Abstract
Granzyme B and perforin, two of the most important components, have shown anticancer properties in various cancers, but their effects in laryngeal cancer remain unexplored. Here we decided to examine the effects of Granzyme B and perforin in Hep-2 cells and clarify the role of perforin and granzyme B in the tumorigenicity of laryngeal cancer cell line. Hep-2 cells were transfected with pVAX1-PIG co-expression vector (comprising perforin and granzyme B genes), and then the growth and apoptosis of these Hep-2 cells were evaluated. The tumorigenicity of Hep-2 cell line co-expressing perforin and granzyme B genes was tested in BALB/c nu/nu mice. We found that the co-expression of perforin and granzyme B genes could obviously inhibit cell focus formation and induce cell apoptosis in Hep-2 cells. Furthermore, after subcutaneous injection of Hep-2 cells transfected with pVAX1-PIG, an extensive delay in tumor growth was observed in BALB/c-nu/nu mice. Moreover, our studies demonstrated that the anticancer activity of perforin and granzyme B was sustainable in vivo as tumor development by inducing cell apoptosis. Taken together, our data indicate that the co-expression of perforin and granzyme B genes exhibits anticancer potential, and hopefully provide potential therapeutic applications in laryngeal cancer.
Keywords: Perforin, granzyme B, Hep-2 cells, cancer gene therapy, apoptosis
Introduction
Many studies have established that cell-mediated innate and adaptive immunity are essential for preventing primary tumor outgrowth and for rejecting transplanted tumors [1,2]. Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells play an important role in tumor control [3-5]. There are two pathways (cell death receptor-mediated pathway and granule exocytosis pathway) by what CTLs and NK cells affect cytotoxicity against the target cells. Of the two pathways, the granule exocytosis pathway is the principal pathway and involves granzyme B and perforin, which are two of the most important components exerting cytotoxicity against target cells [6-10].
Perforin release is stimulated upon contact and polymerizes into the target cell membrane forming pores which are utilized by granzyme to enter the cytosol of the target cell [6,9,11,12]. Granzymes are serine proteases that are capable of cleaving proteins on the carboxy side of aspartate residues. Granzyme B mediates a rapid induction of the target cell apoptosis, whereas other members of the family elicit a delayed response. These cytotoxic molecules are produced constitutively in CTLs and NK cells. Perforin-deficient mice are more susceptible to the development of spontaneous lymphomas, suggesting that the perforin-granzyme pathway is an important component of tumor surveillance [13-15].
In this study, we investigate the anticancer potential of perforin and granzyme B genes co-expression on Hep-2 cell proliferation, apoptosis as well as tumorigenesis. In order to explore the effects of perforin and granzyme B on cell proliferation and apoptosis in vitro, the pVAX1-PIG vector comprising perforin and granzyme B genes was transfected into Hep-2 cells. Vector cassette transfected and parental Hep-2 cells served as controls. Furthermore, we examined the roles of perforin and granzyme B for tumor clearance in BALB/c nu/nu mice by subcutaneous injection of three kinds of Hep-2 cell lines respectively. This is the first study to report the inhibitory potential of perforin and granzyme B co-expression on the tumorigenicity of Hep-2 cell line.
Materials and methods
Reagents and materials
Recombinant plasmid pVAX1-PIG co-expression vector (comprising perforin and granzyme B genes) was constructed and stored in our laboratory. The pVAX1 vector was purchased from Invitrogen (USA). The granzyme B goat polyclonal antibody was purchased from Santa Cruz Biotechnology (USA). The biotinylated rabbit anti-goat Ig G antibody was obtained from Boster (China). The human laryngeal carcinoma Hep-2 cell line was purchased from Shanghai Institute of Cellular Biology of Chinese Academy of Sciences (China). All cells were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 100 U of penicillin-streptomycin per ml, and 10% calf serum (Invitrogen Life Technologies, USA). Athymic BALB/c nu/nu mice were obtained from the Animal Laboratory of Sun Yat-Sen University (China).
