Abstract
Background: The prognostic value of CD117 expression in cancers has been evaluated for several years while the results remain controversial. We thus performed a systematic review and meta-analysis of studies assessing the impact of CD117 expression on overall survival (OS) and disease-free survival (DFS) to clarify this issue. Methods: We searched Pubmed, Embase and Web of Science to identify studies on the prognostic impact of CD117 expression in cancers. A total of 4,458 patients from 39 eligible studies were included in the analysis. Pooled risk ratios (RRs) with 95% confidence interval (95% CI) were calculated to estimate the effect using random-effects model. Results: The analysis indicated that CD117 had significant association with poor OS of osteosarcoma (OR=1.36, 95% CI=1.03-1.79, I2=0%, fixed model) and renal carcinoma (OR=4.86, 95% CI=2.72-8.67, I2=0%, fixed model).However, no significant association between CD117 and DFS was found in overall studies. Conclusions: CD117 expression might be a predictive factor of poor prognosis in some surgically treated cancers, particularly in renal carcinoma.
Keywords: CD117, immunohistochemistry, survival, meta-analysis
Introduction
Despite the recent reduction in incidence and mortality, cancer is still a worldwide health burden and leads to more deaths than heart disease in some regions [1]. Surgical resection can be performed to remove the tumor if neither lymph node nor distant metastasis were present, while recurrence rate after surgery remains high [2]. Moreover, numerous cancers at the time of diagnosis are at advanced stage and the treatment options are limited, resulting in the persistent high mortality of cancers.
A lot of efforts have been made to investigate the prognostic biomarkers in cancers, helping to identify high-risk cancer patients who might need adjuvant treatment after surgery.
CD117, encoded by the proto-oncogene c-kit, is a transmembrane protein belonging to the type III subfamily of the receptor tyrosine kinases [3]. It has extracellular, intramembranous and intracellular domains. By binding to its ligand, called stem cell factor (SCF), this molecule plays an important part in regulating cellular activities, such as apoptosis, cell differentiation, proliferation, and cell adhesion [4].
Although the impact of CD117 expression on prognosis of patients with cancer has been explored recently, the prognostic value of CD117 expression in different tumor types remains conflicting because heterogeneous results were reported in studies and some of them included a small number of patients. To elucidate this issue, we performed this systematic review and meta-analysis to assess the prognostic significance of CD117 expression in various types of cancer.
Materials and methods
Publication search
We searched Pubmed, Embase and Web of Science to identify studies that assessed the prognostic value of CD117 expression in patients with carcinomas who underwent surgical resection of a tumor. The search strategy was the following terms: “CD117”, “c-kit”, “cancer”, “carcinoma”, “prognosis”, “prognostic”, and “survival”. The search ended in August, 2013, and no lower date limit was applied. References cited in selected articles were also searched manually to identify other relevant studies. Although our search did not have language limits initially, for the full-text reading and final evaluation we only performed the review of the studies published in English language. Conference abstracts were not selected for our analysis due to the insufficient data reported in them. Letters to the editor, reviews, and articles published in a book or papers were excluded. Criteria that an eligible study has to meet were as follows: (a) to evaluate the relationship between CD117 and overall survival (OS) or disease-free survival (DFS) of patients with carcinoma; (b) to assess CD117 expression using immunohistochemistry (IHC); (c) to determine CD117 expression in surgically resected primary tumor tissues (not in normal tissues or in body fluids such as blood and sputum). Two reviewers independently judged if studies screened were eligible. Disagreements were resolved by discussion. If the results reported in identified studies have the possible overlap (e.g., same authors, institutions), only the most informative study was involved in the analysis. The following information was extracted from each publication and used as a supplement if available: author, publication year, patient’s country, tumor stage, number of patients, research technique used, definition of positivity (cutoff value), and survival data. If data from any of the above categories were not reported in the primary study, items were treated as “not document”. A lower limit of number of patients included in each study was not set for inclusion in the meta-analysis.
Statistical analysis
Included studies were divided into two groups for analysis: those with data regarding OS and those regarding DFS. For the quantitative aggregation of the survival results, we measured the impact of CD117 expression on survival by risk ratio (RR) between the two survival distributions. RRs and 95% confidence intervals (CIs) were used to combine as the effective value. For those RRs that were not given directly in the published articles, the published data and figures from original papers were used to assess the RR according to the methods described by Parmar et al [5]. The heterogeneity assumption was calculated by the Q-test and P-values greater than 0.05 indicated a lack of heterogeneity among studies, so the RR was calculated by a fixed-effects model (the Mantel-Haenszel method and chi-squared tests). Otherwise, a random-effects model (the DerSimonian-Laird method) was used. Evidence of publication bias was sought using the methods of Egger et al. [6] and of Begg et al [7]. Intercept significance was determined by the t test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias). Kaplan-Meier curves were read by GetData Graph Digitizer 2.24. All the statistical analyses were performed using Stata 12.0 for Windows (Stata Corporation, College Station, TX, USA).
