Abstract
Objective: Wnt5a has been shown to be involved in cancer progression in a variety of tumor types. Previous experimental studies have indicated that it has been shown to be down-regulated in hepatocellular carcinoma (HCC). The goal of this study was to explore the effect of Wnt5a overexpression in an HCC cell line. Methods: We transfected the human HCC cell line Huh7 with a pcDNA3.1-Wnt5a overexpression vector or an empty vector control. The integration of the plasmid DNA and the expression of Wnt5a in Huh7 cells were confirmed by real-time RT-PCR and Western blot. A plate colony formation test was used to calculate the clone formation rate and the cell cycle was analyzed by flow cytometry. The effect of Wnt5a overexpression on cell migration was studied in vitro using a scratch assay and in vivo by xenograft studies in nude mice. Results: Our results showed that in Huh7 cells with overexpression of Wnt5a, the fraction of cells in the G1 and S phases of the cell cycle was significantly increased compared with untransfected cells. In agreement with this finding, overexpression of Wnt5a was associated with a lower colony formation rate compared with control cells. In our xenograft studies, nude mice injected with Huh7 cells with overexpression of Wnt5a had decreased tumor volumes compared with controls. The vitro scratch assay revealed that Wnt5a overexpression cells had a diminished capacity for cell migration. Furthermore, we studied the expression of important proteins associated with Wnt5a signaling pathway, and it was found that Ror2 and E-cadherin were both increased in Huh7 cells with overexpression of Wnt5a, whereas p53 expression was unaffected. Conclusion: Overexpression of Wnt5a in Huh7 cells was associated with decrease of cell proliferation and migration. Wnt5a may act as a tumor-suppressor gene in HCC, which works through the non-canonical Wnt signaling pathway by binding to the Ror2 and E-cadherin receptor.
Keywords: Hepatocellular carcinoma, Wnt5a, Huh7, proliferation, migration
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequent neoplasm worldwide and its incidence is steadily increasing [1]. Although the major risk factors contributing to the development of HCC are known, the molecular mechanisms underlying the carcinogenesis of HCC, including those associated with dedifferentiation and invasiveness, are not fully understood. In this regard, the Wnt family of proteins has been shown to include important regulators of malignant carcinogenesis [2]. The Wnts have historically been divided into two classes, those that signal through the canonical signaling pathway, and those that signal through the non-canonical signaling pathway. Furthermore, it has been established that signaling through the non-canonical pathway can antagonize signaling through the canonical pathway [3,4]. Canonical Wnts are thought to activate a signal-transduction that induces the nuclear accumulation and transcriptional activation of β-catenin [5]. The most well-recognized examples of non-canonical signaling are the planar cell polarity pathway and the Wnt/Ca2+ signaling pathway [3,4]. Wnt5a is a member of the large Wnt family and has been demonstrated to play an important role in malignant progression of tumors. Recently research provide evidence that the Wnt5a gene produces two protein isoforms, WNT5A-long (WNT5A-L) and WNT5A-short (WNT5A-S). Modulation of these two WNT5A isoforms, either through ectopic expression or knockdown, demonstrates that they exert distinct activities in cancer cell lines: while WNT5A-L inhibits proliferation of tumor cell lines and WNT5A-S promotes their growth [6]. Previous experimental studies have indicated that Wnt5a acts as a tumor-suppressor in the development of HCC [7]. The purpose of this study was to further explore the role of Wnt5a in HCC; this was achieved through the use of a Wnt5a expression vector to overexpress Wnt5a in the human HCC cell cline—Huh7.
Materials and methods
Cell lines
The human HCC cell line Huh7 was used in all experiments. Cells were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Life Technologies Co, Grand Island, NY, USA). All cell cultures were incubated at 37°C in 5% CO2, and the growth medium was replaced every second day.
