Abstract
This study investigated VE-statin/Egfl7 expression and its role and regulatory mechanism in malignant glioma progression. Forty-five paraffin-embedded glioma (grade I-II: n=24; grade III-IV: n=21) were examined. VE-statin/Egfl7 protein expression was detected via immunohistochemistry, and its correlation with pathological grade was evaluated. Three-dimensional cell culture was then performed to investigate the influence of VE-statin/Egfl7 on the angiogenesis of umbilical vein endothelial cells. Microarray detection was used to molecularly profile VE-statin/Egfl7 and relevant signaling pathways in malignant glioma (U251 cells). Data showed that VE-statin/Egfl7 protein was mainly expressed in the cytoplasm of cancer and vascular endothelial cells and was significantly related to the degree of malignancy (t=4.399, P<0.01). Additionally, VE-statin/Egfl7 expression was low in certain gray-matter neurons but undetectable in glial cells. VE-statin/Egfl7 gene silencing significantly inhibited angiogenesis in umbilical vein endothelial cells. The following microarray results were observed in VE-statin/Egfl7-silenced U251 cells: 1) EGFR family members showed the highest differential expression, accounting for 5.54% of differentially expressed genes; 2) cell survival-related signaling pathways changed significantly; and 3) the integrin ανβ3 signaling pathway was markedly altered. Thus, malignant glioma cells and glioma vascular endothelial cells highly express VE-statin/Egfl7, which is significantly correlated with the degree of malignancy. Moreover, VE-statin/Egfl7 plays an important role in glioma angiogenesis. Microarray results indicate that VE-statin/Egfl7 may regulate EGFR and integrins to influence the FAK activity of downstream factors, triggering the PI3K/Akt and Ras/MAPK cascades and subsequent malignant glioma development.
Keywords: Epidermal growth factor-like domain 7, glioma, pathological grade, angiogenesis, microarray
Introduction
Glioma, the most common type of intracranial tumor, is a malignancy of glial cells derived from neuroectoderm. Although great progress has been achieved in past years, the prognosis of malignant glioma patients remains poor, with a mean survival time of only 12-15 months [1]. Parker et al. [2] and Soncin et al. [3] independently identified novel vascular endothelium-specific genes in 2004 and 2003, respectively, which were named vascular endothelial-statin (VE-statin) and epidermal growth factor-like domain 7 (Egfl7). It has been confirmed that VE-statin and Egfl7 are the same gene, which is now known as VE-statin/Egfl7. VE-statin/Egfl7 is highly expressed during embryonic development and plays a crucial regulatory role in angiogenesis. However, VE-statin/Egfl7 is not expressed in most human tissues, with the exception of tissues rich in blood vessels, including the lung, ovary, and uterus [4]. Our group [5] found that VE-statin/Egfl7 is also highly expressed in liver cancer and malignant glioma, and increasing evidence shows that VE-statin/Egfl7 gene expression is re-activated in multiple malignancies. Based on our previous findings, the present study aimed to investigate the role of VE-statin/Egfl7 in malignant glioma angiogenesis using a small interfering RNA (siRNA) technique and a microarray assay as well as to explore the potential signaling pathways involved in regulating VE-statin/Egfl7 expression.
Materials and methods
Instrumentation and reagents
An ABC Kit (Vector Labs, Burlingame, California, USA); a concentrated DAB kit (Beijing Zhongshan Biotech Co., Ltd.); rabbit anti-human VE-statin/Egfl7 antibody (Beijing Biosynthesis Biotech Co., Ltd.); TRIzol reagent (Invitrogen, USA); reagents for RT-PCR (TAKARA); fetal bovine serum (FBS), RPMI 1640, and Opti-MEM (Gibco); a plasmid extraction kit (Qiagen); primers (Shanghai GeneChem Biotech Co., Ltd.); a fluorescence microscope (Olympus); protein electrophoresis equipment and protein transfer equipment (Shanghai Tanon Biotech Co., Ltd.); an inverted phase-contrast microscope (Nikon TMS-F); Transwell chambers (Costar, USA); multiwell plates (Corning, USA); DNA electrophoresis equipment (Bio-Rad); and a microarray (HOA v6.1 Human OneArray) were used in this study.
Sample collection and immunohistochemistry
Glioma tissues (low malignant potential [grade I-II]: n=24; high malignant potential [grade III-IV]: n=21) were collected from the Department of Pathology of Xiangya Hospital, Central South University [6]. Additionally, cerebral cortex (n=2) and subcortical white-matter (n=4) samples were collected from patients with brain contusions in frontal non-functional areas. These tissues were embedded in paraffin and sectioned (4 μm). Immunohistochemistry was performed using an ABC Kit according to the manufacturer’s instructions. The concentration of anti-VE-statin/Egfl7 antibody used was 1:500, and the primary antibody was replaced with 0.01 M PBS in the negative-control group. After immunohistochemistry, VE-statin/Egfl7-positive cells exhibited brown-yellow or brown granules that contrasted with the background.