Results
Characteristics of the studies
Using the search criteria (Figure 1), a total of 39 publications met the criteria for this analysis [8-46]. Of them, eight were on lung cancer, two were on colorectal cancer, three were on ovarian cancer, three were on malignant melanoma, two were on uterine sarcomas, two were on Merkel cell carcinoma two were on osteosarcoma, two were on renal cancer, one was on endometrial adenocarcinoma, one was on cervical cancer, one was on vulvar cancer, one was on breast cancer, one was on adenoid cystic carcinoma, one was on germ cell tumors, one was on extrapulmonary small cell carcinoma, one was on prostate cancer, one was on thymic epithelial tumors, and one was on acute myeloid leukemia. The total number of patients included was 4,458 ranging from 26 to 522 patients per study. Among the 39 studies, seven studies were performed in Asian populations, and the remaining 32 studies followed non-Asian patients. All patients in the eligible studies were determined by pathological stage. All of the studies reported the prognostic value of CD117 status for survival in patients with cancer. Of the 39 studies, 6 directly reported survival data, while the other thirty-three studies provided survival curves. Estimation using survival curves were segregated according to either DFS or OS. A RR on DFS or OS could be extracted for all enrolled studies. Eleven of the 39 studies identified CD117 expression as an indicator of poor DFS or OS, three studies demonstrated that CD117 was a favorable prognostic factor, and the other twenty-five studies showed no statistically significant impact of CD117 overexpression on DFS or OS. Of the 39 studies, 2 studies detected the CD117 expression by flow cytometry, other 37 studies performed by immunohistochemistry. The characteristics of the included studies are listed in Table 1.
Figure 1.
Flow chart of study inclusion.
Table 1.
Main characteristic of the included studies
| Study | Patient’s country | Year | Tumor type | Tumor stage (UICC) | Technique | Location | Number of patients | Cut off (IHC) | RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Rehfeld | Germany | 2006 | Small cell lung cancer | I-IV | IHC | Cytoplasm | 195 | ≥1% | OS 1.03 (0.91-1.17) |
| Camps | Spain | 2006 | Small cell lung cancer | I-IV | IHC | ND | 85 | >1% | OS 0.89 (0.78-1.00) |
| DFS 1.00 (0.88-11.14) | |||||||||
| Erler | USA | 2010 | Small cell lung cancer | I-IV | IHC | Cytoplasm | 97 | >25% | OS 1.00 (0.94-1.07) |
| L’opez-Martin | Spain | 2007 | Small cell lung cancer | ND | IHC | membrane | 204 | >1% | OS 0.96 (0.88-1.06) |
| DFS 0.96 (0.88-1.04) | |||||||||
| Potti | USA | 2003 | Small cell lung cancer | ND | IHC | ND | 193 | >10% | OS 0.96 (0.90-1.02) |
| Rohr | Germany | 2004 | Small cell lung cancer | I-IV | IHC | ND | 203 | >25% | OS 0.98 (0.92-1.04) |
| Rossi | Italy | 2003 | Small cell lung cancer | ND | IHC | Cytoplasm and membrane | 27 | >25% | OS 1.14 (0.72-1.80) |
| Pelosi | Italy | 2004 | Lung cancer | I-III | IHC | Cytoplasm | 66 | >5% | OS 3.00 (0.54-16.77) |
| Scobie | USA | 2003 | Endometrial adenocarcinomas | I-IV | IHC | ND | 72 | >10% | DFS 1.90 (1.04-3.49) |
| Erdogan | Turkey | 2007 | Ovarian cancer | I-IV | IHC | Cytoplasm | 68 | >10% | OS 1.12 (0.44-2.85) |
| Tonary | Canada | 2000 | Ovarian cancer | I-IV | IHC | Cytoplasm and membrane | 50 | ND | OS 0.49 (0.30-0.82) |
| DFS 0.71 (0.52-0.96) | |||||||||
| Lassus | Finland | 2004 | Ovarian cancer | I-IV | IHC | Cytoplasm and membrane | 522 | >1% | OS 1.34 (1.11-1.61) |
| Huh | USA | 2010 | Uterine sarcomas | II-IV | IHC | ND | 26 | >25% | OS 1.00 (0.80-1.26) |