Cell transfections
Cells were seeded in slide flasks and were allowed to reach 70% confluence. They were then transfected with either the pcDNA3.1-Wnt5a expression vector or an empty vector, which served as a negative control. Cell transfections were performed using Lipofectamine 2000 (Invitrogen, Life Technologies Co., Grand Island, NY, USA) according to the manufacturer’s instructions. Four hours after transfection, the medium was replaced with fresh serum-containing medium. For stable transfection, the medium was replaced after 48 h with G418-containing medium, and cells which were stably transfected were preferentially selected by the antibiotic resistance cassette in the vector.
Real-time RT-PCR analysis
Cells were grown to about 85% confluence and were harvested for RNA isolation using Trizol reagent (Invitrogen). Isolated RNA was quantified and a total of 0.5 mg of total RNA was then subjected to RT-PCR using the PrimeScript RT reagent Kit and SYBR Premix Ex Tap. The primers used for Wnt5a amplification were followed: forward primer: 5’-ATTCTTGGTGGTCGCTAGG-3’; reverse primer: 5’-CTGTCCTTGAGAAAGTCCTG-3’.
Plate colony formation assay
Control and Wnt5a-transfected Huh7 cells were trypsinized, counted and seeded into six-well plates at a density of 2000 cells per well, in regular culture medium. After 10 days, cells were washed with PBS, fixed in 10% methanol for 15 min, and stained with Giemsa for 10 min. Visualized colonies were then photographed and scored. Each plate colony formation experiment was repeated at least three times.
Cell cycle analysis
In order to determine the effect of Wnt5a overexpression on the cell cycle, control and Wnt5a-transfected Huh7 cells were harvested, fixed in 70% ethanol and stained with 0.05 mg/ml of propidium iodide, as well as 0.05 mg/ml of RNase A. The cell cycle of stained cells was then analyzed by flow cytometry.
Xenograft studies in nude mice
Four-week-old male BALB/c nude mice were housed in a sterile environment. Animals were maintained under specific pathogen-free conditions in the Animal Laboratory Unit of the Second Military Medical University. All animals used in this study were handled in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the Bioethics Committee of General Hospital of Jinan Military Command. Mice were injected with 5 × 106 Huh7 cells which were transfected with the pcDNA3.1-Wnt5a vector or the control vector. Cells were injected into the anterior subcutaneous tissue of the hind leg (n = 3/group). After 15 days, the mice were sacrificed and all tumors were excised, photographed and had their dimensions measured. Tumor volume (V) was calculated using the formula as V = (A × B2)/2; where A is the largest diameter of the tumor and B is the smallest diameter. We also performed standard histological (hematoxylin and eosin) and immunohistochemical studies on excised tumors that were formalin-fixed, paraffin-embedded, and cut into 5-μm tissue sections. Immunohistochemical staining was performed to evaluate the expression of Hep-1 and Ki-67, using anti-Hep-1 (1:400) and anti-Ki-67 (1:100) primary antibodies, respectively. Immunopo-sitive cells were visualized with the Envision Plus System according to a previously described protocol [6].
Wound-healing assay
To assess cell migration, wound-healing scratch assays were performed by plating cells in slide flasks. The cells were then allowed to attach and reach confluence, before a scratch was made in the cell monolayer. Photographs of cells invading the scratch were taken at the indicated time points and were used to assess the effect of Wnt5a overexpression on cell migration.
Western blot analysis
Cells were grown to 80% confluence and were then harvested on ice using lysis buffer. Cells were then Dounce homogenized and centrifuged at 12,000 rpm for 15 min. The protein concentration in the supernatant was then quantified using a BCA protein assay. A total of 50 ng of each lysate was run out on a SDS-PAGE gel and then transferred onto a 0.4 μm nitrocellulose membrane. Membranes were then probed with antibodies, and the protein was visualized using an ECL system. The Western blotting detection kit (ECL Plus) was obtained from Santa Cruz Biotechnology (Delaware Avenue Santa Cruz, CA, USA). The antibodies included: polyclonal anti-Wnt5a (1:1000; Lifespan), anti-Ror2 (1:500; Abnova), anti-E-cadherin (1:1000; Santa Cruz Biotechnology), anti-p53 (1:1000; Santa Cruz Biotechnology).
