Abstract
p63 protein is widely used to identify myoepithelial cells in breast disease. There have been no comparative studies of the p63 antibodies which detect different isoforms. In this study, we examine the expression profiles of p63 protein in benign proliferative diseases and malignant tumors of the breast using pan-p63 and p40 antibodies, and analyze their diagnostic utility and clinical implications. We selected 32 adenoses, 34 intraductal papillomas, 31 ductal carcinoma in situ (DCIS), 257 invasive ductal carcinoma (IDC), and 36 metaplastic carcinomas, and created tissue microarray blocks from them. Immunohistochemical assays for p63 protein were performed on these samples. We investigated the expression patterns of the pan-p63 (TP63, 4A4, Dako, 1:700), p40 antibody [5-17, CalBiochem/EMD Biosciences, 1:2000, p40 (CB)], and p40 antibody [polyclonal, Diagnostic BioSystems, 1:100, p40 (DB)] in various forms of breast disease. We determined that p63 and p40 (DB) expression in myoepithelial cells was broadly similar and showed cognate clinicopathologic features, unlike p40 (CB). p40 (CB) was more sensitive (99.0%) but less specific (85.8%), and p63 was less sensitive (93.8%) in adenosis, IP, and DCIS. In IDCs, p63 and p40 (DB) had similar expression in cancer cells; p40 (CB) expression, however, was statistically different. In metaplastic carcinomas, both p63 and p40 (DB) had distinct expression profiles, according to their histologic subtypes. We conclude that p40 antibodies as well as pan-p63 antibody are specific and sensitive myoepithelial cell markers. Interpretation of p40 positivity in cancer cells, however, should be considered carefully, due to their relatively lower specificity.
Keywords: p63, p40, breast
Introduction
Immunohistochemical analysis is one of the most commonly used methods of pathologic diagnosis in a broad spectrum of breast diseases. For detecting individually specified cell types on immunohistochemical analysis, p63 is often used as a sensitive marker to identify myoepithelial cells [1,2]. Particularly, p63 with nuclear activity is believed to be more specific and sensitive than other myoepithelial cell markers such as CD10, SHHCH, and calponin, which show cytoplasmic positivity [2]. As p63 can be used to discriminate between invasive ductal carcinoma and sclerosing adenosis or to identify myoepithelial cells in papillary neoplasm, p63 is helpful in diagnosing metaplastic carcinoma of the breast, revealing nuclear positive over 90% of metaplastic carcinoma cases [3].
p63 exists in several isoforms; we studied TAp63 and ΔNp63 (p40), which have different N-terminal domains, such as transactivation domain (TA domain) and transcriptionally inactive ΔN domain, respectively [4]. In immunohistochemical analysis of p63, 4A4 antibody, which could detect both of TAp63 and ΔNp63 isoforms, was previously the most widely used pan-p63 marker in pathological diagnosis. Lately, however, it is being replaced by the p40 antibody, which can selectively detect ΔNp63 isoforms and has only recently become available [5]. Some recent studies for p40 utility in lung cancer diagnosis reported that the p40 antibody was more specific than p63 in distinguishing pulmonary squamous cell carcinoma from adenocarcinoma [6,7]. However, the application of p40 antibodies for diverse breast tumors remains somewhat ambiguous.
In this study, we explored the expression profiles of p40 and p63 in an array of breast diseases, and found that these may be valuable myoepithelial markers for detecting myoepithelial cells or cancer cells in the diagnosis of particular breast diseases.
Materials and methods
Case selection
We selected surgical tissue paraffin blocks from the pathology archives of Severance hospital, using 32 cases of adenosis, 34 cases of intraductal papilloma, 31 cases of ductal carcinoma in situ (DCIS), 257 cases of invasive ductal carcinoma (IDC), and 36 cases of metaplastic carcinoma.