| DFS 1.00 (0.80-1.26) | |||||||||
| Winter | USA | 2003 | cancer of uterine corpus | I-IV | IHC | ND | 39 | >30% | OS 0.40 (0.18-0.92) |
| DFS 0.30 (0.09-1.02) | |||||||||
| Sukpan | Thiland | 2011 | Cervical cancer | I-IV | IHC | Cytoplasm | 100 | >5% | DFS 0.90 (0.34-2.38) |
| de Melo Maia1 | Brazil | 2012 | Vulvar cancer | ND | IHC | Cytoplasm or membrane | 139 | ≥score 2 | OS 0.31 (0.16-0.59) |
| DFS 1.04 (0.46-2.34) | |||||||||
| Tanaka | Japan | 2006 | Gallbladder cancer | I-IV | IHC | Cytoplasm | 47 | >20% | OS 0.93 (0.49-1.77) |
| Fan | China | 2013 | Oesophageal cancer | I-IV | IHC | Cytoplasm and membrane | 157 | >1% | OS 1.76 (1.08-2.85) |
| DFS 2.16 (1.18-3.96) | |||||||||
| Friederichs | Germany | 2010 | Colorectal cancer | I-IV | IHC | Cytoplasm | 263 | >1% | OS 1.48 (1.04-2.11) |
| Medinger | Germany | 2010 | Colorectal carcinoma (1); Breast cancer (2) | ND | IHC | Cytoplasm or membrane | 282 | >1% | OS (1) 1.46 (0.94-2.28) |
| OS (2) 1.17 (0.94-1.45) | |||||||||
| Murakami | Japan | 2011 | Malignant melanoma | ND | IHC | Cytoplasm | 39 | >10% | OS 0.78 (0.56-1.08) |
| Potti | USA | 2004 | Malignant melanoma | ND | IHC | ND | 202 | >10% | OS 0.57 (0.07-4.58) |
| Bataille | France | 2008 | Malignant melanoma | I-IV | FC | / | 62 | ≥10% | OS 2.62 (1.74-3.95) |
| Wei | China | 2008 | Osteosarcoma | ND | IHC | Cytoplasm | 40 | >1% | OS 1.24 (0.99-1.56) |
| Miiji | Brazil | 2011 | Osteosarcoma | ND | IHC | Cytoplasm | 52 | >10% | OS 1.69 (0.75-3.81) |
| Waltari | Finland | 2010 | Merkel cell carcinoma | I-IV | IHC | Cytoplasm | 207 | ≥score 1 | OS 1.18 (0.88-1.58) |
| DFS 1.26 (0.93-1.71) | |||||||||
| Aleodor | UK | 2010 | Merkel cell carcinoma | I-IV | IHC | Cytoplasm | 40 | >30% | OS 1.27 (0.60-2.70) |
| Schwarz | Germany | 2008 | Salivary gland carcinomas | I-IV | IHC | membrane | 101 | >10% | DFS 1.09 (0.68-1.73) |
| Bar-Sela | Israel | 2003 | Nasopharyngeal carcinoma | I-IV | IHC | Cytoplasm and membrane | 49 | ≥10% | OS 0.79 (0.34-1.84) |
| Zhang | China | 2009 | Renal cancer | I-IV | IHC | membrane | 119 | >10% | OS 4.08 (2.02-8.22) |
| Jones | UK | 2007 | Pediatric renal tumour | II-III | IHC | Cytoplasm | 226 | >10% | OS 5.80 (2.29-14.67) |
| DFS 3.05 (2.09-4.45) | |||||||||
| Aslan | USA | 2005 | Adenoid cystic carcinoma | ND | IHC | Cytoplasm and membrane | 45 | >25% | DFS 1.27 (0.45-3.60) |
| Durán | Canada | 2010 | Germ cell tumors | ND | IHC | Cytoplasm and membrane | 84 | >1% | DFS 0.94 (0.46-1.94) |
| Kurt | Turkey | 2005 | Extrapulmonary small cell carcinoma | ND | IHC | ND | 28 | >5% | OS 1.00 (0.87-1.15) |
| Di Lorenzo | Italy | 2004 | Prostate cancer | ND | IHC | Cytoplasm or membrane | 94 | >1% | DFS 3.66 (1.28-10.51) |
| Petrini | Italy | 2010 | Thymic epithelial tumors | I-IV | IHC | membrane | 120 | >1% | DFS 4.23 (1.90-9.44) |
| Sharawat | India | 2013 | Acute myeloid leukemia | ND | FC | / | 115 | / | OS 16.50 (3.11-87.47) |
Meta-analysis results
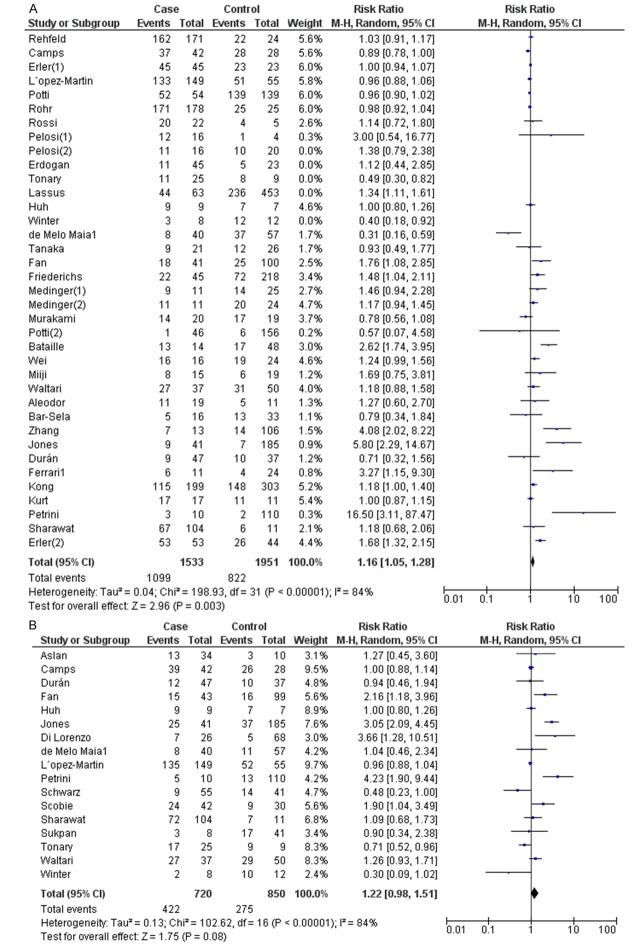
The meta-analysis was performed for cancer types on which more than 2 studies were eligible for inclusion. For studies evaluating overall survival (OS), there appeared to have heterogeneity among studies for CD117 expression (I2=80%). Accordingly, a random model was applied to calculate a pooled RR and its 95% CI (Table 2). We found that CD117 significantly predicted poorer OS, with the pooled RR being 1.12 (95% CI: 1.02-1.22, Figure 2A). In the tumor type subgroup analysis, CD117 expression was significantly associated with poor OS in patients with osteosarcoma (OR=1.36, 95% CI=1.03-1.79, I2=0%, fixed model) and renal carcinoma (OR=4.86, 95% CI=2.72-8.67, I2=0%, fixed model) (Table 2).
