly defined. Immunohistochemical staining of paraffin-embedded bone marrow biopsies from 97 patients with AML, treated between February 1994 and May 2011, for NOTCH-1 was performed according to standardized procedures. Immunological, cytological, pathological, molecular and clinical data were obtained from the hospitals database and patient records. Hyperexpression of NOTCH-1 was seen in 7/97 AML specimens, the other patients showed some expression of NOTCH-1. There was a significant correlation between hyperexpression of NOTCH-1 and the morphological subgroup M0/1 - AML without morphologic maturation (p<0.001). Significant correlation between NOTCH-1 hyperexpression and coexpression of CD7, a phenotypic marker of immaturity (p<0.001) was also seen. Patients with hyperexpression of NOTCH-1 were found to have an inferior overall survival in this retrospective study. Our results indicate that a specific subgroup of AMLs may be associated with hyperexpression of components of the Notch signaling pathway. Better knowledge in pathway signaling in AML could help to identify patient subsets that may benefit from administration of pathway inhibitors and could also contribute to tailored treatment.
Keywords: Acute myeloid leukemia, NOTCH-1, embryonic pathway
Introduction
The Notch signaling pathway is a cell program that is active during early development of multicellular organisms and is required for the formation of basic structures in the growing embryo [1]. Notch signaling requiring direct cell to cell interaction plays a crucial role in precursor cells making binary cell fate decisions [2-4] and regulates boundary formation between cell populations [5]. The signal affects the expression of hundreds of genes and has profound phenotypic consequences. Drosophila possesses only one Notch protein, whereas four Notch paralogues (designated N1-4) have been identified in mammals, playing both unique and redundant roles. Notch family receptors bind to Notch ligands encoded by the Jagged (JAG1, JAG2) and Delta-like gene families (DLL1, DLL3, DLL4) [6].
Notch family receptors are large single-pass type I transmembrane proteins. Notch receptors have a functional extracellular domain, a transmembrane domain, and an intracellular domain (NICD). The extracellular domain contains multiple epid ermal growth factor (EGF)-like repeats followed by a negative regulatory region (NRR) and the intracellular region contains the RAM domain, ankyrin repeats, and a C-terminal PEST domain [7]. Activation of Notch involves intramembrane proteolysis catalyzed by the gamma-secretase (Presenilin) complex and the release of the NICD from the membrane [8]. NICD then translocates to the nucleus, complexes with CSL (CBF1, Suppressor of Hairless, Lag-1), displaces a histone deacetylase (HDAc)-co-repressor (CoR) complex, and recruits components of an activation complex, such as MAML1 and histone acetyltransferases (HAc), resulting in activation of gene transcription [9]. Well established target genes of this transcription activation complex include the Hes and Hey gene family.
Scientific evidence which has accumulated during the last years clearly indicates that aberrant pathway activation may be critical for the pathogenesis of malignant disease [1]. Despite some limited information on Notch signaling in acute myeloid leukemia (AML) from gene expression profiling, immunohistochemical expression of Notch-1 in bone marrow biopsies from AML patients has not been investigated. Therefore, immunohistochemical staining of paraffin-embedded bone marrow biopsies from 97 patients with AML, treated at our institution between February 1994 and May 2011, for NOTCH-1 was performed according to standardized procedures.
Patients and methods
The cohort in this non-interventional retrospective study comprised 97 patients with newly diagnosed AML who were treated at Hospital Hietzing, Vienna, between February 1994 and May 2011 and in whom paraffin-embedded bone marrow samples were available in the Institute of Pathology. In this cohort 47 of the patients were males and 50 were women, the mean age at diagnosis was 64 years (range 20 - 93 years). Immunological, cytological, pathological, molecular and clinical data (including treatment regimens) were obtained from the hospitals database and from patient records. The use of samples and associated data was approved by the Ethics Committee of the Gemeinde Wien.
Information on morphology was available in all patients. AML samples in this retrospective study were classified according to morphology based on criteria defined in the FAB classification [10]. In more recent samples AML with multilinear dysplasia was included as a distinct entity [11]. Figure 1 shows the distribution of morphological subgroups in these patients. Information on karyotype, immunophenotype, and molecular genetics was only available in a subgroup of patients due to various reasons including not testing in very early patients of our series and inaccessibility of data. 54 patients who were fit enough received induction chemotherapy. Remission rate is calculated only from these patients.