Cell lines and the detection of VE-statin/Egfl7 expression
Cell culture
Human umbilical vein endothelial cells (HUVECs) and a human astrocytoma cell line (U251; WHO grade IV pleomorphic glioblastoma) were maintained in the Xiangya Central Laboratory of Central South University. These cells were cultured in RPMI 1640 containing 12% FBS at 37°C in an environment with 5% CO2.
Real-time fluorescence quantitative PCR
VE-statin/Egfl7 mRNA expression was detected in human glioma cell lines (U251, U373, and U87), a human prostate cancer cell line (PANC-1), and a human hepatocellular carcinoma cell line (HepG2). In brief, cells were harvested, and total RNA was extracted using TRIzol and reverse transcribed into cDNA, followed by fluorescence quantitative PCR. The primers for the target genes were synthesized by GeneChem and were as follows: GAPDH (121 bp), 5′-TGACTTCAACAGCGACACCCA-3′ (forward) and 5′-CACCCTGTTGCTGTAGCCAAA-3′ (reverse); VE-statin/Egfl7 (231 bp), 5′-AGCACCTACCGAACCATCTA-3′ (forward) and 5′-ATCCACATCTGACTGGCAAG-3′ (reverse).
Construction of VE-statin/Egfl7-siRNA lentiviral vectors and cell infection
A target in the VE-statin/Egfl7 gene for RNA interference was identified in our previous study, and siRNA lentiviral vectors expressing a green fluorescent protein (GFP)-conjugated reporter gene were successfully constructed. The results showed that these vectors could effectively inhibit VE-statin/Egfl7 expression in U251 cells and HUVECs. The construction of VE-statin/Egfl7-siRNA lentiviral vectors and cell infection were performed as previously reported [7,8].
Detection of the angiogenic capability of HUVECs
Rat tail collagen was prepared and mixed with 10× RPMI 1640, 0.1 N sodium hydroxide, and FBS at a volume ratio of 7:1:1:1 in a sterilized flask, and the pH was adjusted to 7.4. Cells in the logarithmic growth phase were harvested and maintained in 24-well plates (pore size: 0.4 μm), and the cell density was adjusted. HUVECs were added to the lower chamber of 24-well plates (4×104 cells/well), and U251 cells were added to the upper chamber of 24-well plates (4×104 cells/well). After lentivirus infection, the cells were incubated at 37°C in an environment with 5% CO2. When HUVEC confluence reached nearly 100% and single-layer cells were observed, collagen was added to the lower chamber containing HUVECs (300 μl/well), followed by incubation at 37°C in an environment with 5% CO2. HUVEC angiogenesis was then observed under a microscope.
Gene expression profiling
A microarray assay was performed with the Axon GenePix™ 4100 microarray, and the Rosetta Resolver analysis system was employed for data analysis. The raw data underwent mean normalization, and the fold changes were compared between different groups after the standardization of the means. Differentially expressed genes were defined once two requirements were met: 1) the standardized spot signal value was transformed into the binary logarithm, and the absolute value of the binary logarithm was ≥0.585. 2) After adjusting the control and experimental groups, the P value was <0.05 in a t test. Unsupervised hierarchical clustering of the differentially expressed genes was then performed with Cluster 3.0 software. The data regarding the differentially expressed genes were input into the REACTOME, Pathway Interaction, Biocarta, and KEGG databases for signaling pathway analysis and into the Gene Ontology (GO) database for an analysis of gene functions. The mRNA expression of TRIB3 and FBN1, two differentially expressed genes, was detected by real-time fluorescence quantitative PCR. The primers were as follows: TRIB3 (430 bp), 5′-AAGAAGCGGTTGGAGTTGG-3′ (forward) Figure 1. VE-statin/Egfl7 expression in normal brain tissue, glioma and U251 cells in glioma. A: There is no Egfl7 expression in gliocytes of normal brain tissue. B: There is Egfl7 expression in neurons of normal brain tissue. C: Negative control; D: There is Egfl7 expression in WHO Grade I glioma. E: There is Egfl7 expression in WHO Grade II glioma. F: There is no significant Egfl7 expression in U87 glioma cells. G: There is Egfl7 expression in WHO Grade III glioma. H: There is Egfl7 expression in WHO Grade IV glioma. I: There is Egfl7 expression in U251 glioma cells. and 5′-CCTCAGGCTCAGGGATACG-3′ (reverse); FBN1 (400 bp), 5′-CCATAACTGCCGGCTGGGAG-3′ (forward) and 5′-GCTGGTGGGGCGCACTCATC-3′ (reverse).
Figure 1.