Table 2.
Meta-analysis of effects of CD117 expression on OS and DFS of different cancer types
| Cancer type | Studies | Odds ratio | Heterogeneity | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR (95% CI) | POR | Model | I2 (%) | P | ||
| OS | ||||||
| Total studies | 37 | 1.12 (1.02-1.22) | 0.01 | Random | 80 | <0.001 |
| Lung cancer | 9 | 0.99 (0.95-1.04) | 0.75 | Fixed | 11 | 0.34 |
| Colorectal cancer | 2 | 1.28 (0.95-1.74) | 0.11 | Random | 55 | 0.13 |
| Ovarian cancer | 3 | 0.91 (0.44-1.88) | 0.79 | Random | 85 | 0.001 |
| Malignant melanoma | 3 | 1.23 (0.43-3.51) | 0.7 | Random | 91 | <0.001 |
| Uterine sarcomas | 2 | 0.66 (0.14-3.80) | 0.59 | Random | 92 | 0.0003 |
| Merkel cell carcinoma | 2 | 1.20 (0.91-1.58) | 0.21 | Fixed | 0 | 0.84 |
| Osteosarcoma | 2 | 1.36 (1.03-1.79) | 0.03 | Fixed | 0 | 0.36 |
| renal cancer | 2 | 4.86 (2.72-8.67) | <0.001 | Fixed | 0 | 0.54 |
| DFS | ||||||
| Total studies | 17 | 1.22 (0.98-1.51) | 0.08 | Random | 84 | <0.001 |
| Lung cancer | 2 | 0.97 (0.90-1.04) | 0.39 | Fixed | 0 | 0.59 |
| Uterine sarcomas | 2 | 0.57 (0.05-6.11) | 0.64 | Random | 93 | 0.0001 |
Figure 2.
Meta-analysis of the effects of CD117 expression on OS (A) and DFS (B).
For studies evaluating DFS, a random model was also applied due to the heterogeneity among studies (Table 2). The expression of CD117 was not significantly correlated with DFS, with 1.22 (95% CI: 0.98-1.51, Figure 2B) as the pooled RR.
We also performed subgroup analysis by tumor histological type, the region of reports or cut off value in lung cancer. There was still no significant association detected in all stratified analysis (Table 3). Due to the limited number of eligible studies (n=2 to 3) included in the analysis of cancer types other than lung cancer, we did not conduct the subgroup analysis for these carcinomas.
Table 3.
Subgroup analysis of the studies reporting the prognostic value of CD117 expression on OS of lung cancer
| Stratified analysis | Studies | Odds ratio | Heterogeneity | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR (95% CI) | POR | Model | I2 (%) | P | ||
| OS | ||||||
| Lung cancer overall | 9 | 0.99 (0.95-1.04) | 0.75 | Fixed | 11 | 0.34 |
| SCLC | 8 | 0.98 (0.94-1.02) | 0.89 | Fixed | 0 | 0.5 |
| LCNEC | 1 | 1.38 (0.79-2.38) | 55 | 0.13 | ||
| Region | ||||||
| Germany | 2 | 1.00 (0.94-1.07) | 0.93 | Fixed | 0 | 0.34 |
| Spain | 2 | 0.94 (0.87-1.01) | 0.1 | Fixed | 7 | 0.3 |
| USA | 2 | 0.97 (0.93-1.02) | 0.19 | Fixed | 0 | 0.32 |
| Italy | 3 | 1.44 (0.97-2.14) | 0.07 | Fixed | 0 | 0.42 |
| Cut off | ||||||
| >25% | 3 | 1.00 (0.94-1.06) | 0.95 | Fixed | 0 | 0.68 |
| <25% | 6 | 0.99 (0.94-1.05) | 0.75 | Fixed | 38 | 0.15 |
| DFS | ||||||
| Lung cancer overall | 2 | 0.97 (0.90-1.04) | 0.39 | Fixed | 0 | 0.59 |
SCLC: Small cell lung cancer. LCNEC: large-cell neuroendocrine carcinomas.
Potential publication bias
Publication bias of the included studies was evaluated by funnel plots and Egger’s tests. As shown in Figure 3, the funnel plots were symmetric. The results of Egger’s test showed there was no evidence of publication bias on DFS (P=0.433) and OS (P=0.680).