Focus formation assay
In a 6-well plate, a bottom feeder layer (0.6% agar) was prepared with RPMI 1640 media containing 20% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. A top layer (0.25% agar) was prepared with RPMI 1640 and the same media as described above but containing 1 × 105 Hep-2 cells. The pVAX1-PIG transfected Hep-2 cells were used for studies. The pVAX1-PIG vector which is the recombinant eukaryotic co-expression vector comprising perforin and granzyme B genes that our group constructed previously and the construction of pVAX1-PIG vector is described in detail elsewhere. Vector transfected and parental Hep-2 cells were used as controls. Fresh medium was then added to each well and the cells incubated at 37°C and 5% CO2 atmosphere for 3 weeks. The medium was replaced once in two days. Cells were viewed regularly under the microscope for colony formation [16,17]. Focus formation was visually detected by observing dense foci of intensive cell growth, consisting of refractive cells that rounded up and piled on top of each other. Colonies, containing at least 50 cells, were counted. The assay was repeated a minimum of three times (with triplicates for each group) and standard error of the mean was determined.
Hoechst 33342 staining to assess cell apoptosis
Controlled treatment of cells with the membrane-permeable dye Hoechst 33342 has been shown to effectively stain apoptotic nuclei and detect chromatin condensation. Parental Hep-2 cells, Hep-2 cells transfected with pVAX1 or transfected with pVAX1-PIG were cultured on poly-L-lysine-coated coverslips, washed twice with PBS, and fixed with PBS containing 4% glutaldehyde for 30 min. The cells were fixed and permeabilized and then assessed for chromatin condensation and disintegration of the nucleus by Hoechst staining. A 5 μg/ml concentration of Hoechst 33342 was used to label the cells for 30 min, following which the dye was washed out with PBS and then pictures shot by an Olympus fluorescence microscope (Olympus, Japan). The apoptotic cells were identified by their disintegrated nuclear structure and counted to assess the proportion of healthy and apoptotic cells in multiple fields.
Flow cytometric sorting and analysis
Parental Hep-2 cells, Hep-2 cells transfected with pVAX1 or transfected with pVAX1-PIG were trypsinized and collected. For flow cytometry analysis after propidium iodide staining, cells were resuspended in PBS containing 3 mM sodium citrate, 0.1% Triton X-100, and 50 μg/ml propidium iodide and incubated for 2-4 h. The propidium iodide-stained cells were subjected to flow cytometric analysis on a flow cytometer (BD Biosciences, USA).
Tumorigenicity assay in nude mice
The tumorigenicity of Hep-2 cell line co-expressing perforin and granzyme B genes was tested in BALB/c nu/nu mice (5-7 weeks old). Athymic BALB/c-nu/nu mice were housed in a specific pathogen-free rodent facility under a standard 12 h light/12 h dark cycle, and were fed a standard rodent chow and water ad libitum. The control animals were treated with pVAX1 transfected Hep-2 cell line or parental Hep-2 cell line. In all cases 5 × 106 cells were inoculated subcutaneously into the right flank of BALB/c-nu/nu mice (n = 6 per group). The mice were monitored every 2 or 3 days for the appearance of tumors, and tumor volume was measured on days 7, 10, 13, 16, 19, 22, 25, and 28 post inoculation. Tumor sizes were evaluated by tumor volume (0.5 × length × width2). Four weeks after inoculation, the mice were sacrificed. The tumors were removed and weighed [18,19]. Data were pooled from the three experiments and reported. The inhibitory rate of tumor was calculated by the following formula: inhibitory rate = (tumor weight of control group - tumor weight of treatment group)/tumor weight of control group × 100%. The tumor tissues were fixed with formalin for pathology analysis. All animal procedures were approved by the Animal Ethics Committee of Guangzhou Medical University.
Histology and immunohistochemistry analysis
Tumor specimens were harvested and fixed in 10% buffered formalin for 24 h, and then transferred to 70% ethanol. For paraffin sections, tumors were dehydrated in graded ethanol and histoclear and embedded. The specimens were subsequently paraffin-embedded and cut into 5 μm-thick sections. Briefly, sections were dewaxed in xylene, rehydrated and stained with hematoxylin and eosin (Sigma, USA), or used for immunohistochemistry.