Figure 1.
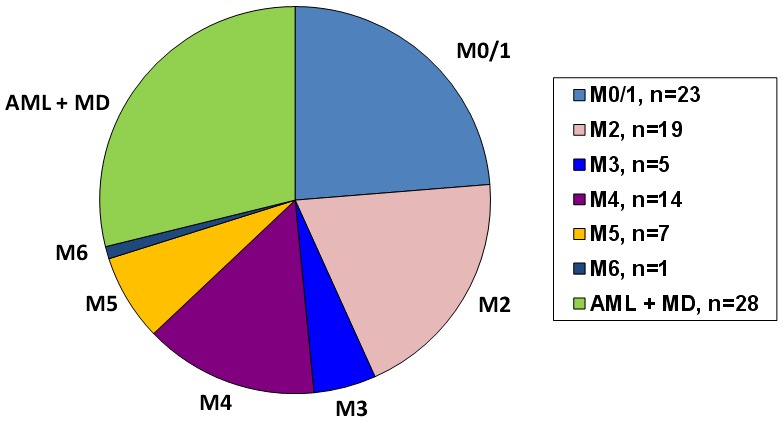
Distribution of the morphological AML subgroups according to the FAB-classification. The morphological subgroup AML with multilineage dysplasia according to the WHO-classification was also included.
In order to look for a potential correlation of NOTCH-1 expression with morphology, immunophenotype, cytogenetics, molecular aberrations and clinical outcome the following parameters were used as far as available. Immunophenotype parameters included CD2, 3, 5, 7, 8, 10, 11B, 13, 14, 15, 16, 19, 20, 30, 34, 36, 38, 41, 56, 61, 64, 71, 79a, 117, 138, TdT, MPO, DR. The molecular characterization of AMLs was performed by multiplex PCR including molecular aberrations such as t(X;11), t(4;11), t(6;11), t(11;19), t(10;11), t(1;11), t(11;17), t(9;11), t(1;19), t(17;19), t(12;21), TAL1, t(8;21), t(3;21), t(16;21), t(15;17), t(6;9), t(9;9), inv(16), t(9;22)(major+minor); t(9;12), t(5;12), t(12;22), t(3;5). As clinical outcome parameters the rate of complete remission (CR) after intensive chemotherapy and overall survival (OS) were used.
Immunohistochemistry
The paraffin-embedded bone marrow biopsies from the patients of interest were collected and sectioned into parts appropriate for immunohistochemical staining. In order to preserve the tissue, prevent autolysis and inhibit the growth of bacteria and molds all samples went through a formaldehyde-based fixation. The old paraffin was liquefied, the samples were covered with new paraffin and after that sliced and mounted on slides. Samples required additional steps to make the epitopes available for antibody binding, including deparaffinization and hydration as well as heat-induced epitope retrieval. Prior to antibody staining, endogenous peroxidase was inhibited by 3% hydrogen peroxide for 30 min.
A monoclonal anti-human NOTCH-1 antibody was used as a primary antibody (EP1238Y, EPITOMICS, Burlingame, California). For detection and visualization of the primary antibody the Dako EnVisionTM detection system was used (DAKO, Glostrup, Denmark). The EnVisionTM FLEX/HRP detection reagent consists of a dextran backbone to which a large number of peroxidase (HRP) molecules and secondary antibody molecules have been coupled. The substrate system consists of two components, the FLEX DAB+ Chromogen, a concentrated diaminobenzidine (DAB) solution and the FLEX Substrate Buffer containing hydrogen peroxide. The substrate system produces a crisp brown end product at the site of target antigen. Finally the slides were counterstained with Hämalaun which provides a clear blue nuclear staining. After mounting stained tissue sections with a mounting medium and coverslips slides were ready for microscopy.