Statistical analysis
All statistical analyses were carried out using SPSS13.0 software (SPSS Inc., Chicago, IL, USA). If not otherwise stated, means of at least three independent experiments ± SD are shown. Statistically significances between control and experimental group were calculated by independent t-test, a P-value < 0.05 was considered to be statistically significant.
Results
The Huh7 HCC cell line was selected for these experiments based on its low baseline Wnt5a gene and protein expression levels. Huh7 cells were transfected with plasmid vectors capable of constitutively driving expression of Wnt5a or an empty vector to serve as a control. The mRNA expression level of Wnt5a was measured to assess whether transfection with the pcDNA3.1-Wnt5a expression vector was successful. The Wnt5a gene expression level was found to be low in untransfected Huh7 cells and Huh7/pcDNA3.1 cells.
Effect of Wnt5a overexpression on colony forming potential of Huh7 cells
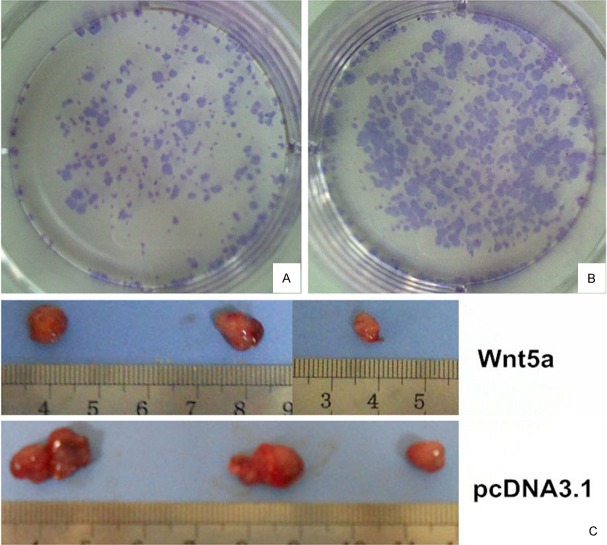
To investigate whether Wnt5a expression affected the ability of Huh7 cells to form colonies, the same number of Huh7/Wnt5a cells and Huh7/pcDNA3.1 cells were seeded at a low density in six-well plates. After 10 days, the existing colonies were visualized and counted microscopically (Figure 1). The number and size of colonies formed by Huh7/Wnt5a cells was lower than by Huh7/pcDNA3.1 cells. Statistical analysis revealed that the colony formation rate of Huh7/Wnt5a cells was 8.07%±0.37%, which was significantly lower than the rate of 16.47%±0.39% observed for Huh7/pcDNA3.1 cells (T = -27.174; P < 0.01).
Figure 1.
A, B: Effect of Wnt5a on clonogenicity of Huh7 cells. The Huh7/Wnt5a cells displayed less clonogenicity than Huh7/pcDNA3.1 cells after 10 Days culture. C: Xenograft studies in nude mice. Nude mice injected Huh7/Wnt5a cells had decreased tumor volumes compared with Huh7/pcDNA3.1 cells.
Effects of Wnt5a overexpression on the cell cycle of Huh7 cells
To examine the mechanism underlying the effect of Wnt5a on Huh7 cell proliferation, we evaluated the cell cycle in control and Wnt5a-overexpressing cells by flow cytometry. As shown in Table 1, our results indicated that the proportion of Huh7/Wnt5a cells in the G1 phase (62.76±1.01%) was significantly increased relative with Huh7/pcDNA3.1 cells (55.82±1.70%; P = 0.004). Conversely, the proportion of Huh7/Wnt5a cells in the S phase (30.64±1.45%) was significantly decreased relative than Huh7/pcDNA3.1 cells (38.03±1.14%; P = 0.002). This data showed that there was a suppression of cell cycle progression from G0/G1 to the S phase in Huh7/Wnt5a cells, compared with the control group, and a blockade in the G1 phase was observed in the cells transfected with Wnt5a expression vectors.