Figure 3.
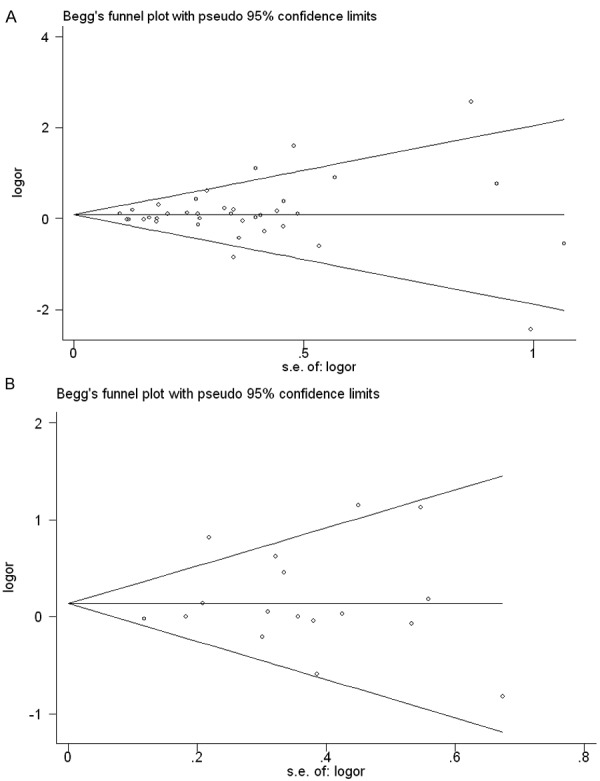
Funnel plots for the evaluation of potential publication bias in the impact of CD117 expression on OS (A) and DFS (B). The Egger test for publication bias was not significant for OS (p=0.433) and DFS (p=0.680).
Discussion
Kit protein, a cell-surface transmembrane receptor for stem cell factor, have been identified as a key oncogenic driver in a variety of solid tumours [47]. KIT has been shown to plays a role in the proliferation of a number of cell types, including mast cells, melanocytes, germ cells, and hematopoietic stem cells [48]. Recently, KIT, as a biomarker of prognosis in malignancies, has generated much interest. But the conclusions for its prognostic value are controversial. In gastrointestinal stromal tumors, myeloid leukemias and mast cell disorders, for example, c-KIT gene gain-of-function mutations result in constitutive tyrosine kinase activity and are considered to play a central role in oncogenesis and sustained tumor growth [49]. Coexpression of c-KIT and its ligand in small-cell lung cancer, for example, appears to result in an autocrine growth loop sustaining tumor cell proliferation [50]. On the other hand, a markedly better outcome has already been demonstrated in tumors that expressed c-KIT compared with those that did not, such as nasopharyngeal carcinomas [20] and multiple myeloma [21]. Thus, we performed a meta-analysis to veritably evaluate the role of KIT in the prognosis of cancer.
To our knowledge, this meta-analysis is the first study to systematically assess the association between CD117 expression and prognosis of various cancer types. It is reported that CD117 is expressed in a high proportion of patients with SCLC (28%-100%) [33,51]. And between 79% and 88% of SCLC cell lines were found to express CD117 [52]. Recently, a number of studies have focused on the association of CD117 expression with the survival of SCLC cancer patients. In this study, Totally 8 studies with the follow-up time of more than three years are involved. The pooled analysis shows that positive expression of CD117 is not significantly associated with the poor OS and DFS of patients with SCLC. In the subgroup analysis by tumor histological type, the region of reports or cut off value, there was still no significant association detected in all stratified analysis.
Interestingly, pooled analysis of the included studies exhibited a significant correlation between CD117 expression and poor OS of patients with osteosarcoma and renal carcinoma, suggesting that CD117 might be an independent prognostic factor of poor survival in patients with osteosarcoma and renal carcinoma. However, considering the limited studies included in the meta-analysis, our results should be interpreted with caution. More studies with a larger sample size on those cancer types are thus needed to further elucidate the prognostic value of CD117 in them.
Some limitations of this meta-analysis should be acknowledged. First, we found the heterogeneity in our meta-analysis. The heterogeneity could be explained by the fact that the technique of detecting CD117 is not comparable among the studies. Most studies (77.4%) in the meta-analysis used IHC staining to study expressions of CD117. Although IHC staining is simple and cost-effective to perform, results are highly dependent on a variety of methodological factors, such as storage time, fixation method of paraffin-embedded tissues, different primary antibodies, the revelation protocols and different levels of positive (0, 10, 50%, different scores combining intensity and percentage, intensity only) [53]. Immunostaining cutoff point were arbitrarily selected and varied between studies. So, variability in protein expression assessment must be considered a potential source of bias. Second, potential publication bias was a concern. We restricted our review to articles published in English or Chinese language because other languages were not accessible to the readers. This selection could favor the positive studies that are more often published in English while the negative ones tend to be more often reported in native languages [54].
To sum up, CD117 expression exhibited the significant association with OS of osteosarcoma and renal carcinoma. Whereas, the large prospective clinical studies based on homogeneous series of patients are needed to further confirm the prognostic value of CD117 in different types of cancer.