Evaluation of immunohistochemical staining
Two reviewers including an experienced hematopathologist independently scored the expression of NOTCH-1 expression in leukemic cells. NOTCH-1 expression was assessed with regard to staining intensity (0, background; +1, weak; +2, moderate; +3, strong) and localization (cytoplasmatic or intranuclear). NOTCH-1 was considered as hyperexpressed if both reviewers agreed, either initially or following discussion of disagreements that Notch-1 staining was strongly positive (+3). Likewise absent expression was only assumed, if both reviewers agreed and negativity was observed consistently by both reviewers. All other samples that were not categorized as hyperexpressed or negative were considered to show some expression of NOTCH-1.
Statistical analysis
The statistical analyses were performed using SPSS 20.0 software for Windows. Using Kaplan-Meier method the cumulative survival was analyzed and compared by the long-rank test. Chi square test was used for testing significance of differences in proportions.
Results
In normal BM NOTCH-1 was strongly expressed in the cytoplasm of megakaryocytes and endothelial cells (Figure 2). Isolated nuclear expression of NOTCH-1 was not observed in these cells. In all other hematopoietic cells including immature and mature myeloid cells, erythroid cells and lymphoid cells NOTCH-1 was not expressed.
Figure 2.
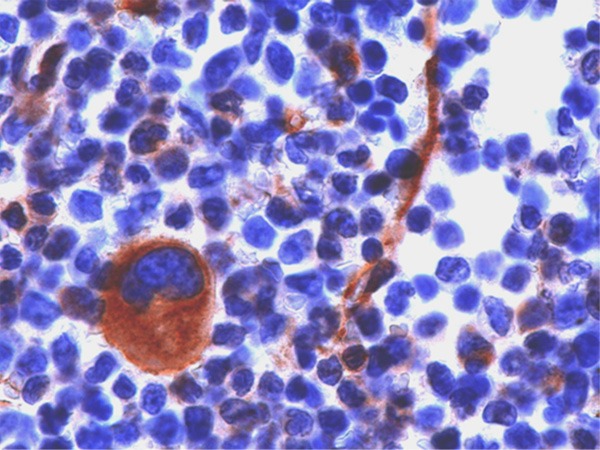
Immunohistochemical staining of normal bone marrow cells showing strong expression of NOTCH-1 in the cytoplasm (brown staining) of megakaryocytes (large cell) and endothelial cells (small spindle like cells).
Hyperexpression of NOTCH-1 was seen in 7/97 AML specimens (Figure 4). One of these patients is shown in Figure 3. In these samples cytoplasmic as well as nuclear expression of this protein was observed. Based on morphological criteria all patients with NOTCH-1 hyperexpression were classified as AML without morphological maturation (M0 or M1). Hyperexpression of NOTCH-1 was also observed in 2/2 patients with T-ALL. These patients served as positive controls, since it is known that in the majority of T-ALLs hyperactivity of the Notch pathway can be demonstrated. There was only 1 AML-patient who was classified as completely NOTCH-1 negative. All other patients (n=89) showed some expression of NOTCH-1 protein.
Figure 4.
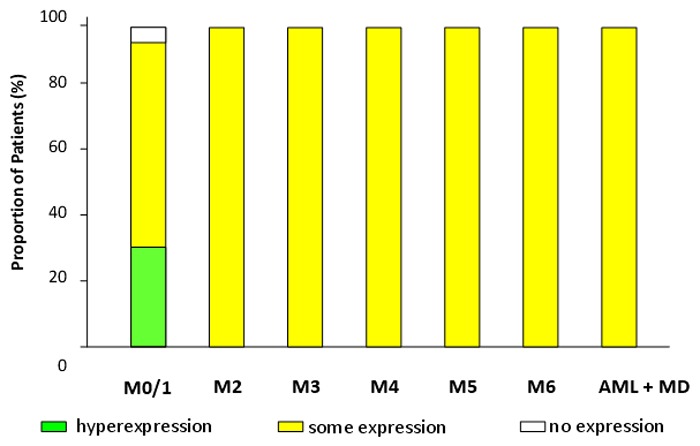
Proportion of patients regarding expression of NOTCH-1 in the morphological AML subgroups (FAB classification) and AML with multilineage dysplasia (WHO classification).
Figure 3.