Table 1.
Flow cytometry analysis of cell cycle
| Group | G1 phase (%) | S phase (%) | G2 phase (%) |
|---|---|---|---|
| Huh7/Wnt5a | 62.76±1.01 | 30.64±1.45 | 6.59±0.50 |
| Huh7/pcDNA3.1 | 55.82±1.70 | 38.03±1.14 | 7.15±0.53 |
Xenograft studies in nude mice
To test the tumor-suppressing efficiency of Wnt5a overexpression in vivo, we established a xenograft model in nude mice by subcutaneous transplantation of Huh7/Wnt5a cells and Huh7/pcDNA3.1 cells. As shown in Figure 1C, tumor volume was decreased in nude mice injected with Huh7/Wnt5a cells compared with Huh7/pcDNA3.1 cells (P = 0.063). Immunohistochemical staining of tumor sections showed that Hep-1 was equally expressed in each group, suggesting that the tumor cells maintained the phenotypic characteristics of HCC cells (Figure 2A). Consistent with our in vitro data, we observed a significant decrease in the number of tumor cells stained positive for the cell proliferation marker Ki-67 in Huh7/Wnt5a group compared with Huh7/pcDNA3.1 group (P < 0.05; Figure 2B and 2C).
Figure 2.

A: Immunohistochemical staining showed that hep-1 was expressed in each group without obvious difference; B: The expression of Ki-67 in Huh7/Wnt5a cells (37.74%±2.455%); C: The expression of Ki-67 in Huh7/pcDNA3.1 cells (66.1%±2.162%).
Effects of Wnt5a expression on the cell motility of Huh7 cells
We examined whether Wnt5a could regulate cell motility by performing an in vitro wound-healing assay (Figure 3). 48 h after a scratch was made in the Huh7 cell monolayer, we observed that control cells had migrated into the scratch zone and the boundary area had become unclear. In contrast, the scratch area was still clear in cell cultures containing Huh7/Wn5a cells. These results confirmed that Wnt5a overexpression resulted in a decreased capacity for cell migration in Huh7 cells.
Figure 3.
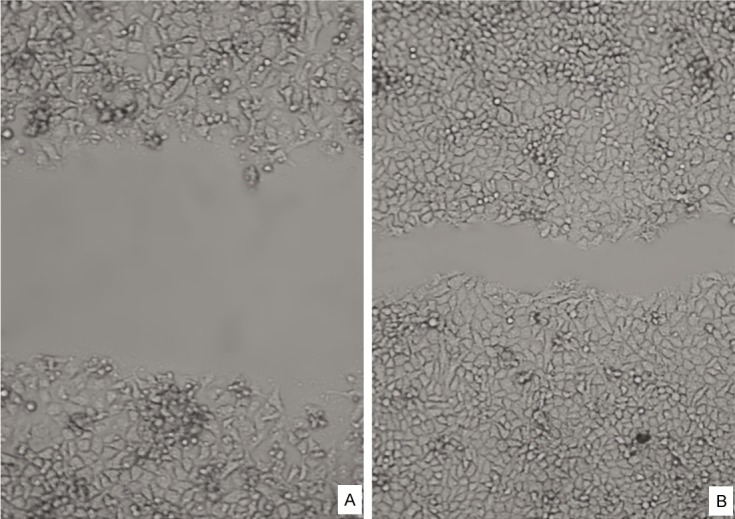
The Huh7/pcDNA3.1 and Huh7/Wnt5a cells motility were determined by wound migration assay. Huh7/Wnt5a cell (A) spreading along the edges of the wound was significantly decrease as compared with the Huh7/pcDNA3.1 cells (B) (scratch after 48 hr).