For immunohistochemical detection, sections were microwaved in antigen retrieval solution for 30 min, blocked with biotin, avidin and 5% normal goat serum. Sections were incubated with a granzyme B goat polyclonal antibody (1:1000) followed by a biotinylated rabbit anti-goat Ig G antibody (1:500). Positive cells were visualized by a streptavidin-HRP color reaction with DAB (Sigma, USA). Sections were counter-stained with hematoxylin [20].
Morphology analysis by transmission electron microscopy
For transmission electron microscopy analysis, tumor specimens were cut into small pieces (about 1 × 1 × 1 mm), and fixed in a cacodylate buffer (0.1 M) containing 3% glutaraldehyde and 2% paraformaldehyde for 1 h, washed, and treated with 0.1% tannic acid for 20 min. The samples were then incubated in 1% buffered osmium tetroxide for 1 h and stained with 1% aqueous uranyl acetate for 1 h. The samples were dehydrated in increasing concentrations of ethanol [(50-70-90-100-100-100)% (v/v); 10 min/step], and then infiltrated and embedded in Spurr’s low viscosity medium. The blocks were polymerized in a 60°C oven overnight. Thin sections were cut, stained with uranyl acetate and lead citrate, and examined using a Jeol 1200-EX transmission electron microscope (Jeol, USA).
Statistics
All data were analyzed by SPSS12.0 statistical software. Experimental data were expressed as the mean ± standard deviation (SD). Student’s t-test was used for statistical analysis. A value of P < 0.05 was regarded as statistically significant.
Results
Inhibition of focus formation by perforin and granzyme B genes co-expression
In order to monitor the effect of perforin and granzyme B on tumor formation, we investigated focus formation by Hep-2 cell line as an index of a neoplastic phenotype. Focus formation was observed as dense foci of intensive cell growth in culture, consisting of refractive cells that rounded up and piled on top of each other. Three Hep-2 cell lines were used in this study: pVAX1-PIG transfected, vector cassette transfected, and parental Hep-2 cell line. For each cell line, 1 × 105 cells/well was seeded and grown to confluence. Focus formation was examined after 3 weeks. The results of this study showed a drastic reduction in focus formation by Hep-2 cells co-expressing perforin and granzyme B (Student’s t-test, P < 0.05). The number of foci was 5 ± 2.4 (mean ± SD) in co-expressing perforin and granzyme B Hep-2 cell line, 26 ± 4.2 in parental Hep-2 cell line, and 25 ± 2.8 in vector cassette transfected Hep-2 cell line, respectively (Table 1). The results shown in Table 1 suggest that perforin and granzyme B may exhibit anti-tumor activity in vitro.
Table 1.
Inhibition of focus formation by Hep-2 cell line co-expressing perforin and granzyme B
| Cell line | No. of foci/6 cm dish | P value |
|---|---|---|
| Parental Hep-2 | 26 ± 4.2 | |
| pVAX1 transfected Hep-2 | 25 ± 2.8 | 0.656a |
| pVAX1-PIG transfected Hep-2 | 5 ± 2.4 | 0.0005b |
Student’s t-test (pVAX1 transfected Hep-2 vs Parental Hep-2).
Student’s t-test (pVAX1-PIG transfected Hep-2 vs Parental Hep-2).
Effects of perforin and granzyme B co-expression in Hep-2 cells
After staining with Hoechst 33342, the typical apoptotic change such as nuclear fragmentation in the cells transfected with pVAX1-PIG plasmid was found (Figure 1A) and the number of such apoptotic cells was significantly greater than that of the cells in control groups, as shown by Hoechst 33342 staining at 72 h after the transfection. Thus, the average apoptosis cells was significantly higher in cells transfected with the pVAX1-PIG plasmid compared to control groups (Student’s t-test, P < 0.01, Figure 1B).
Figure 1.