Acknowledgements
We are supported by Project of Anhui Province for Excellent Young Talents in Universities (2013SQRL049ZD) and Project of Anhui Provincial Department of Health (KJ2012Z251).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Wang B, Chen X, Bi J. The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis. 2011;32:411–416. doi: 10.1093/carcin/bgq266. [DOI] [PubMed] [Google Scholar]
- 3.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 4.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–266. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 5.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 8.Rehfeld N, Geddert H, Atamna A, Gabbert HE, Steidl U, Fenk R, Kronenwett R, Haas R, Rohr UP. Coexpression of fragile histidine triad and c-kit is relevant for prediction of survival in patients with small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2232–2238. doi: 10.1158/1055-9965.EPI-06-0342. [DOI] [PubMed] [Google Scholar]
- 9.Andea AA, Patel R, Ponnazhagan S, Kumar S, DeVilliers P, Jhala D, Eltoum IE, Siegal GP. Merkel cell carcinoma: correlation of KIT expression with survival and evaluation of KIT gene mutational status. Hum Pathol. 2010;41:1405–1412. doi: 10.1016/j.humpath.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslan DL, Oprea GM, Jagush SM, Gulbahce HE, Adams GL, Gaffney PM, Savik K, Pambuccian SE. c-kit expression in adenoid cystic carcinoma does not have an impact on local or distant tumor recurrence. Head Neck. 2005;27:1028–1034. doi: 10.1002/hed.20306. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Sela G, Kuten A, Ben-Eliezer S, Gov-Ari E, Ben-Izhak O. Expression of HER2 and C-KIT in nasopharyngeal carcinoma: implications for a new therapeutic approach. Mod Pathol. 2003;16:1035–1040. doi: 10.1097/01.MP.0000089778.48167.91. [DOI] [PubMed] [Google Scholar]
- 12.Bataille R, Pellat-Deceunynck C, Robillard N, Avet-Loiseau H, Harousseau JL, Moreau P. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leuk Res. 2008;32:379–382. doi: 10.1016/j.leukres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Camps C, Sirera R, Bremnes RM, Garde J, Safont MJ, Blasco A, Berrocal A, Sánchez JJ, Calabuig C, Martorell M. Analysis of c-kit expression in small cell lung cancer: prevalence and prognostic implications. Lung Cancer. 2006;52:343–347. doi: 10.1016/j.lungcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Durán I, García-Velasco A, Ballestín C, García E, Martínez-Tello F, Pond GR, García-Carbonero R, Cortés-Funés H, Paz-Ares L. Expression of EGFR, HER-2/neu and KIT in germ cell tumours. Clin Transl Oncol. 2010;12:443–449. doi: 10.1007/s12094-010-0532-6. [DOI] [PubMed] [Google Scholar]
- 15.Erdogan G, Bassorgun CI, Pestereli HE, Simsek T, Karaveli S. C-kit protein expression in uterine and ovarian mesenchymal tumours. APMIS. 2007;115:204–209. doi: 10.1111/j.1600-0463.2007.apm_419.x. [DOI] [PubMed] [Google Scholar]
- 16.Erler BS, Presby MM, Finch M, Hodges A, Horowitz K, Topilow AA, Matulewicz T. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumor Biology. 2011;32:107–111. doi: 10.1007/s13277-010-0104-y. [DOI] [PubMed] [Google Scholar]
- 17.Fan H, Yuan Y, Wang J, Zhou F, Zhang M, Giercksky KE, Nesland JM, Suo Z. CD117 expression in operable oesophageal squamous cell carcinomas predicts worse clinical outcome. Histopathology. 2013 Jun;62:1028–37. doi: 10.1111/his.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari L, Torre SD, Collini P, Martinetti A, Procopio G, De Dosso S, Bajetta R, Catena L. Kit protein (CD117) and proliferation index (Ki-67) evaluation in well and poorly differentiated neuroendocrine tumors. Tumori. 2006;92:531. doi: 10.1177/030089160609200611. [DOI] [PubMed] [Google Scholar]
- 19.Friederichs J, von Weyhern CW, Rosenberg R, Doll D, Busch R, Lordick F, Siewert JR, Sarbia M. Immunohistochemical detection of receptor tyrosine kinases c-kit, EGF-R, and PDGF-R in colorectal adenocarcinomas. Langenbecks Arch Surg. 2010;395:373–379. doi: 10.1007/s00423-009-0478-8. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Liao LM, Wang HY, Zheng M. Clinicopathologic characteristics and prognostic factors of ovarian fibrosarcoma: the results of a multi-center retrospective study. BMC Cancer. 2010;10:585. doi: 10.1186/1471-2407-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh WK, Sill MW, Darcy KM, Elias KM, Hoffman JS, Boggess JF, Alvarez RD, Long HJ, O’Malley DM, Birrer MJ. Efficacy and safety of imatinib mesylate (Gleevec®) and immunohistochemical expression of c-Kit and PDGFR-β in a Gynecologic Oncology Group Phase Il Trial in women with recurrent or persistent carcinosarcomas of the uterus. Gynecol Oncol. 