Breast cancer cases which had been surgically resected in Severance hospital were diagnosed as IDC, not specific type (NST) (from January 2006 to December 2006) and metaplastic carcinoma (from January 2005 and December 2011). Patients who received pre-operation neoadjuvant chemotherapy or hormonal treatment were excluded. We retrieved various clinicopathologic factors, such as patient age, survival, tumor recurrence, tumor stage, lymph node metastasis, histologic grade, expression status of estrogen receptor (ER)/progesterone receptor (PR)/HER-2, and Ki-67 labeling index (LI). The histological grade of IDC and metaplastic carcinoma were assessed using the Nottingham grading system [8]. We subdivided metaplastic carcinomas into several groups, according to the histologically dominant features: squamous cell differentiation, spindle cell metaplasia, rhabdoid differentiation, and matrix-producing. Pathologic parameters such as ER, PR, and HER-2 status were obtained from patients’ pathologic reports. A cut-off value of 1% or more positively stained nuclei was used to define ER and PR positivity [9]. HER-2 staining was analyzed, according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines, using the following categories: 0 = no immunostaining; 1+ = weak incomplete membranous staining, less than 10% of tumor cells; 2+ = complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+ = uniform intense membranous staining in at least 30% of tumor cells [10]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 1+ were regarded as negative. The cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization (FISH).
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital. The authors, including a breast pathologist (Koo JS), retrospectively reviewed the histology of all cases using H&E stained slides.
Tumor phenotype classification
In this study, we classified breast cancer phenotypes according to immunohistochemistry results for ER, PR, HER-2 and Ki-67 and FISH results for HER-2 as follows [11]: luminal A type: ER or/and PR positive and HER-2 negative and Ki-67 LI < 14%, Luminal B type: (HER-2 negative) ER or/and PR positive and HER-2 negative and Ki-67 LI ≥ 14%, (HER-2 positive) ER or/and PR positive and HER-2 overexpressed or/and amplified, HER-2 overexpression type: ER and PR negative and HER-2 overexpressed or/and amplified, TNBC type: ER, PR, and HER-2 negative.
Tissue microarray
We reviewed H&E-stained slides and retrospectively selected formalin-fixed paraffin-embedded (FFPE) tumor tissue samples. We choose the most representative tumor areas, and the corresponding FFPE sample cores that were removed were as small as 3 mm. We made two cores for each case. These separate tissue cores were assembled in an array fashion.
Immunohistochemistry
Immunohistochemical staining was performed by Ventana Discovery XT automated stainer (Ventana Medical System, Tucson, AZ, USA) after antigen retrieval using CC1 buffer (Cell Conditioning 1; tris-base buffer PH 8.0, Ventana Medical System). Antibodies used for detecting p63 protein were as follows: p40 antibody [5-17, CalBiochem/EMD Biosciences, 1:2000, p40 (CB)], p40 antibody [polyclonal, Diagnostic BioSystems, 1:100, p40 (DB)], and p63 (TP63, 4A4, Dako, 1:700). The proper positive and negative control samples were included in the TMA blocks, and the immunohistochemistry was performed under the same conditions for all samples. We assess p63 or p40 positivity when nuclear staining was identified in the myoepithelial cells or the cancer cells.
Interpretation of immunohistochemical staining
We made a detailed assessment for the expression of the antibodies listed above for p63 protein in the various breast diseases. We evaluated each of the expression profiles in the myoepithelial cells of adenosis, intraductal papilloma, and DCIS, and the cancer cells of IDC and metaplastic carcinoma. Only nuclear staining was interpreted as positive for p63 and p40 expression in the myoepithelial cells of adenoses, intraductal papillomas, and DCIS. In addition, 5% was used as the positive cut off of p63 or p40 positivity in IDC and metaplastic carcinoma [3].
Sensitivity, specificity, Positive Predictive Palue (PPV), Negative Predictive Value (NPV), and accuracy
We calculated the values of sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and accuracy, in analyzing p40 and p63 expressions in the myoepithelial cells and luminal ductal cells, using the following definition: sensitivity = 100 × true positive/(true positive + false negative); specificity = 100 × true negative/(true negative + false positive); PPV = 100 × true positive/(true positive + false positive); NPV = 100 × true negative/(true negative + false negative); and accuracy = 100 × (true positive + true negative)/(true positive + true negative + false positive + false negative). Table 1 presents the definitions of true positive, false positive, true negative, false negative for p63 and p40 immunostaining. These measurement methods were applied to adenosis, intraductal papilloma, and DCIS.