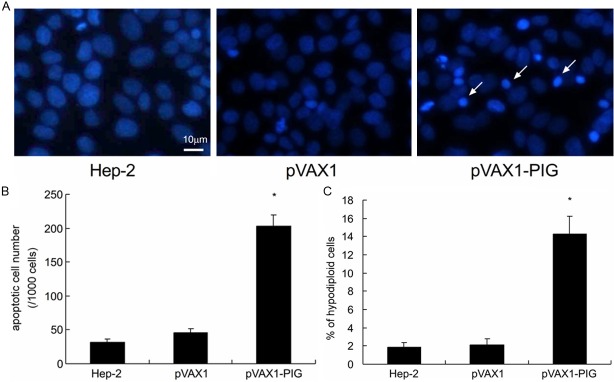
Cell apoptosis analysis in Hep-2 cell lines. A: After staining with Hoechst 33342, the typical apoptotic change in the cells transfected with pVAX1-PIG plasmid was found. Hep-2 cell line transfected with pVAX1 plasmid and parental Hep-2 cell line served as controls. Fragmented nuclei stained with Hoechst 33342 (arrows) indicated apoptotic cells (× 400). B: The number of apoptotic cells in pVAX1-PIG plasmid transfected cells was significantly greater than that of the control cells (P < 0.01). C: Cells were fixed and stained with propidium iodide and analyzed by flow cytometry. Compared to cells transfected with the pVAX1 plasmid (2.1%) and parental Hep-2 cells (1.9%), 14.5% of Hep-2 cells transfected with the pVAX1-PIG plasmid had undergone apoptosis. The percentage of cells with hypodiploid DNA content was higher in pVAX1-PIG transfected cells than in control cells (P < 0.05). The data are presented as mean ± SD of three independent experiments.
In order to confirm this observation, Hep-2 cells were evaluated by flow cytometry. As shown in Figure 1C, it is a summary of at least three independent flow cytometry analyses. Compared to cells transfected with the pVAX1 plasmid (2.1%) and parental Hep-2 cells (1.9%), 14.5% of Hep-2 cells transfected with the pVAX1-PIG plasmid had undergone apoptosis. As the result, pVAX1-PIG transfected cells showed a higher percentage of hypodiploid cells than the control cells (Student’s t-test, P < 0.05). These results suggest that perforin and granzyme B co-expression in Hep-2 cells leads to an inhibition of cell growth.
Co-expression of perforin and granzyme B inhibits tumorigenicity of Hep-2 cell line in athymic nude mice
In order to determine whether perforin and granzyme B co-expression inhibits the tumorigenicity of Hep-2 cell line in vivo, we inoculated 5 × 106 Hep-2 cells (pVAX1-PIG plasmid transfected cells as test, parental Hep-2 cell line and pVAX1 vector transfected cells as controls) subcutaneously into the right flank of BALB/c-nu/nu mice. Animals were examined for tumor formation on days 7, 10, 13, 16, 19, 22, 25, and 28 after inoculation. Our results showed that tumor formation was inhibited in mice that were inoculated with Hep-2 cell line co-expressing perforin and granzyme B (Figure 2 and Table 2). The control animals that were inoculated with parental Hep-2 cell line and pVAX1 vector transfected cells developed tumors, and tumor size increased progressively with time as shown in Figure 2. Statistical analysis, by Student’s t-test, demonstrated that tumor volume in test and control animals were significantly different (Student’s t-test, P < 0.01). The average tumor weight of test group was 164.4 ± 24.4 mg. In contrast, this weight was 499.8 ± 44.5 mg in the pVAX1 vector group and 518.1 ± 55.3 mg in the parental Hep-2 cell line group, respectively. A comparison of tumor weight between test and control mice by Student’s t-test, further showed that tumor weight were significantly different (P < 0.01, Table 2). The inhibitory rate of tumor was 68.3%. These findings demonstrate that perforin and granzyme B significantly inhibited the tumorigenicity of Hep-2 cell line in athymic nude mice, and corroborate our data from focus formation assay (Table 1).
Figure 2.