2010;117:248–254. doi: 10.1016/j.ygyno.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Jones C, Rodriguez-Pinilla M, Lambros M, Bax D, Messahel B, Vujanic GM, Reis-Filho JS, Pritchard-Jones K. c-KIT overexpression, without gene amplification and mutation, in paediatric renal tumours. J Clin Pathol. 2007;60:1226–1231. doi: 10.1136/jcp.2007.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurt E, Sezgin C, Evrensel T, Yalcinkaya U, Kanat O, Veral A, Demiray M, Arslan M, Karabulut B, Ercan I. Therapy, outcome and analysis of c-kit expression in patients with extrapulmonary small cell carcinoma. Int J Clin Pract. 2005;59:537–543. doi: 10.1111/j.1368-5031.2005.00447.x. [DOI] [PubMed] [Google Scholar]
- 24.Lassus H, Sihto H, Leminen A, Nordling S, Joensuu H, Nupponen N, Butzow R. Genetic alterations and protein expression of KIT and PDGFRA in serous ovarian carcinoma. Br J Cancer. 2004;91:2048–2055. doi: 10.1038/sj.bjc.6602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Lorenzo G, Autorino R, D’Armiento F, Mignogna C, De Laurentiis M, De Sio M, D’Armiento M, Damiano R, Vecchio G, De Placido S. Expression of proto-oncogene c-kit in high risk prostate cancer. Eur J Surg Oncol. 2004;30:987–992. doi: 10.1016/j.ejso.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 26.de Melo Maia B, Lavorato-Rocha AM, Rodrigues IS, Baiocchi G, Cestari FM, Stiepcich MM, Chinen LT, Carvalho KC, Soares FA, Rocha RM. Prognostic significance of c-KIT in vulvar cancer: bringing this molecular marker from bench to bedside. J Transl Med. 2012;10:150. doi: 10.1186/1479-5876-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Martin A, Ballestín C, Garcia-Carbonero R, Castaño A, Lopez-Ríos F, López-Encuentra A, Sánchez-Cespedes M, Castellano D, Bartolomé A, Cortés-Funes H. Prognostic value of KIT expression in small cell lung cancer. Lung Cancer. 2007;56:405–413. doi: 10.1016/j.lungcan.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Medinger M, Kleinschmidt M, Mross K, Wehmeyer B, Unger C, Schaefer HE, Weber R, Azemar M. c-kit (CD117) expression in human tumors and its prognostic value: an immunohistochemical analysis. Pathol Oncol Res. 2010;16:295–301. doi: 10.1007/s12253-010-9247-9. [DOI] [PubMed] [Google Scholar]
- 29.Miiji LN, Petrilli AS, Di Cesare S, Odashiro AN, Burnie MN Jr, de Toledo SR, Garcia RJ, Alves MTS. C-kit expression in human osteosarcoma and in vitro assays. Int J Clin Exp Pathol. 2011;4:775. [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami A, Mori T, Sakai H, Murakami M, Yanai T, Hoshino Y, Maruo K. Analysis of KIT expression and KIT exon 11 mutations in canine oral malignant melanomas. Vet Comp Oncol. 2011;9:219–224. doi: 10.1111/j.1476-5829.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 31.Pelosi G, Masullo M, Leon ME, Veronesi G, Spaggiari L, Pasini F, Sonzogni A, Iannucci A, Bresaola E, Viale G. CD117 immunoreactivity in high-grade neuroendocrine tumors of the lung: a comparative study of 39 large-cell neuroendocrine carcinomas and 27 surgically resected small-cell carcinomas. Virchows Archiv. 2004;445:449–455. doi: 10.1007/s00428-004-1106-1. [DOI] [PubMed] [Google Scholar]
- 32.Petrini I, Zucali PA, Lee HS, Pineda MA, Meltzer PS, Walter-Rodriguez B, Roncalli M, Santoro A, Wang Y, Giaccone G. Expression and mutational status of c-kit in thymic epithelial tumors. J Thorac Oncol. 2010;5:1447–1453. doi: 10.1097/JTO.0b013e3181e96e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potti A, Moazzam N, Ramar K, Hanekom D, Kargas S, Koch M. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol. 2003;14:894–897. doi: 10.1093/annonc/mdg253. [DOI] [PubMed] [Google Scholar]
- 34.Potti A, Moazzam N, Langness E, Sholes K, Tendulkar K, Koch M, Kargas S. Immunohistochemical determination of HER-2/neu, c-Kit (CD117), and vascular endothelial growth factor (VEGF) overexpression in malignant melanoma. J Cancer Res Clin Oncol. 2004;130:80–86. doi: 10.1007/s00432-003-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohr UP, Rehfeld N, Pflugfelder L, Geddert H, Müller W, Steidl U, Fenk R, Gräf T, Schott M, Thiele KP. Expression of the tyrosine kinase c-kit is an independent prognostic factor in patients with small cell lung cancer. Int J Cancer. 2004;111:259–263. doi: 10.1002/ijc.20252. [DOI] [PubMed] [Google Scholar]