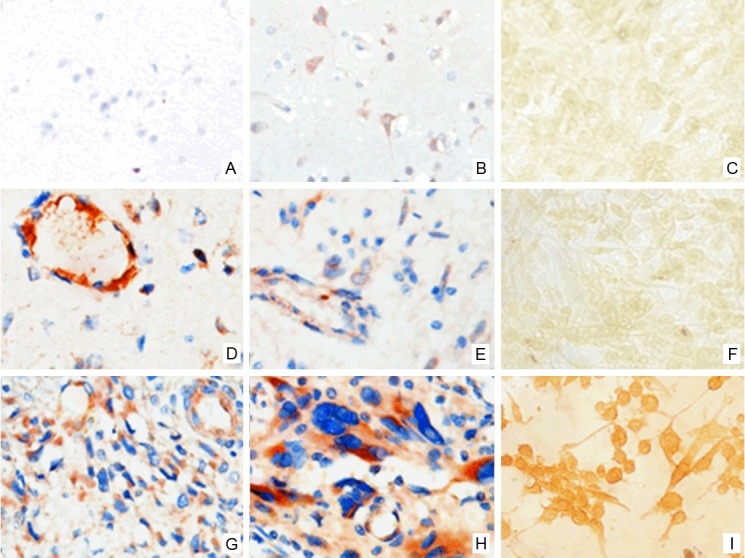
VE-statin/Egfl7 expression in normal brain tissue, glioma and U251 cells in glioma. A: There is no Egfl7 expression in gliocytes of normal brain tissue. B: There is Egfl7 expression in neurons of normal brain tissue. C: Negative control; D: There is Egfl7 expression in WHO Grade I glioma. E: There is Egfl7 expression in WHO Grade II glioma. F: There is no significant Egfl7 expression in U87 glioma cells. G: There is Egfl7 expression in WHO Grade III glioma. H: There is Egfl7 expression in WHO Grade IV glioma. I: There is Egfl7 expression in U251 glioma cells.
Statistical analysis
A statistical analysis was performed with SPSS version 13.0, and the data were expressed as the mean ± standard deviation (x̅±s). A value of P<0.05 was considered statistically significant. Comparisons of the means between two groups were performed with a t test and among multiple groups were performed using one-way analysis of variance (ANOVA) and a subsequent SNK-q test.
Results
Significant correlation between VE-statin/Egfl7 expression and the pathological grade of glioma
VE-statin/Egfl7 expression was positively related to the pathological grade of glioma (P<0.05) but showed no relationship with age or gender (P>0.05) (Table 1). Immunohistochemistry showed that VE-statin/Egfl7 was expressed in both cancer cells and vascular endothelium. To confirm that VE-statin/Egfl7 is indeed expressed in cancer cells, expression was detected in different cancer cell lines, and the results showed that U251, U373, PANC-1, and HepG2 cells were positive and that U87 cells were negative for VE-statin/Egfl7. In addition, gray-matter neurons exhibited VE-statin/Egfl7 expression, whereas the glial cells in both the gray matter and the white matter were negative for VE-statin/Egfl7 expression (Figure 1).
Table 1.
Expression of EGFL7 in gliomas
| n | - | + | ++ | +++ | Positive rate | |
|---|---|---|---|---|---|---|
| Male | 25 | 8 | 6 | 6 | 5 | 68.0% |
| Female | 20 | 6 | 4 | 7 | 3 | 70.0% |
| <40 years | 19 | 7 | 5 | 5 | 2 | 63.2% |
| ≤40 years | 26 | 7 | 5 | 8 | 6 | 73.1% |
| Grade I-II | 24 | 11 | 7 | 6 | 0 | 54.2% |
| Grade III-IV | 21 | 3 | 3 | 7 | 8 | 85.7%a |
vs. Grade I-II, χ2=5.021, P<0.05.
Infection of cells with VE-statin/Egfl7-siRNA lentiviral vectors
Lentivirus expressing VE-statin/Egfl7 siRNA was used to infect U251 cells and HUVECs for 48 h. Under an inverted phase-contrast fluorescence microscope, the infected cells showed a strong green fluorescence; the transfection efficiency was >80%. In contrast, no fluorescence was observed in the control group. Moreover, the fluorescence quantitative PCR results showed that VE-statin/Egfl7 expression was inhibited by >80% compared with the control group (Figure 2).
Figure 2.
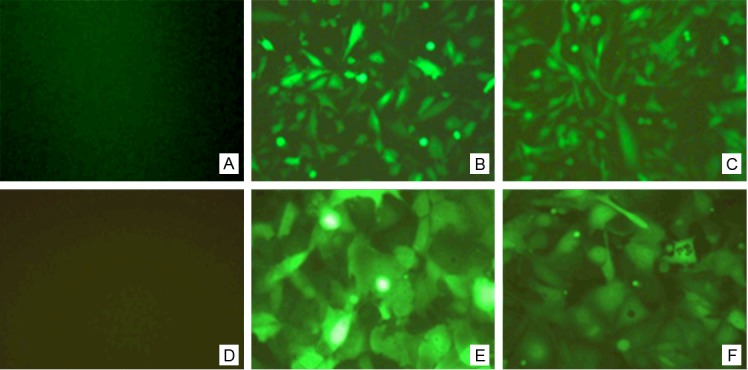
Fluorescence detection of HUVEC and U251 cells infected with the lentiviral expression vectors of siRNA targeting VE-statin/Egfl7. A: Normal negative control U251 cells; B: U251 cells infected with universal negative control lentiviral; C: U251 cells infected with the lentiviral expression vectors of siRNA targeting VE-statin/Egfl7; D: Normal negative control HUVECs; E: HUVECs infected with universal negative control lentiviral; F: HUVECs infected with the lentiviral expression vectors of siRNA targeting VE-statin/Egfl7.