Western blot analysis of Wnt5a overexpressing Huh7 cells
The protein expression levels of Wnt5a, Ror2, E-cadherin and p53 were assessed in control and Wnt5a-overexpressing Huh7 cells by Western blotting (Figure 4). Consistent with our gene expression data, the protein expression level of Wnt5a was higher in Huh7/Wn5a cells relative to control. Similarly, the protein expression levels of Ror2 and E-cadherin were also increased in Huh7/Wn5a cells; however, the expression of p53 was unaffected.
Figure 4.
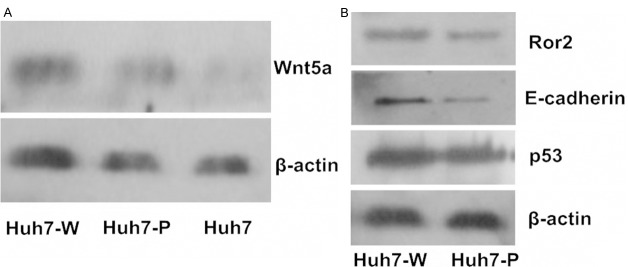
A: Expression of Wnt5a in Huh7 cell lines. The overexpression of Wnt5a in huh7-W revealed the successful transfection. β-actin detection confirmed equal loading. B: Upregulation of Ror2 and E-cadherin protein in Huh7/Wnt5a cells, while P53 had the same level expression in both Huh7/Wnt5a and Huh7/pcDNA3.1 cells. (Huh7-W: Huh7 cells transfected with Wnt5a expression plasmid; Huh7-P: Huh7 cells transfected with pcDNA3.1 plasmid.).
Discussion
Recent work studying various human tumors has indicated that Wnt5a has a critical role in malignant progression of cancer. For example, Wnt5a has been shown to be downregulated in neuroblastoma [8] and thyroid carcinoma [9]. Downregulation of Wnt5a has been associated with higher tumor grade and has been shown to be an independent factor indicating poor prognosis in a number of different tumor subtypes [10]. This tumor-suppressor role was further evidenced by studies that reintroduced Wnt5a into breast cancer cell lines MDA-MB-231 and 4T1 and FTC-133 cells, a thyroid cancer cell line, resulting in decreased invasion, migration, clonogenicity and proliferation [9,11]. But recently research reveal that Wnt5a promotes breast cancer cell migration via Dvl2/Daam1/RhoA [12], then they demonstrated that Wnt5a promotes breast cancer cell migration via Dvl2/Rab35/Rac1 signaling pathway in MCF-7 cells [13]. Although there was evidence that Wnt5a acted as a tumor-suppressor, a few studies have indicated an oncogenic role for Wnt5a in tumors arising from a variety of different tissues. For example, increased expression of Wnt5a has been identified in gastric cancer [14], pancreatic cancer [15] and non-small-cell lung cancer [16]. A cancer-promoting function for Wnt5a was also shown in human gastric cancer cells (SGC-7901) [17], and human pancreatic cancer cell lines [18] where over expression of Wnt5a promoted cell proliferation and invasion.
Previous experimental studies have demonstrated that Wnt5a mRNA expression was significantly upregulated in HCC and in cases of non-tumorous liver disease such as chronic hepatitis and cirrhosis. However, these effects were not completely corroborated by immunohistochemical staining, where strong Wnt5a staining was observed in some cases of chronic hepatitis and cirrhosis, although there was a reduction or loss of staining in liver tumors [19]. Previously, we investigated the expression and clinical significance of Wnt5a and Ror2 in HCC [7], our results indicated that all chronic hepatitis, cirrhosis and dysplastic liver cells exhibited strong positive immunostaining for Wnt5a. Contrastingly, in 76.5% of HCC patients, Wnt5a immunostaining was reduced or absent compared with the expression level in adjacent noncancerous tissue. Furthermore, we also found a significant negative correlation between Wnt5a expression and tumor stage [7]. Consistent with previous reports [19,20], the loss of Wnt5a protein expression in tumors was frequently observed in patients with HCC (71%-81%), which correlated with increased AFP and poor histological grade. Based on the existing literature, we speculated that Wnt5 may play important roles in the control of differentiation, proliferation and invasiveness of HCC. To test this hypothesis, we examined the effect of Wnt5a overexpression in the HCC cell line, Huh-7. We successfully established a Huh7 cell line that overexpressed Wnt5a, which was confirmed at the mRNA and protein level.