- 36.Rossi G, Cavazza A, Marchioni A, Migaldi M, Bavieri M, Facciolongo N, Petruzzelli S, Longo L, Tamberi S, Crinò L. Kit expression in small cell carcinomas of the lung: effects of chemotherapy. Mod Pathol. 2003;16:1041–1047. doi: 10.1097/01.MP.0000089780.30006.DE. [DOI] [PubMed] [Google Scholar]
- 37.Ettl T, Schwarz S, Kleinsasser N, Hartmann A, Reichert T, Driemel O. Overexpression of EGFR and absence of C-KIT expression correlate with poor prognosis in salivary gland carcinomas. Histopathology. 2008;53:567–577. doi: 10.1111/j.1365-2559.2008.03159.x. [DOI] [PubMed] [Google Scholar]
- 38.Scobie JV, Acs G, Bandera CA, Blank SV, Wheeler JE, Pasha TL, Salscheider M, Zhang PJ. C-kit immunoreactivity in endometrial adenocarcinomas and its clinicopathologic significance. Int J Gynecol Pathol. 2003;22:149–155. doi: 10.1097/00004347-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sharawat SK, Gupta R, Raina V, Kumar L, Sharma A, Iqbal S, Bakhshi R, Vishnubhatla S, Bakhshi S. Increased coexpression of c-KIT and FLT3 receptors on myeloblasts: Independent predictor of poor outcome in pediatric acute myeloid leukemia. Cytometry B Clin Cytom. 2013 Nov-Dec;84:390–7. doi: 10.1002/cyto.b.21098. [DOI] [PubMed] [Google Scholar]
- 40.Sukpan K, Settakorn J, Khunamornpong S, Cheewakriangkrai C, Srisomboon J, Siriaunkgul S. Expression of survivin, CD117, and C-erbB-2 in neuroendocrine carcinoma of the uterine cervix. Int J Gynecol Cancer. 2011;21:911–917. doi: 10.1097/IGC.0b013e31821a2567. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S, Tanaka H, Yamamoto T, Shuto T, Takemura S, Hai S, Sakabe K, Uenishi T, Hirohashi K, Kubo S. Immunohistochemical demonstration of c-Kit protooncogene product in gallbladder cancer. J Hepatobiliary Pancreat Surg. 2006;13:228–234. doi: 10.1007/s00534-005-1074-0. [DOI] [PubMed] [Google Scholar]
- 42.Tonary AM, Macdonald EA, Faught W, Senterman MK, Vanderhyden BC. Lack of expression of c-KIT in ovarian cancers is associated with poor prognosis. Int J Cancer. 2000;89:242–250. doi: 10.1002/1097-0215(20000520)89:3<242::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Waltari M, Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619–628. doi: 10.1002/ijc.25720. [DOI] [PubMed] [Google Scholar]
- 44.Wei H, Zhao M, Dong W, Yang Y, Li J. Expression of c-kit protein and mutational status of the c-kit gene in osteosarcoma and their clinicopathological significance. J Int Med Res. 2008;36:1008–1014. doi: 10.1177/147323000803600518. [DOI] [PubMed] [Google Scholar]
- 45.Winter WE, Seidman JD, Krivak TC, Chauhan S, Carlson JW, Rose GS, Birrer MJ. Clinicopathological analysis of c-kit expression in carcinosarcomas and leiomyosarcomas of the uterine corpus. Gynecol Oncol. 2003;91:3–8. doi: 10.1016/j.ygyno.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Ye D, Yao X, Dai B, Zhang S, Shen Y, Zhu Y, Mao H. Role of KIT expression in the prognosis of clear cell renal cell carcinomas in Chinese patients. J Cancer Res Clin Oncol. 2009;135:249–253. doi: 10.1007/s00432-008-0447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tay C, Ong C, Lee V, Pang B. KIT gene mutation analysis in solid tumours: biology, clincial applications and trends in diagnostic reporting. Pathology. 2013;45:127. doi: 10.1097/PAT.0b013e32835c7645. [DOI] [PubMed] [Google Scholar]
- 48.Krams M, Parwaresch R, Sipos B, Heidorn K, Harms D, Rudolph P. Expression of the c-kit receptor characterizes a subset of neuroblastomas with favorable prognosis. Oncogene. 2004;23:588–595. doi: 10.1038/sj.onc.1207145. [DOI] [PubMed] [Google Scholar]
- 49.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 2002;20:1692–1703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 50.DiPaola RS, Kuczynski WI, Onodera K, Ratajczak MZ, Hijiya N, Moore J, Gewirtz AM. Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene Ther. 1996;4:176–182. [PubMed] [Google Scholar]
- 51.Natali PG, Nicotra MR, Sures I, Santoro E, Bigotti A, Ullrich A. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res. 1992;52:6139–6143. [PubMed] [Google Scholar]
- 52.Rygaard K, Nakamura T, Spang-Thomsen M. Expression of the proto-oncogenes c-met and c-kit and their ligands, hepatocyte growth factor/scatter factor and stem cell factor, in SCLC cell lines and xenografts. Br J Cancer. 1993;67:37. doi: 10.1038/bjc.1993.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacquemier J, Moles J, Penault-Llorca F, Adelaide J, Torrente M, Viens P, Birnbaum D, Theillet C. p53 immunohistochemical analysis in breast cancer with four monoclonal antibodies: comparison of staining and PCR-SSCP results. Br J Cancer. 1994;69:846. doi: 10.1038/bjc.1994.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]