Inhibition of HUVEC angiogenesis after VE-statin/Egfl7-siRNA lentivirus infection
At 6 h after the addition of collagen, the morphology of HUVECs in the control and negative-control groups was altered: most cells were long and spindle shaped and had pseudopodia, and the cells appeared extended, with a cell body that was long and thin. The transformed cells moved continuously over time, and a clustering phenomenon was observed along the longitudinal axis. After culturing for 24 h, capillary lumen-like structures formed by single-layer HUVECs were observed and increased with time. These lumen-like structures were more evident after culturing for 3 d. Conversely, no lumen-like structures were observed in the cells transfected with the VE-statin/Egfl7 siRNA-expressing lentivirus compared with the control group (Score 0). Images were captured after 24 h of culture and quantified with Image-Pro Plus 6.0. The results showed that the area of the lumen-like structures was 0.599±0.052 and 0.587±0.036 and that the number of lumens was 25.20±2.51 and 23.8±2.32 in the control and negative-control groups, respectively, with no significant differences (P>0.05) (Figure 3).
Figure 3.
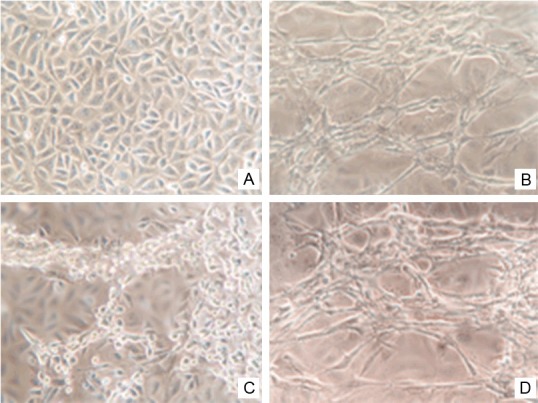
Effect of VE-statin/Egfl7 siRNA on the tube formation in vitro of HUVEC cells. A: Normal negative control HUVECs; B: Capillary lumen-like structures formed by HUVECs with rat tail collagen; C: HUVECs infected with the lentiviral expression vectors of siRNA targeting VE-statin/Egfl7 cannot form intact capillary lumen-like structures added with rat tail collagen. D: Capillary lumen-like structures formed by HUVECs infected with universal negative control lentiviral added with rat tail collagen
Microarray assay of the gene expression profile of VE-statin/Egfl7
Quality control of total RNA and microarray assay
The absorption of total RNA was detected in all samples. The ratio of A260 to A280 was ≥1.8 and of A260 to A230 was ≥1.5 in all samples, and the RIN was >6 for RNA integrity. Two bands, corresponding to 28S and 18S RNA, were observed in the proper proportions. These findings suggest that the extracted RNA had a favorable purity and no degradation, thus meeting the requirements for the microarray assay. The detection of the RNA Cy5 incorporation rate using a NanoDrop ND-1000 showed >10 stained molecules/1000 bases, which indicates that the fluorescence conjugation met the requirement for detection. The mean background intensity was 78.1 and 67.5 (normal: <100), the spacing between spots was even, the signal-to-noise ratio was high, the internal hybridization control (IHC) was >15000, and the IHC CV was >15%, showing that the overall hybridization was suitable. Additionally, detection of the external target quality control (ETQC) demonstrated that the hybridization was specific. These findings suggest that the microarray was of good quality and that the results were reliable.
Bioinformatics analysis of differentially expressed genes
A total of 288 differentially expressed genes were identified, and 17 genes with conserved probes in the microarray were excluded. An unsupervised hierarchical clustering analysis of six samples showed these genes were divided into two clusters in the columns: a normal control group and an experimental group. This finding suggested that these groups were clearly separated and that the differentially expressed genes were specific to the experimental group. These genes were also divided into two clusters: genes with up regulation (red) and genes with down regulation (green). Among the 141 genes showing down regulation, seven had no known function, and 134 genes had identified functions. Among the 130 genes displaying up regulation, three had no known function, and 127 had identified functions. The GO analysis showed 150 functions of related genes, and the following functions were the most related characteristics of these genes: (1) genes related to cell proliferation, differentiation, and apoptosis (n≈50); the regulation of PI3K activity, RAS-related GTPase activity, and the MAPK cascade; the dissociation and phosphorylation of intracellular protein complexes; and cell survival, accounting for 20.3% of all genes, and (2) genes related to adherence to the extracellular matrix (n≈20), accounting for 9.2% of all genes. An analysis of signaling pathways revealed 50 pathways related to these genes, and ERBB regulation was involved in 18 of these pathways (36%). The P value was adjusted with multiple detection to control for the false discovery rate (FDR) and revealed the following significant pathways: 1) epidermal growth factor receptor (EGFR) ERBB family members had the highest differential expression and accounted for 5.54% of the differentially expressed genes, 2) cell survival-related signals had the most evident change in expression, and 3) the integrin ανβ3 signaling pathway was markedly altered (Table 2).
Table 2.