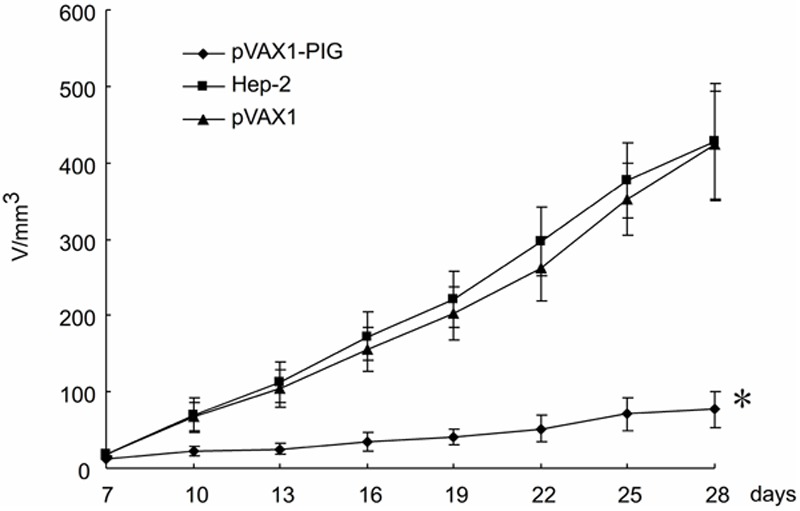
Tumorigenicity of Hep-2 cell lines in nude mice. The tumorigenicity of Hep-2 cell line co-expression of perforin and granzyme B was tested in BALB/c nu/nu mice. The control animals were treated with pVAX1 transfected Hep-2 cell line or parental Hep-2 cell line. Confluent Hep-2 cells (5 × 106 cells/100 μl) were inoculated subcutaneously into the right flank of mice, and monitored for tumor production (n = 6 per group). Tumor size was measured on days 7, 10, 13, 16, 19, 22, 25, and 28 post inoculation. Data from three animal experiments were pooled. The average tumor volume in cubic centimeters was plotted against time in days. The error bars represent standard deviation. Notice the significant reduction in tumor size in animals inoculated with co-expression of perforin and granzyme B recombinant Hep-2 cell line. A repeated measures analysis of variance demonstrated a significant difference in tumor volume between test and control animals (P < 0.01).
Table 2.
The effect of perforin and granzyme B co-expression on tumor weight and inhibition rate in mice (mean ± SD)
| Cell line | Tumor weight (mg) | Tumor inhibitory rate (%) | P value |
|---|---|---|---|
| parental Hep-2 | 518.1 ± 55.3 | ||
| pVAX1 transfected Hep-2 | 499.8 ± 44.5 | 0.18a | |
| pVAX1-PIG transfected Hep-2 | 164.4 ± 24.4 | 68.3 | 0.000b |
Student’s t-test (pVAX1 transfected Hep-2 vs Parental Hep-2).
Student’s t-test (pVAX1-PIG transfected Hep-2 vs Parental Hep-2).
Perforin and granzyme B co-expression inhibits tumor growth in nude mice
The tumorigenicity of Hep-2 cell line co-expressing perforin and granzyme B was tested in BALB/c-nu/nu mice. After 28 days of inoculation, mice were killed and necropsied, and tumors were harvested and processed for routine histology and immunohistochemistry analyses of cell proliferation and apoptosis using anti-granzyme B antibodies.
The histologic and immunohistochemical staining analyses of tumors treated with pVAX1-PIG showed a progressive accumulation of dying cells with clear apoptotic features, such as cell shrinkage and nuclear fragmentation. Only a few apoptotic cells, however, were found in control groups. In contrast, the tumor cells of control mice showed elongated nuclei and plump cytoplasm with cellular atypia and frequent mitotic figures (Figure 3A). Abundant granzyme B-positive cells were detected by light microscopic analysis as brown stained cells with condensed or fragmented nuclei in mice tumors treated with pVAX1-PIG (Figure 3B, data not shown). As the result, it indicated that the co-expression of perforin and granzyme B actively inhibited cells proliferation and tumor growth.
Figure 3.