Our results demonstrated that overexpression of Wnt5a in Huh7 cells decreases cell proliferation, which was associated with a block in cell cycle progression in the G1 phase. Consistent with this finding, Huh7 cells with overexpressing Wnt5a had a diminished colony forming potential compared to control cells. In agreement with our in vitro data, xenografts of Wnt5a overexpressing Huh7 cells into nude mice yielded tumors with smaller volumes than control Huh7 cells. We also noted that the tumors derived from Wnt5a overexpressing Huh7 cells contained less Ki-67 immunopositive cells, reflecting decreased cell proliferation.
In the context of the existing literature and our own data, it was indicated that the expression of Wnt5a was correlated with the rate of cell proliferation in Huh7 cells. The association between high expression of Wnt5a and decreased cell proliferation suggests that Wnt5a is involved in the development of HCC. It has been reported that knockdown of Wnt5a results in a significantly increase in drug-induced apoptosis and overexpression of Wnt5a or addition of recombinant Wnt5a mediates resistance to apoptosis [21].
With regard to cell migration and metastasis, it has been reported that WNT5A knockdown in human colon cancer cells caused reduced directional migration, deregulated focal adhesion site formation and reduced invasion, whereas Wnt5a administration promoted the directional migration of colon cancer cells [22]. It has also been reported that increased H.pylori is a Wnt5a inducer to macrophages; and macrophage-derived Wnt5a enhances gastric cancer cell migration through upregulating CXCR4 expression in a paracrine manner [23].
In our studies, we observed that Huh7/Wnt5a cells had a decreased capacity for wound-healing in an in vitro scratch assay, indicating that increased Wnt5a expression decreases the motility of Huh7 cells.
Emerging evidence suggests that the function of Wnt5a can be altered depending on the availability of key receptors [24-26]. It was recently reported that an alternative Wnt receptor, receptor tyrosine kinase-like orphan receptor 2 (Ror2) mediates Wnt5a-initiated non-canonical signaling and is required for Wnt5a-mediated inhibition of canonical Wnt signaling [26,27]. The Ror2 receptor belongs to the receptor tyrosine kinase superfamily [26]. This large protein family is involved in regulating diverse cellular process such as the cell cycle, migration, proliferation and differentiation [27]. Previously we found that Ror2 gene transcription and protein translation were both suppressed in tumor tissues of HCC, and that the reduced expression of Ror2 in tumor tissue correlated with decreased Wnt5a expression [7]. In the current study, overexpression of Wnt5a was also associated with an increase in Ror2 expression. We interpret our results to indicate that Wnt5a acts as a tumor-suppressor during the development of HCC, which is mediated via the non-canonical Ror2 signaling pathway.
E-cadherin mediated intercellular adhesion limits cell motility and establishes cell apical-basal polarity [28]. Loss of E-cadherin expression and disassembly of the E-cadherin/catenin complex on the cell surface induces a transition from a stationary to a motile phenotype and enables tumor cells to disseminate and metastasize [29]. Previous studies show that in pancreatic cancer cells WNT5A/JNK signaling promoted the mRNA expressions of vimentin, but decreased in E-Cadherin expression, which suggested its regulatory effects on the EMT processes [30]. Kanzawa also find that Wnt5a regulates the induction of epithelial-mesenchymal transition (EMT) and the maintenance of caner stem cell (CSC) properties in MKN-7 cells [31]. In agreement with the literature, we found that Huh7/Wnt5a cells have an elevated level of E-cadherin protein expression, which was associated with decreased migration of these cells.