Top 10 enriched pathway terms from the gene set enrichment analysis
| Gene set name | Gene symbol | P valuea |
|---|---|---|
| BIOCARTA_BCELLSURVIVAL_PATHWAY | PIK3CGb, CASP3b, BIRC5 | 0.005 |
| REACTOME_SHC1_EVENTS_IN_ERBB4_SIGNALING | NRASb, NRG1, NRG2, PIK3CGb, PAK3, ABL2, APH1Ab, TRIB3, NEDD4, RALB, WASF1b, ACTG2, PLEKHA1b, F2Rb | 0.010 |
| REACTOME_GRB2_EVENTS_IN_ERBB2_SIGNALING | 0.013 | |
| KEGG_ERBB_SIGNALING_PATHWAY | 0.015 | |
| REACTOME_SIGNALING_BY_ERBB4 | 0.017 | |
| REACTOME_DOWNREGULATION_OF_ERBB2_ERBB3_SIGNALING | 0.030 | |
| REACTOME_P38 MAPK_EVENTS | 0.035 | |
| PID_INTEGRIN3_PATHWAY | TGFβR2, SDC4b, THY1, FBN1b, SMURF2, PIK3CGb | 0.016 |
| PID_P75NTRPATHWAY | CASP3b, APH1Ab, ARHGDIAb, MMP3b, BEX1 | 0.021 |
| KEGG_RENAL_CELL_CARCINOMA | PIK3CGb, NRASb, PAK3, VHLb, PGF | 0.022 |
P-values were adjusted by FDR, which was calculated for the top 50 significant pathway terms;
Downregulated genes.
Real-time fluorescence quantitative PCR for selected genes
Two differentially expressed genes (TRIB3 and FBN1), which had the most obvious change in expression and were related to regulation of the extracellular matrix, were selected for the detection of mRNA expression by RT-PCR. The results showed that the mRNA expression of TRIB3 and FBN1 was significantly different between the control and the experimental groups (P<0.05): 0.58±0.12 and 0.72±0.14, respectively, in the control group, and 1.43±0.20 and 0.37±0.09, respectively, in the experimental group. These findings are consistent with the results of the microarray assay.
Discussion
Similar to other solid tumors, the growth of glioma is highly dependent on angiogenesis. Our group found that VE-statin/Egfl7 was highly expressed in vascular endothelial cells and malignant human glioma cells, which was positively related to the degree of malignancy, cell proliferation, and angiogenesis [6]. In addition, we found that VE-statin/Egfl7 expression in the neurons of rat spinal-cord gray matter (particularly the anterior horn neurons) was at a high level, whereas glial cells were negative for VE-statin/Egfl7 expression. Schmidt et al. [9] found that the VE-statin/Egfl7 protein is secreted from certain neurons of the cerebral cortex and could influence the self-renewal and differentiation of neural stem cells via the Notch signaling pathway. We further investigated VE-statin/Egfl7 expression in the cerebral cortex and subcortical white matter of patients with brain contusion in frontal non-functional areas. We found that VE-statin/Egfl7 expression in gray-matter neurons was at a low level and that the glial cells of both the gray matter and the white matter were negative for VE-statin/Egfl7 expression. These findings suggest that VE-statin/Egfl7 is expressed in a fraction of neurons in the central nervous system but not in glial cells. However, VE-statin/Egfl7 is reactivated in malignant glioma, and expression increases with increasing glioma pathological grade, consistent with previous findings [6], suggesting that VE-statin/Egfl7-positive glioma might have a different cellular origin than VE-statin/Egfl7-negative glioma.
VE-statin/Egfl7 was previously regarded as a new vasoactive factor. In particular, under physiological or pathological conditions, VE-statin/Egfl7 is reactivated and involved in angiogenesis [4]. Furthermore, VE-statin/Egfl7 might be reactivated in certain blood vessel-dependent malignancies, so antiangiogenesis therapy may be promising in the treatment of cancer. Accordingly, over the past 3 years, the correlation between VE-statin/Egfl7 and the occurrence and development of cancer has attracted much attention. Along these lines, in our previous study, a siRNA with a short hairpin structure of VE-statin/Egfl7 was introduced into a lentivirus to construct a vector expressing a GFP-conjugated siRNA targeting VE-statin/Egfl7. The results showed that this vector could significantly inhibit VE-statin/Egfl7 expression in HUVECs and U251 cells [7,8]. Based on these findings, the present study aimed to investigate the role and mechanism of VE-statin/Egfl7 in the endothelium-induced angiogenesis of malignant glioma. Our results revealed that endothelial cells failed to generate capillary lumen-like structures following VE-statin/Egfl7 interference, suggesting that VE-statin/Egfl7 plays an important role in lumen formation during glioma angiogenesis.