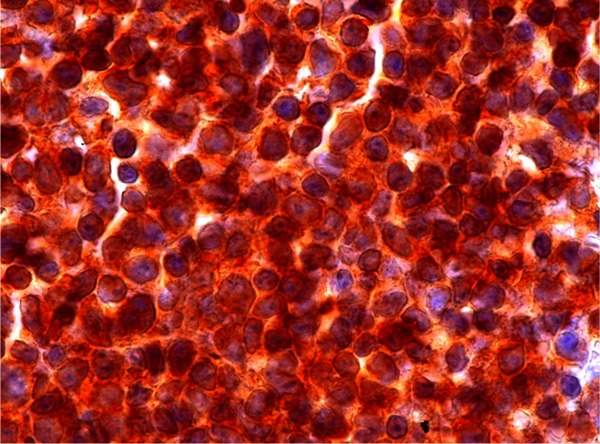
NOTCH-1 immunohistochemical staining of bone marrow cells in a patient with AML M1.
As shown in Figure 5 there was a significant correlation between hyperexpression of NOTCH-1 and the morphological AML subgroup M0/1 (p<0.001 by the Chi square test). All 4 patients with NOTCH-1 hyperexpression and in which immunological data were available showed coexpression of CD7. As shown in Figure 6 there was a significant correlation between NOTCH-1 hyperexpression and coexpression of CD7 (p<0.001 by the Chi square test).
Figure 5.
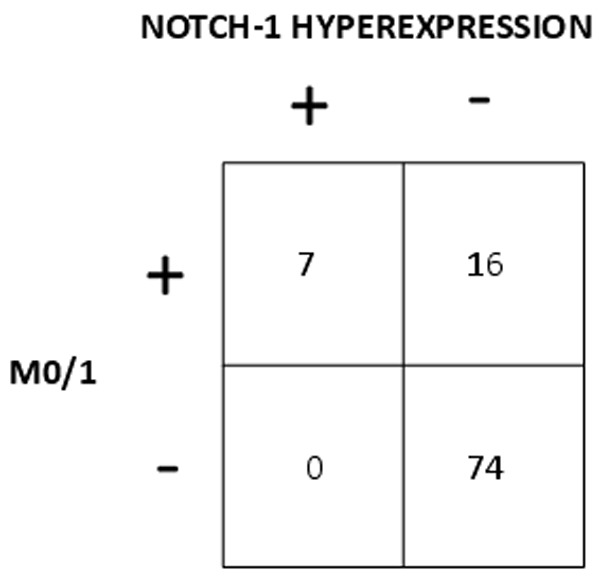
Correlation between hyperexpression of NOTCH-1 and AML without morphological maturation (M0/1).
Figure 6.
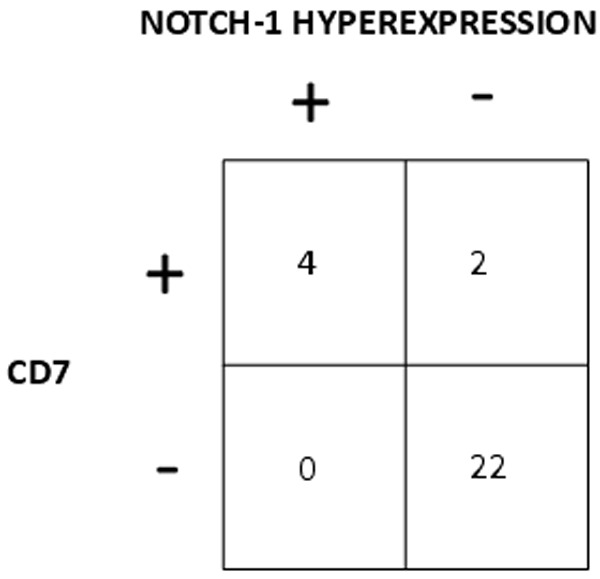
Correlation between hyperexpression of NOTCH-1 and coexpression of CD7.
In order to investigate the prognostic value of NOTCH-1 hyperexpression Kaplan-Meier survival curves were calculated for patients with and without overexpression of NOTCH-1. As shown in Figure 7 patients who had overexpressed NOTCH-1 protein in their blast cells had a significant inferior OS as compared to patients without NOTCH-1 hyperexpression. (p=0.006 by the Log Rank test). The probability of survival after 1 year was 14% for patients with NOTCH-1 hyperexpression and 48% for patients without NOTCH-1 hyperexpression.