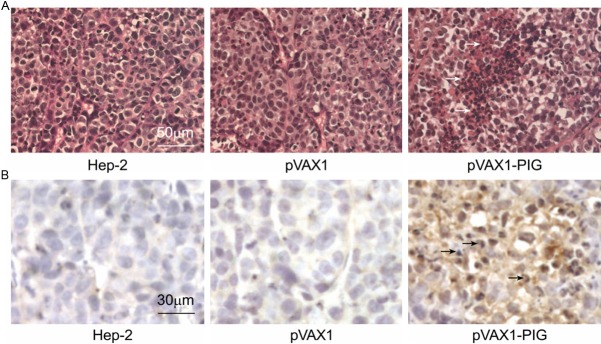
Histologic and immunohistochemical staining analyses. A: The tumorigenicity of Hep-2 cell line co-expressing perforin and granzyme B was tested in BALB/c nu/nu mice. Histologic analysis revealed that tumors treated with pVAX1-PIG contained large areas filled with dying cells with clear apoptotic features. Only a few apoptotic cells, however, were found in the control groups. Representative apoptotic cells were indicated by arrows and are presented at 400 × magnification. B: Histological sections from the same tumors were immunostained using an antibody recognizing granzyme B and are presented at 400 × magnification. Abundant granzyme B-positive cells were detected by light microscopic analysis as brown stained cells with condensed or fragmented nuclei in mice tumors treated with pVAX1-PIG. Representative granzyme B-positive cells are indicated by arrows. As the result, it further indicated that perforin and granzyme B was expressed efficiently in the tumors treated with pVAX1-PIG and inhibited tumor growth.
Perforin and granzyme B co-expression induces apoptosis in nude mice
To further confirm that perforin and granzyme B co-expression inhibits tumor growth by inducing apoptosis in nude mice, we analyzed the cellular morphology of tumor specimens. Transmission electron microscopy analysis of tumor specimens treated with pVAX1-PIG showed typical apoptotic morphology with plasma membrane blebbing, chromatin condensation, and cytoplasmic vascularization. In addition, nuclear changes consistent with apoptosis, such as condensation and segregation of chromatin into compact masses aligning with the inner side of the nuclear membrane were also apparent. In contrast, control group treated with pVAX1 did not show these changes (Figure 4).
Figure 4.
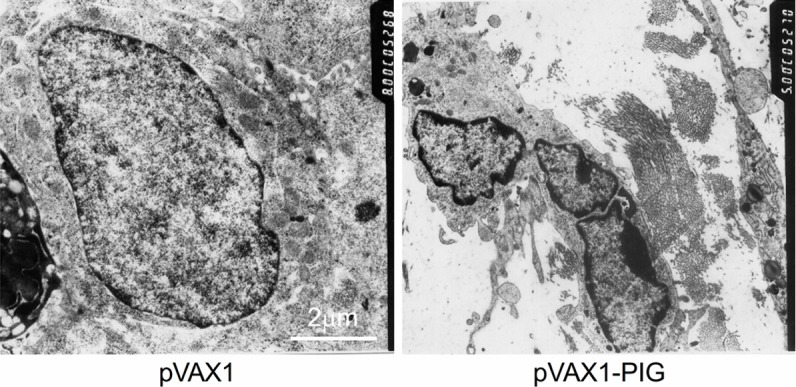
Apoptosis analysis by transmission electron microscopy. The morphological changes were observed by TEM in vivo: tumor specimens treated with pVAX1-PIG showed typical apoptotic morphology with plasma and nuclear membrane blebbing, chromatin condensation, and cytoplasmic vacuolization. In contrast, control group treated with pVAX1 did not show these changes (× 8000).
Discussion
Conventional chemotherapy is relatively ineffective in the treatment of patients with laryngeal carcinoma tissue, and many patients die of this disease each year [21]. Current treatments often have far reaching negative side effects. The systemic toxicity of chemotherapy regimens, while not as severe as they once were, still often result in acute and delayed nausea, mouth ulcerations and mild cognitive impairments. Therefore, new therapy options are called for in order to alleviate the death and suffering caused by cancers. The emerging field of cancer gene therapy offers a number of exciting potential treatments. The term gene therapy encompasses a wide range of treatment types that all use genetic material to modify cells (either in vitro or in vivo) to help effect a cure [22]. Numerous in vitro and preclinical animal models, testing a wide variety of gene therapy agents, have shown remarkable efficacy [23,24].
Since it was proposed in the 1980s, the granule exocytosis mechanism for lymphocyte cytotoxicity has become well accepted as a major immune effector mechanism. Its key elements are the regulated receptor-triggered secretory pathway, which delivers mediators to the target cell, critical mediators including perforin and the granzymes in the target cell [25,26]. Granzyme B-induced apoptosis is dependent on the presence of perforin because granzyme B cannot initiate binding to lipid cell membranes, and granzyme B internalization into target cells requires perforin-mediated membrane pore formation [27]. In this study, we found that perforin and granzyme B co-expression could inhibit Hep-2 cells proliferation.