Angiogenesis is a crucial event in the rapid growth, invasion, and metastasis of cancers. The results of the present study showed that VE-statin/Egfl7 could regulate angiogenesis in glioma, playing an important role in the malignant development of glioma. Our study further elucidated the significance and mechanism of high VE-statin/Egfl7 expression in malignant human glioma. There is evidence showing that VE-statin/Egfl7 can activate EGFR to increase the phosphorylation of focal adhesion kinase (FAK), which promotes the migration and metastasis of liver cancer cells [5]. In Drosophila, VE-statin/Egfl7 may form complexes with the integrin ligand talin to regulate the activity of the integrin αPS2βPS [10], and the binding of an integrin to talin may activate the FAK signaling pathway. In humans, VE-statin/Egfl7 in the extracellular matrix binds to the integrin ανβ3, which is involved in angiogenesis [11]. During embryonic vascular development and the repair of vascular injury, VE-statin/Egfl7 functions to induce the formation of lumen-like structures, which may be attributed to the clustering migration and the spatiotemporal arrangement of vascular endothelial cells [12]. Previous findings, together with the present results, thus indicate that VE-statin/Egfl7 may regulate glioma angiogenesis, one of molecular mechanisms underlying the malignant development of glioma.
Studies have shown that VE-statin/Egfl7 is expressed in several human malignancies. Moreover, VE-statin/Egfl7 may promote the invasion and metastasis of cancers, and its expression is associated with poor prognosis [13-15]. In the present study, real-time fluorescence quantitative PCR was performed to detect VE-statin/Egfl7 expression in human glioma cell lines (U251, U373, and U87 cells), a human prostate cancer cell line (PANC-1 cells), and a human hepatocellular carcinoma cell line (HepG2 cells), and immunohistochemistry was performed to detect the expression of VE-statin/Egfl7 protein in a human pleomorphic glioblastoma cell line (U251 cells). The results showed that VE-statin/Egfl7 was highly expressed in U251, U373, PANC-1, and HepG2 cells, whereas U87 cells were negative for VE-statin/Egfl7 expression. These findings suggest that VE-statin/Egfl7 is not an endothelium-specific factor, as shown in previous studies, and can be synthesized in certain types of cancer cells [6]. Indeed, VE-statin/Egfl7 expression might be cell-type dependent. In addition to its role in angiogenesis, VE-statin/Egfl7 is involved in the complex regulation of the occurrence and development of cancer. Thus, we employed a microarray assay to investigate the gene expression profile of U251 cells after VE-statin/Egfl7 silencing, aiming to elucidate the molecular mechanisms underlying the regulation of the VE-statin/Egfl7 gene. A total of 271 differentially expressed genes were identified, among which 141 were down regulated, and 130 were up regulated. Further bioinformatics analyses indicated that the genes with the most evident changes in expression and with the highest proportion were related to signaling pathways involved in cell proliferation, invasion, and apoptosis. This group included genes regulating the PI3K and Ras signaling pathways (20.3%) and genes related to adherence to the extracellular matrix (9.2%). Our results showed that high VE-statin/Egfl7 expression was closely related to the proliferation and invasion of cancer cells, which is consistent with our previous findings [6]. The downstream factors of VE-statin/Egfl7 are involved in the EGFR family ERBB signaling pathway, the integrin ανβ3-dominant pathway associated with regulation of the extracellular matrix, and pathways related to apoptosis.
The human ERBB receptor family includes four types of transmembrane tyrosine kinase receptors: ERBB1-4. ERBB1 is also known as EGFR, which functions in the form of homodimers or heterodimers, and there is evidence that EGFR/ERBB1 mRNA expression increases by >50% in primary malignant glioma and plays an important role in the development of this cancer [1]. ERBB2/ERBB3 expression also increases in glioma, whereas ERBB4 expression remains unchanged. These proteins function via PI3K and Ras signaling [16]. After silencing the VE-statin/Egfl7 gene, we found that the mRNA expression of PIK3CG and Ras was significantly down regulated (P<0.001), suggesting that VE-statin/Egfl7 promotes the development of glioma via these two signaling pathways. Surprisingly, the ERBB2/ERBB3 ligand NRG1/2 exhibited down regulation after VE-statin/Egfl7 silencing, even though the microarray results showed that the ERBB signaling pathway was closely related to VE-statin/Egfl7. This finding indicates that the VE-statin/Egfl7 gene promotes the development of glioma via both signaling pathways and that VE-statin/Egfl7 may activate PI3K and Ras in an ERBB1-dependent manner that is not associated with the ERBB2/ERBB3 pathway. These results are consistent with findings reported by Wu et al. [5]. The PI3K and Ras signaling pathways are downstream factors of FAK. Our results indicate that ERBB1 is a target of VE-statin/Egfl7 and that VE-statin/Egfl7 may directly or indirectly bind to ERBB1, resulting in FAK phosphorylation, which subsequently activates the PI3K and Ras signaling pathways and a series of changes in gene expression. In our future studies, co-immunoprecipitation and pull-down assays will be employed to detect interactions between these factors.