Table 1.
Definition of True Positive, False Positive, True Negative, False Negative in p63 and p40 Immunostaining
| Cell compartment | Myoepithelial cell | Luminal ductal cell |
|---|---|---|
| p63 or p40 positive | True positive | False positive |
| p63 or p40 negative | False negative | True negativetests |
Statistical analysis
Data were processed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Student’s t and Fisher’s exact tests were used to examine differences in continuous and categorical variables, respectively. Two-tailed Mann-Whitney tests were used for comparing p63, p40 (DB), and p40 (CB) immunohistochemical expression profiles in IDCs and metaplastic carcinomas. P-values of less than 0.05 were used to determine significance.
Results
Immunohistochemical analysis of p63, p40 (DB), and p40 (CB) for breast disease according to the composed cell compartment
We collected and reviewed cases diagnosed as adenosis, intraductal papilloma, DCIS, IDC, and metaplastic carcinoma in the breast and made tissue microarray blocks. Then, we performed immunohistochemical analysis of the various breast diseases, using p63 and p40 primary antibodies, for identifying and comparing immunohistochemical profiles (Table 2).
Table 2.
Immunohistochemical Profiles of p63, p40 (DB), and p40 (CB) in Each Cell Component According to the Breast Disease
| Diagnosis | Adenosis n = 32 (%) | Intraductal papilloma n = 34 (%) | DCIS n = 31 (%) | IDC n = 257 (%) | Metaplastic carcinoma n = 36 (%) | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||
| Cell compartment | Myoepithelial cell | Luminal cell | Myoepithelial cell | Luminal cell | Myoepithelial cell | Cancer cells | Cancer cells | Cancer cells |
| p63 | ||||||||
| Positive | 32 (100.0) | 0 (0.0) | 31 (91.2) | 0 (0.0) | 28 (90.3) | 1 (3.2) | 7 (2.7) | 9 (25.0) |
| Negative | 0 (0.0) | 32 (100.0) | 3 (8.8) | 34 (100.0) | 3 (9.7) | 30 (96.8) | 250 (97.3) | 27 (75.0) |
| P40 (DB) | ||||||||
| Positive | 32 (100.0) | 0 (0.0) | 31 (91.2) | 0 (0.0) | 29 (93.5) | 1 (3.2) | 5 (1.9) | 10 (27.8) |
| Negative | 0 (0.0) | 32 (100.0) | 3 (8.8) | 34 (100.0) | 2 (6.5) | 30 (96.8) | 252 (98.1) | 26 (72.2) |
| P40 (CB) | ||||||||
| Positive | 32 (100.0) | 0 (0.0) | 34 (100.0) | 1 (2.9) | 30 (96.8) | 9 (29.0) | 30 (11.7) | 26 (72.2) |
| Negative | 0 (0.0) | 32 (100.0) | 0 (0.0) | 33 (97.1) | 1 (3.2) | 22 (71.0) | 227 (88.3) | 10 (27.8) |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
First, we verified the expression patterns of different myoepithelial markers in proliferative lesions (adenosis) and benign tumors (intraductal papilloma). In cases of adenosis, the markers p63, p40 (DB), and p40 (CB) were all expressed in the myoepithelial cells, not in the luminal ductal cells (Figure 1, top panels). In intraductal papillomas, luminal ductal cells did not express p63 and p40 (DB), however, most cases except three expressed these two markers in the myoepithelial cells (Figure 1, middle panels). The myoepithelial cells in two out of these three cases did not show activity for p63 or p40 (DB). On the other hand, p40 (CB) showed positive activity in the myoepithelial cells of every intraductal papilloma, and even in the luminal ductal cells of one intraductal papilloma.
Figure 1.