Figure 7.
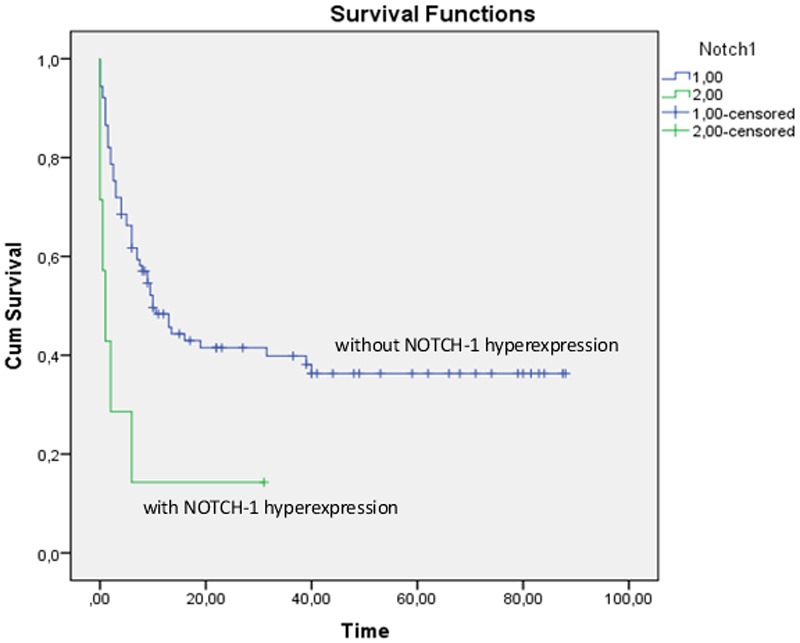
Kaplan-Meier curve showing overall survival of patients with (lower line) and without (upper line) NOTCH-1 hyperexpression. The x-axis shows the time of survival in months, from the day of diagnosis until death/end of study.
Discussion
Adult hematopoietic differentiation is a developmental process that employs many of the same molecular mechanisms as embryogenesis [1]. We explored the possibility that the Notch signaling is involved in the pathogenesis of AML by performing immunohistochemistry staining of NOTCH-1 in bone marrow samples of patients with AML at the time of diagnosis. The demonstration of pathway hyperactivation could help to identify patient subsets that may benefit from the administration of specific pathway inhibitors in future. Moreover, by correlating signal pathway component expression with immunological parameters and clinical parameters we hoped to find potential biomarkers of pathway expression and to get also some prognostic information associated with pathway signaling.
In normal bone marrow we observed significant NOTCH-1 expression in megakaryocytes and endothelial cells whereas the other bone marrow cells were negative for this staining. The positive staining of both cell types is not unexpected since Notch signaling has been implicated in megakaryopoiesis and vasculogenesis. The impact of Notch signaling on megakaryocyte development was confirmed by a study using heterotypic cocultures of hematopoietic cells with OP9 cells, a line of bone marrow-derived mouse stromal cells, expressing Delta-like 1 [12] as well as by coculturing human CD34+ cells with Dll-4-expressing stroma cells [13]. The role of the Notch pathway in vasculogenesis has been demonstrated by studies showing that Notch1 -/- mice exhibit defects in angiogenic vascular remodeling [14,15].
The potential role of Notch in human malignancy has been discovered for the first time by the report of a translocation t(7;9) in a case of T-ALL by Ellisen [16]. Following this initial observation screening more patients with T-ALL with regard to molecular aberrations revealed in more than 50% of human T-ALLs activating mutations that involve the extracellular heterodimerization domain of NOTCH-1 [17]. Therefore leukemic cells from patients T-ALL were used as positive controls in our study and were helpful to validate our immunohistochemical staining in AML. Both patients with T-ALL were strongly stained with the NOTCH-1 antibody in our study (data not shown).
Our major findings was that although some expression of NOTCH-1 seems to be a common finding in AML hyperexpression of the NOTCH-1 was relatively rare and restricted to AMLs without morphological maturation. In fact most of the AMLs showed some expression of NOTCH-1 but strongly positive staining of NOTCH-1 was seen in only 7/97 AML specimens.