The impetus for study on anti-tumor activity of perforin and granzyme B in Hep-2 cells was given by our previous findings that their co-expression suppressed cancer cell proliferation in vitro. Thus previous findings suggest that perforin and granzyme B co-expression is not toxic to cells, and we speculate that the same would be true in this study. In the current investigation, we examined the effect of co-expression of perforin and granzyme B on focus formation, a malignant phenotype, by recombinant Hep-2 cell line. Widespread and large foci were formed by control Hep-2 cell lines in contrast to background foci formed by Hep-2 cells transfected with pVAX1-PIG vector. Focus formation by Hep-2 cell line co-expressing perforin and granzyme B was inhibited drastically in comparison to that of control parental and vector transfected cell lines (Table 1).
How does perforin and granzyme B inhibit Hep-2 cells growth? As shown in Figure 1A and 1B, widespread and typical apoptotic change of Hep-2 cells transfected with pVAX1-PIG plasmid were found in contrast to control Hep-2 cells. For the inhibition of cell growth, pVAX1-PIG transfected cells showed a higher percentage of hypodiploid cells than the control cells (Figure 1C). These results suggest that perforin and granzyme B co-expression leads to an inhibition of cell growth by inducing Hep-2 cells apoptosis.
Furthermore, our studies demonstrated that the anti-tumor activity of perforin and granzyme B co-expression was sustainable in vivo as tumor development was significantly inhibited in mice that were treated with perforin and granzyme B recombinant Hep-2 cell line (Figure 2, P < 0.01). The subcutaneous tumor from Hep-2 cell line co-expressing perforin and granzyme B was significantly smaller compared with control animals (Figure 2 and Table 2). As shown in Figure 3A and 3B, the histologic analyses revealed that lots of brown stained cells with condensed or fragmented nuclei in mice tumors treated with pVAX1-PIG, and few in control groups. It indicated that the co-expression of perforin and granzyme B actively inhibited proliferation and stimulated the apoptotic cascade in Hep-2 tumor cells by inducing tumor cells apoptosis. Transmission electron microscopy analysis of tumor specimens treated with pVAX1-PIG showed cells with typical apoptotic morphology (Figure 4). As the result, it confirmed that perforin and granzyme B co-expression inhibits tumor growth by inducing apoptosis in nude mice. Therefore, these data from the inhibition of focus formation and tumorigenicity in our study seem to support the anti-tumor hypothesis of perforin and granzyme B previously reported by other workers [4,6,27,28].
In conclusion, the findings in this study suggest that perforin and granzyme B co-expression exhibits strong anti-tumor potential in Hep-2 cell line. The dramatic inhibition and abundant apoptosis in perforin and granzyme B co-expressing Hep-2 cell line can be interpreted as evidence of perforin and granzyme B anti-tumor activity. These findings will contribute to further investigate perforin and granzyme B genes, and might lead to novel therapeutic strategies in laryngeal cancer.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81102158) and Doctor Start-up Foundation of Guangzhou Medical University (L110518).
Disclosure of conflict of interest
Authors declare no conflict of interest.