In summary, our experiments demonstrated that Wnt5a affects the biological behavior of the HCC cell line Huh7. Specifically, increased Wnt5a expression decreased the proliferative and migratory capacity of this HCC cell line. As such, we presumed that Wnt5a might be a potential therapeutic target for the inhibition of HCC progression.
Acknowledgements
This work was supported by Grants from the National Science Foundation of China (No. 81172261) and the Nature Science Foundation of Shandong Province (No. ZR2009CM041).
Disclosure of Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 2.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 5.Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer M, Benard J, Gaasterland T, Willert K, Cappellen D. WNT5A Encodes Two Isoforms with Distinct Functions in Cancers. PLoS One. 2013;8:e80526. doi: 10.1371/journal.pone.0080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH. Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol. 2012;18:1328–1338. doi: 10.3748/wjg.v18.i12.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc E, Roux GL, Benard J, Raguenez G. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene. 2005;24:1277–1283. doi: 10.1038/sj.onc.1208255. [DOI] [PubMed] [Google Scholar]
- 9.Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 10.Roman-Gomez J, Jimenez-Velasco A, Cordeu L, Vilas-Zornoza A, San Jose-Eneriz E, Garate L, Castillejo JA, Martin V, Prosper F, Heiniger A, Torres A, Agirre X. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer. 2007;43:2736–2746. doi: 10.1016/j.ejca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Crossman DK, Mitchell EH, Sohn P, Crowley MR, Serra R. WNT5A inhibits metastasis and alters splicing of Cd44 in breast cancer cells. PLoS One. 2013;8:e58329. doi: 10.1371/journal.pone.0058329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J, Gu L. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One. 2012;7:e37823. doi: 10.1371/journal.pone.0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Shen T, Liu J, Zheng J, Zhang Y, Xu R, Sun C, Du J, Chen Y, Gu L. Rab35 is required for Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7 breast cancer cells. Cell Signal. 2013;25:1075–1085. doi: 10.1016/j.cellsig.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, Downward J, Gress T, Michl P. WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 15.Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, Ueno M. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8765–8773. doi: 10.1200/JCO.2005.02.2871. [DOI] [PubMed] [Google Scholar]
- 16.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Zhang Y, Xu R, Du J, Hu Z, Yang L, Chen Y, Zhu Y, Gu L. PI3K/Akt-dependent phosphorylation of GSK3beta and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal. 2013;25:447–456. doi: 10.1016/j.cellsig.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Bo H, Zhang S, Gao L, Chen Y, Zhang J, Chang X, Zhu M. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer. 2013;13:496. doi: 10.1186/1471-2407-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–416. [PubMed] [Google Scholar]
- 20.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 21.Griesmann H, Ripka S, Pralle M, Ellenrieder V, Baumgart S, Buchholz M, Pilarsky C, Aust D, Gress TM, Michl P. WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic cancer. Neoplasia. 2013;15:11–22. doi: 10.1593/neo.121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, Van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis. 2013;34:2629–2638. doi: 10.1093/carcin/bgt215. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Ma H, Bu X, Wang W, Zhang N. SFRP5 inhibits gastric epithelial cell migration induced by macrophage-derived Wnt5a. Carcinogenesis. 2013;34:146–152. doi: 10.1093/carcin/bgs309. [DOI] [PubMed] [Google Scholar]
- 24.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59:83–96. doi: 10.1007/s00018-002-8407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403–411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- 28.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 29.Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, Li H, Li N, Sun H, Li Q, Shen X. WNT5A/JNK signaling regulates pancreatic cancer cells migration by Phosphorylating Paxillin. Pancreatology. 2013;13:384–392. doi: 10.1016/j.pan.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80:235–244. doi: 10.1159/000346843. [DOI] [PubMed] [Google Scholar]