There is limited information on the role of the Notch signaling pathway in patients with AML. By performing gene expression profiling in a large number of AMLs Wouters [18] demonstrated Notch-1 gene hyperexpression in several specimens of an AML subgroup characterized by a gene signature resembling that of AMLs with mutations in CEBPA, while lacking such mutations. These leukemias phenotypically showed aberrant expression of T cell genes, of which CD7 was most consistent. It could be shown, that in these leukemias, the CEBPA gene was silenced, which was associated with frequent promoter hypermethylation. Genome-wide epigenetic analysis also delineated a biologically distinct immature acute leukemia with myeloid/T-lymphoid features [19]. Based on their DNA methylation profiles AML patients with silenced CEBPA could be readily segregated from patients harbouring mutations of the CEBPA, since aberrant DNA hypermethylation signature was present in the CEBPA silenced but not in the CEBPA mutant group. Due to the fact that gene expression profiling and epigenetic analyses were not available in our study we have no detailed information on the genetic profile of our patients who showed NOTCH-1 hyperexpression by immunohistochemistry. Therefore we cannot directly answer the question if and how many patients with NOTCH-1 hyperexpression belonged to the CEBPA silenced AML subgroup.
Although it needs to be confirmed that patients with NOTCH-1 hyperexpression by immunohistochemistry reflects CEBPA silencing we think that there is at least some indirect evidence that NOTCH-1 hyperexpression in our study may identify patients with functional CEBPA deficiency. Data in the literature clearly provide evidence for the involvement of CEBPA, the myeloid master regulator in hematopoietic cells, in the active repression of several of the T-cell associated genes. Thus absence of CEBPA led to upregulation of specific T-cell transcripts (ie CD7) in hematopoietic stem cells isolated from conditional CEBPA knockout mice [18]. CD7 expression has been demonstrated to be a common finding in immature AML [19]. Moreover, CD7 expression was a consistent finding in the patients with CEBPA silencing as described by Wouters. Analysis of available diagnostic flow cytometry data in our AML patients with NOTCH-1 hyperexpression revealed myeloid marker expression in combination with CD7 in all 4 cases which showed a significant correlation by the Chi square test. Moreover, an immature phenotype has been also reported by Figueroa in this patient group [20]. In our study NOTCH-1 hyperexpression was highly correlated with leukemia cell morphology lacking maturation. Thus, our findings of a significant correlation of NOTCH-1 hyperexpression with the immature morphological phenotype as well with the coexpression of immunological T cell markers strongly suggests, that hyperexpression of NOTCH-1 in AML seems to mirror functional deficiency of CEBPA.
Another questions, which we wanted to address in our study was to look for a potential prognostic role of embryonic pathway signaling in AML. Interestingly, NOTCH-1 hyperexpression was associated with inferior outcome in the AML patients included in this retrospective study. Of course this retrospective data need to be confirmed and validated by further studies. If the negative prognostic value of NOTCH-1 hyperexpression holds true our preliminary observation may help to extend the list of prognostic factors that are used for treatment decisions in AML.
Considering the poor outcome of patients with NOTCH-1 hyperexpression new treatment modalities are highly desired. A hyperactivated Notch signaling pathway may represent a new treatment target for these patients. In T-ALL patients with increased Notch signaling due to activating Notch-1 mutations gamma secretase inhibitors have shown encouraging effects in preclinical models and are currently tested in clinical trials [21]. Our results show that AML patients with increased Notch signaling may also be another interesting patient group to look at. Moreover, demethylating drugs such as azacytidine [22] and decitabine [23] may be particularly interesting in this leukemia subgroup, if the patients with NOTCH-1 hyperexpression by immunohistochemistry can really identify patients with a hypermethylation profile which is regularly seen in CEBPA silenced patients.
We are aware of the limitations of our study. This study was retrospective in nature and information on karyotype, immunophenotype, and molecular genetics was only available in a subgroup of patients due to various reasons including not testing in very early patients of our series and inaccessibility of data. Moreover a large number of patients, particularly in the early series did not receive intensive chemotherapy due to advanced age and/or significant comorbidities. This may have impact on outcome data but we think that these limitations do not alter our main conclusions.