References
- 1.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007 Oct;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–8. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medina MA, Couturier J, Feske ML, Mahne AE, Turner M, Yu X, Kozinetz CA, Orozco AF, Hutchison AT, Savidge TC, Rodgers JR, Lewis DE. Granzyme B- and Fas ligand-mediated cytotoxic function induced by mitogenic CD28 stimulation of human memory CD4+ T cells. J Leukoc Biol. 2012 May;91:759–71. doi: 10.1189/jlb.0511264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontani K, Sawai S, Hanaoka J, Tezuka N, Inoue S, Fujino S. Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. Eur J Surg Oncol. 2001 Mar;27:180–6. doi: 10.1053/ejso.2000.1060. [DOI] [PubMed] [Google Scholar]
- 5.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping S, Bleackley RC, Kirchhausen T, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–7. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007 Jun;19:339–47. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Trotta R, Ciarlariello D, Dal Col J, Mao H, Chen L, Briercheck E, Yu J, Zhang J, Perrotti D, Caligiuri MA. The PP2A inhibitor SET regulates granzyme B expression in human natural killer cells. Blood. 2011 Feb 24;117:2378–84. doi: 10.1182/blood-2010-05-285130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Betancourt A, Cohen D, Adams A, Sun L, Horohov D. Granzyme B-mRNA expression by equine lymphokine activated killer (LAK) cells is associated with the induction of apoptosis in target cells. Vet Immunol Immunopathol. 2011;143:108–15. doi: 10.1016/j.vetimm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Rauf A, Khatri M, Murgia MV, Saif YM. Expression of perforin-granzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. 2011 May;35:620–7. doi: 10.1016/j.dci.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002 Oct;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 11.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012 May 1;72:2162–71. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolitho P, Street SE, Westwood JA, Edelmann W, Macgregor D, Waring P, Murray WK, Godfrey DI, Trapani JA, Johnstone RW, Smyth MJ. Perforin-mediated suppression of B-cell lymphoma. Proc Natl Acad Sci U S A. 2009 Feb 24;106:2723–8. doi: 10.1073/pnas.0809008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–62. [PubMed] [Google Scholar]
- 14.Leong JW, Fehniger TA. Human NK cells: SET to kill. Blood. 2011 Feb 24;117:2297–8. doi: 10.1182/blood-2011-01-327247. [DOI] [PubMed] [Google Scholar]
- 15.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000 Sep 4;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Samadi AK, Roby KF, Timmermann B, Cohen MS. Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin A. Gynecol Oncol. 2012 Mar;124:606–12. doi: 10.1016/j.ygyno.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia L, Zhang S, Ye Y, Li X, Mercado-Uribe I, Bast RC Jr, Liu J. Paclitaxel inhibits ovarian tumor growth by inducing epithelial cancer cells to benign fibroblast-like cells. Cancer Lett. 2012 Dec 30;326:176–82. doi: 10.1016/j.canlet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Mao Q, Lin Y, Yang K, Xie L. RNA interference targeting mutant p53 inhibits growth and induces apoptosis in DU145 human prostate cancer cells. Med Oncol. 2011 Dec;28(Suppl 1):S381–7. doi: 10.1007/s12032-010-9679-9. [DOI] [PubMed] [Google Scholar]
- 20.Akhmetzyanova I, Zelinskyy G, Schimmer S, Brandau S, Altenhoff P, Sparwasser T, Dittmer U. Tumor-specific CD4+ T cells develop cytotoxic activity and eliminate virus-induced tumor cells in the absence of regulatory T cells. Cancer Immunol Immunother. 2013 Feb;62:257–71. doi: 10.1007/s00262-012-1329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010 Sep;15:355–73. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006 Sep;4:218–27. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong GK, Chiu AT. Gene therapy, gene targeting and induced pluripotent stem cells: applications in monogenic disease treatment. Biotechnol Adv. 2011 Jan-Feb;29:1–10. doi: 10.1016/j.biotechadv.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill MJ, Bourre L, Melgar S, O’Driscoll CM. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011 Mar;16:203–18. doi: 10.1016/j.drudis.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Stewart SE, D’Angelo ME, Bird PI. Intercellular communication via the endo-lysosomal system: translocation of granzymes through membrane barriers. Biochim Biophys Acta. 2012 Jan;1824:59–67. doi: 10.1016/j.bbapap.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008 Oct 6;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 27.Hoves S, Trapani JA, Voskoboinik I. The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol. 2010 Feb;87:237–43. doi: 10.1189/jlb.0909608. [DOI] [PubMed] [Google Scholar]
- 28.Kim TD, Lee SU, Yun S, Sun HN, Lee SH, Kim JW, Kim HM, Park SK, Lee CW, Yoon SR, Greenberg PD, Choi I. Human microRNA-27a* targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood. 2011 Nov 17;118:5476–86. doi: 10.1182/blood-2011-04-347526. [DOI] [PMC free article] [PubMed] [Google Scholar]