Signaling pathway analysis showed that VE-statin/Egfl7 was most closely related to the integrin signaling pathway. The FAK signaling pathway is an important downstream pathway of integrins and is dependent on the binding of talin to integrins. In humans, VE-statin/Egfl7 in the extracellular matrix binds to the integrin ανβ3 to promote angiogenesis [11], and VE-statin/Egfl7 may function in glioma in an integrin/FAK-dependent manner. In the present study, the differentially expressed genes related to integrins included THY1, TGFβR2, and SMURF2 (up regulated) and SDC4, FBN1, PIK3CG, and RAS (down regulated). THY1 is also known as TGFβR2 and is a receptor for TGFβ, and SMURF2 is a Smad-specific E3 ubiquitin ligase 2, a classic molecule mediating the TGFβ signaling pathway. Classic TGFβ signal transduction is a relatively definite linear cascade that involves the ligand TGF-β/BMP, the receptor complex TGFβR1/2, and signal transduction factors of the Smad family. In particular, Smad2,3 is related to TGFβ signal transduction and may regulate the expression of related genes. Our signaling pathway analysis showed that VE-statin/Egfl7 mainly functions to inhibit the TGFβ signaling pathway. However, the role of TGFβ in cancers is very complex and may promote or inhibit cancer growth. In addition, TGFβ is a target of integrins and may interact with ERBB via the PI3K/Akt and Ras/MAPK signaling pathways [17-19]. PI3K/Akt and Ras/MAPK may in turn reduce TGFβ/Smad-induced apoptosis and cell cycle arrest. Additionally, TGFβ may activate signaling pathways (Ras/MAPK, Notch, and PI3K/Akt), including the Smad signaling pathway, which may facilitate the pro-cancer effect of TGFβ in an interaction that is cell-type dependent [17]. Although TGFβ expression was found to be up regulated in glioma, further studies are required to investigate the interaction between TGFβ and ERBB1. Indeed, it remains unclear whether inhibition of the downstream molecules of TGFβ by VE-statin/Egfl7 increases the interaction between TGFβ and other signaling pathways; inhibits cell apoptosis; and promotes the proliferation, invasiveness, survival, and EMT of glioma cells. In addition, our pathway analysis showed that VE-statin/Egfl7 promoted the expression of SDC4 and FBN1. SDC4 is a widely distributed, multi-ligand transmembrane proteoglycan that functions in intercellular and intracellular signal transduction. The ligands of SDC4 include growth factors and the extracellular matrix. SDC4 may promote the signal transduction of growth factors, activate ERK signals via the PKCα pathway, and be involved in the proliferation and migration of cells and endothelium-induced angiogenesis [20,21]. The fibrillin FBN1 is an important component of the extracellular matrix and is closely related to integrins and TGFβ. FBN1 is composed of several EGF-like repeats that are largely calcium dependent, and calcium binding to the EGF-like domain may prevent proteolysis. The RGD domain of FBN1 may bind to the integrins α5β1, αvβ3, and αvβ6, and there is evidence that the binding of the integrins αvβ3 and α5β1 to FBN1 may promote the proliferation and migration of endothelial cells. Moreover, the LTBP-1 domain of TGFβ1 can competitively bind to FBN1 [22,23]. Therefore, TGFβ1, FBN1, and SDC4 play important roles in the formation of integrin complexes and their signal transduction. Studies have also revealed that the EGFR may interact with integrins in an Src-dependent manner, with FAK as the key molecule initiating the downstream cascade [24,25]. The present findings, together with our previous results, show that VE-statin/Egfl7 could regulate important signal molecules (such as TGFβ1, FBN1, and SDC4) to modify the activity of integrin complexes and that the interaction between integrins and ERBB1 increases FAK activity.
Although the results of our previous study indicated that VE-statin/Egfl7 could promote the proliferation of U251 cells (a glioma cell line), flow cytometry revealed that cell apoptosis was unchanged. In the present study, a microarray assay showed that certain pro-apoptotic genes were up regulated (TRIB3) or down regulated (CASP3) and that certain anti-apoptotic genes were up regulated (BIRC5). These findings indicate that VE-statin/Egfl7 may exert both anti-apoptotic and pro-apoptotic effects that maintain a balance. In addition, the regulation of glioma cell apoptosis by VE-statin/Egfl7 is not involved in the role of VE-statin/Egfl7 in gliomas, which is consistent with our previous findings. Therefore, glioma gene therapy targeting VE-statin/Egfl7 (including the ERBB1 gene) requires concomitant anti-apoptotic treatment; otherwise, such therapy may induce drug resistance.
Based on our previous findings, a microarray assay was performed to detect differentially expressed genes after VE-statin/Egfl7 gene silencing in U251 cells. Our results showed that VE-statin/Egfl7 functions via the PI3K/Akt and Ras/MAPK signaling pathways. VE-statin/Egfl7 could regulate the interaction between integrins and ERBB1 to influence FAK activity, triggering the PI3K/Akt and Ras/MAPK cascades. Our findings provide a foundation for future studies on the functions and molecular mechanisms of VE-statin/Egfl7 in glioma.
Acknowledgements
The study was supported by Excellent Youth Project of Bureau of Education of Hunan Province (NO. 11B100) and Scientific Fund of Bureau of Health of Hunan Province (NO.B2010-134), “Western Light” Visiting Scholar Fund Central Ministry of Organization, Scientific Fund of Bureau of Science of Hunan Province (No. 2009JT3049/14JJ2019) and National Natural Science Foundation of China (81272798).