In conclusion our data indicate that a specific subgroup of AML may be associated with hyperexpression of embryonic signaling pathways components as we have shown for NOTCH-1 in this study. Better knowledge in pathway signaling in AML could help to identify patient subsets who may benefit from the administration of pathway inhibitors and could also contribute to tailored treatment, if pathway signaling turns out to be of prognostic value in prospective trials.
Acknowledgements
This work was supported by a Grant from the Österreichische Gesellschaft für Hämatologie und Onkologie.
Disclosure of conflict of interest
None.
References
- 1.Geissler K, Zach O. Pathways involved in Drosophila and human cancer development: the Notch, Hedgehog, Wingless, Runt, and Trithorax pathway. Ann Hematol. 2012;91:645–669. doi: 10.1007/s00277-012-1435-0. doi: 10.1007/s00277-012-1435-0. [DOI] [PubMed] [Google Scholar]
- 2.Seydoux G, Greenwald I. Cell autonomy of lin-12 function in a cell fate decision in C. elegans. Cell. 1989;57:1237–1245. doi: 10.1016/0092-8674(89)90060-3. [DOI] [PubMed] [Google Scholar]
- 3.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 4.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 5.Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowitz D, Lewis J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 2000;408:475–479. doi: 10.1038/35044091. [DOI] [PubMed] [Google Scholar]
- 6.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das I, Craig C, Funahashi Y, Jung KM, Kim TW, Byers R, Weng AP, Kutok JL, Aster JC, Kitajewski J. Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J Biol Chem. 2004;279:30771–30780. doi: 10.1074/jbc.M309252200. [DOI] [PubMed] [Google Scholar]
- 8.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 9.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 11.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 12.Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, Maillard I, Pear WS, Aster JC, Gilliland DG. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3:314–26. doi: 10.1016/j.stem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirault-Chassac S, Six E, Catelain C, Lavergne M, Villeval JL, Vainchenker W, Lauret E. Notch/Delta4 signaling inhibits human megakaryocytic terminal differentiation. Blood. 2010;116:5670–8. doi: 10.1182/blood-2010-05-285957. doi: 10.1182/blood-2010-05-285957. [DOI] [PubMed] [Google Scholar]
- 14.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 15.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 16.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 17.Weng AP, Ferrando AA, Lee W, Morris JP 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 18.Wouters BJ, Jordà MA, Keeshan K, Louwers I, Erpelinck-Verschueren CA, Tielemans D, Langerak AW, He Y, Yashiro-Ohtani Y, Zhang P, Hetherington CJ, Verhaak RG, Valk PJ, Löwenberg B, Tenen DG, Pear WS, Delwel R. Distinct gene expression profiles of acute myeloid/T-lymphoid leukemia with silenced CEBPA and mutations in NOTCH1. Blood. 2007;110:3706–3714. doi: 10.1182/blood-2007-02-073486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo Coco F, De Rossi G, Pasqualetti D, Lopez M, Diverio D, Latagliata R, Fenu S, Mandelli F. CD7 positive acute myeloid leukaemia: a subtype associated with cell immaturity. Br J Haematol. 1989;73:480–485. doi: 10.1111/j.1365-2141.1989.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa ME, Wouters BJ, Skrabanek L, Glass J, Li Y, Erpelinck-Verschueren CA, Langerak AW, Löwenberg B, Fazzari M, Greally JM, Valk PJ, Melnick A, Delwel R. Genome-wide epigenetic analysis delineates a biologically distinct immature acute leukemia with myeloid/T-lymphoid features. Blood. 2009;113:2795–804. doi: 10.1182/blood-2008-08-172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 22.Pleyer L, Stauder R, Burgstaller S, Schreder M, Tinchon C, Pfeilstocker M, Steinkirchner S, Melchardt T, Mitrovic M, Girschikofsky M, Lang A, Krippl P, Sliwa T, Egle A, Linkesch W, Voskova D, Angermann H, Greil R. Azacitidine in patients with WHO-defined AML - results of 155 patients from the Austrian Azacitidine Registry of the AGMT-Study Group. J Hematol Oncol. 2013;6:32. doi: 10.1186/1756-8722-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysák D, Minden M, Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30:2670–7. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]