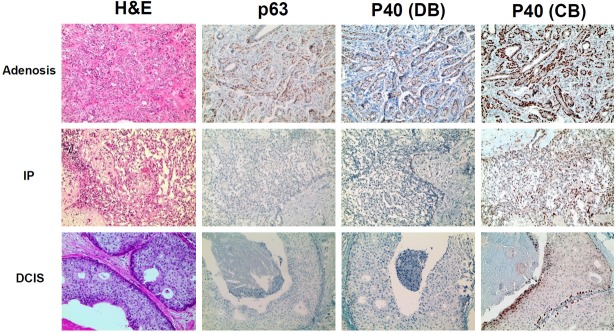
Immunohistochemical expression of p63, p40 (DB), and p40 (CB) in adenosis, intraductal papilloma and ductal carcinoma in situ. In adenosis, all three markers, p63, p40 (DB), and p40 (CB), were expressed in the myoepithelial cells, and not in the luminal ducal cells (top panels). Most intraductal papillomas expressed p63 and p40 (DB) in the myoepithelial cells, but not in luminal ductal cells (middle panels). Most DCISs expressed p63, p40 (DB), and p40 (CB) in the surrounding myoepithelial cells. p40 (CB) showed more frequent positivity in cancer cells of DCISs than the other two myoepithelial markers (bottom panels).
In DCISs, most cases expressed p63 (28 cases, 90.3%) and p40 (DB) (30 cases, 96.8%) in the surrounding myoepithelial cells (Figure 1, bottom panels). On the other hand, p40 (CB) positivity (9 cases, 29.0%) was more frequently observed than p63 and p40 (DB) in the neoplastic cells of DCISs. One DCIS did not show any activity for p63, p40 (DB), or p40 (CB) myoepithelial markers on either myoepithelial cells or neoplastic cells.
Next, we investigated p63, p40 (DB), and p40 (CB) expression profiles in the malignant tumors: IDC and metaplastic carcinoma. In the case of malignant tumors, we examined the expression patterns of myoepithelial markers on the cancer cells, not on the myoepithelial cells, because, by definition, the myoepithelial cells are lost in malignant tumors. Cancer cells expressed p63 in seven cases (2.7%), p40 (DB) in five cases (1.9%), and p40 (CB) in 30 cases (11.7%), respectively, out of 257 invasive ductal carcinomas (Figure 2, top panels). Metaplastic carcinomas more frequently showed nuclear activity in myoepithelial markers in cancer cells: p63 in nine cases (25.0%); p40 (DB) in ten cases (27.8%); and p40 (CB) in 26 cases (72.2%). p40 (CB) had stronger expression in myoepithelial cells than in luminal ductal cells or in cancer cells (Figure 2, middle and bottom panels).
Figure 2.
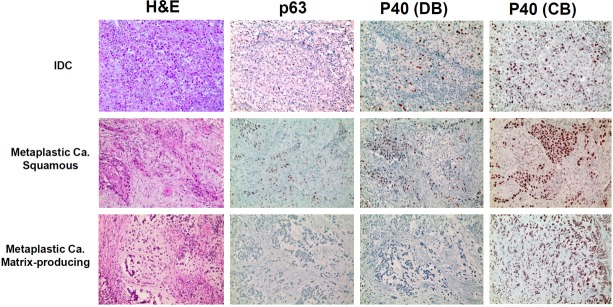
Immunohistochemical expression of p63, p40 (DB), and p40 (CB) in IDC and metaplastic carcinoma. In IDCs, cancer cells showed more frequent nuclear positivity, with p40 (CB) exhibiting the greatest positivity, then p63 and p40 (DB) (top panels). Squamous subtypes of metaplastic carcinomas showed positive activity for all the myoepithelial markers in the cancer cells (middle panels). Matrix-producing types showed positive activity exclusively for p40 (CB) (bottom panels).