Disclosure of conflict of interest
None.
References
- 1.Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, Brennan C, Ligon KL, Furnari F, Cavenee WK, Depinho RA, Chin L, Hahn WC. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 3.Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor- domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009;50:1839–1850. doi: 10.1002/hep.23197. [DOI] [PubMed] [Google Scholar]
- 6.Huang CH, Li XJ, Zhou YZ, Luo Y, Li C, Yuan XR. Expression and clinical significance of EGFL7 in malignant glioma. J Cancer Res Clin Oncol. 2010;136:1737–1743. doi: 10.1007/s00432-010-0832-9. [DOI] [PubMed] [Google Scholar]
- 7.Huang CH, Li XJ, Luo Y, Huang J, Yuan XR. Construction and identification of lentiviral vector-mediated short hairpin RNA of human EGFL7 gene. Chin J Neurosurg Dis Res. 2009;8:513–516. [Google Scholar]
- 8.Zhou GL, Huang CH, Wan Y, He J. Effect of short hairpin RNA targeting human EGFL7 gene on proliferation of glioblastoma cell line U251. Chin J Modern Med. 2011;21:2702–2706. [Google Scholar]
- 9.Schmidt MH, Bicker F, Nikolic I, Meister J, Babuke T, Picuric S, Muller-Esterl W, Plate KH, Dikic I. Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat Cell Biol. 2009;11:873–880. doi: 10.1038/ncb1896. [DOI] [PubMed] [Google Scholar]
- 10.Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 2010;137:785–794. doi: 10.1242/dev.043703. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K, Baumgart J, Simon K, Thal SC, Patra C, Harter PN, Plate KH, Engel FB, Dimmeler S, Eble JA, Mittelbronn M, Schafer MK, Jungblut B, Chavakis E, Fleming I, Schmidt MH. EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood. 2013;121:3041–3050. doi: 10.1182/blood-2011-11-394882. [DOI] [PubMed] [Google Scholar]
- 12.De Maziere A, Parker L, Van Dijk S, Ye W, Klumperman J. Egfl7 knockdown causes defects in the extension and junctional arrangements of endothelial cells during zebrafish vasculogenesis. Dev Dyn. 2008;237:580–591. doi: 10.1002/dvdy.21441. [DOI] [PubMed] [Google Scholar]
- 13.Li JJ, Yang XM, Wang SH, Tang QL. Prognostic role of epidermal growth factor-like domain 7 protein expression in laryngeal squamous cell carcinoma. J Laryngol Otol. 2011;125:1152–1157. doi: 10.1017/S0022215111002441. [DOI] [PubMed] [Google Scholar]
- 14.Philippin-Lauridant G, Baranzelli MC, Samson C, Fournier C, Pinte S, Mattot V, Bonneterre J, Soncin F. Expression of Egfl7 correlates with low-grade invasive lesions in human breast cancer. Int J Oncol. 2013;42:1367–1375. doi: 10.3892/ijo.2013.1820. [DOI] [PubMed] [Google Scholar]
- 15.Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC, Bonneterre J, Trottein F, Faveeuw C, Soncin F. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011;71:7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- 16.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, Wheeler DL, Kuo JS. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia. 2012;14:420–428. doi: 10.1596/neo.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow A, Arteaga CL, Wang SE. When tumor suppressor TGFbeta meets the HER2 (ERBB2) oncogene. J Mammary Gland Biol Neoplasia. 2011;16:81–88. doi: 10.1007/s10911-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti F, Finetti F, Ziche M, Simons M. The syndecan-4/protein kinase Calpha pathway mediates prostaglandin E2-induced extracellular regulated kinase (ERK) activation in endothelial cells and angiogenesis in vivo. J Biol Chem. 2013;288:12712–12721. doi: 10.1074/jbc.M113.452383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang E, Albadawi H, Watkins MT, Edelman ER, Baker AB. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc Natl Acad Sci U S A. 2012;109:1679–1684. doi: 10.1073/pnas.1117885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MR, Summers KM. Structure and function of the mammalian fibrillin gene family: implications for human connective tissue diseases. Mol Genet Metab. 2012;107:635–647. doi: 10.1016/j.ymgme.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Mariko B, Ghandour Z, Raveaud S, Quentin M, Usson Y, Verdetti J, Huber P, Kielty C, Faury G. Microfibrils and fibrillin-1 induce integrin-mediated signaling, proliferation and migration in human endothelial cells. Am J Physiol Cell Physiol. 2010;299:C977–987. doi: 10.1152/ajpcell.00377.2009. [DOI] [PubMed] [Google Scholar]
- 24.Ray RM, Li C, Bhattacharya S, Naren AP, Johnson LR. Spermine, a molecular switch regulating EGFR, integrin beta3, Src, and FAK scaffolding. Cell Signal. 2012;24:931–942. doi: 10.1016/j.cellsig.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomar A, Schlaepfer DD. A PAK-activated linker for EGFR and FAK. Dev Cell. 2010;18:170–172. doi: 10.1016/j.devcel.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