Comparison of p63, p40 (DB), p40 (CB) expression profiles in myoepithelial cells according to the classification of breast disease
We performed a comparative, in-depth analysis of the expression profiles of myoepithelial markers in the various breast disease, based on the above immunohistochemical results. Table 3 presents the sensitivity, specificity, PPV, NPV, and accuracy for each myoepithelial cell marker, according to breast disease: adenosis, intraductal papilloma, and DCIS.
Table 3.
Sensitivity, Specificity, PPV, NPV, and Accuracy of p63, p40 (DB), and p40 (CB) in the Myoepithelial Cells According to Breast Disease
| Category | Adenosis | Intraductal papilloma | DCIS | Total (adenosis + intraductal papilloma + DCIS) |
|---|---|---|---|---|
| p63 | ||||
| Sensitivity | 100.0 | 91.2 | 90.3 | 93.8 |
| Specificity | 100.0 | 100.0 | 96.8 | 99.0 |
| PPV | 100.0 | 100.0 | 96.6 | 98.9 |
| NPV | 100.0 | 91.9 | 90.9 | 94.1 |
| Accuracy | 100.0 | 95.6 | 93.5 | 96.4 |
| p40 (DB) | ||||
| Sensitivity | 100.0 | 91.2 | 93.5 | 94.8 |
| Specificity | 100.0 | 100.0 | 96.8 | 99.0 |
| PPV | 100.0 | 100.0 | 96.7 | 98.9 |
| NPV | 100.0 | 91.9 | 93.8 | 95.0 |
| Accuracy | 100.0 | 95.6 | 95.2 | 96.9 |
| p40 (CB) | ||||
| Sensitivity | 100.0 | 100.0 | 96.8 | 99.0 |
| Specificity | 100.0 | 97.1 | 71.0 | 85.8 |
| PPV | 100.0 | 97.1 | 76.9 | 85.7 |
| NPV | 100.0 | 100.0 | 95.7 | 99.0 |
| Accuracy | 100.0 | 98.5 | 83.9 | 91.9 |
DCIS, ductal carcinoma in situ; PPV, positive predictive value; NPV, negative predictive value.
In adenosis, p63, p40 (DB), p40 (CB) expression profiles received 100% marks for all the categories. In intraductal papillomas, p63 and p40 (DB) showed 100% specificity and PPV, and p40 (CB) showed 100% sensitivity and received the highest accuracy (98.5%). In DCISs, p40 (CB) was the most sensitive (96.8%), and p63 had the lowest sensitivity (90.3%). p40 (CB), however, received the lowest scores in the specificity and accuracy categories (71.0% and 83.9%, respectively). Accuracy was highest for p40 (DB) expression (95.2%).
p40 (CB) was the most sensitive (99.0%), but the least specific (85.8%), and p63 was the least sensitive (93.8%) in adenosis, IP, and DCIS. p40 (DB) ranked highest (96.9%) and p40 (CB) lowest (91.9%) in accuracy.
Clinicopathologic features of IDC according to the expression status of p63, p40 (DB), and p40 (CB)
Next, we investigated the correlation of the expression status of myoepithelial cell markers in cancer cells to the clinicopathologic features of IDC cases (Table 4). We classified 257 IDCs according to pathologic features (histologic grade, tumor size, lymph node metastasis, molecular subtype, predictive marker status [estrogen receptor, progesterone receptor, and HER-2], and proliferative index) and clinical features (patient age, tumor recurrence, and survival).
Table 4.
Clinicopathologic Features of Invasive Ductal Carcinoma, According to the Expression Status of p63, p40 (DB), and p40 (CB)
| Parameters | Total N = 257 | p63 | p40 (DB) | p40 (CB) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Negative n = 249 (%) | Positive n = 7 (%) | P-value | Negative n = 251 (%) | Positive n = 5 (%) | P-value | Negative n = 226 (%) | Positive n = 30 (%) | P-value | ||
| Age (years, mean ± SD) | 51.2 ± 11.1 | 51.3 ± 11.2 | 47.8 ± 7.3 | 0.416 | 51.1 ± 11.1 | 57.6 ± 12.1 | 0.199 | 50.5 ± 10.9 | 56.2 ± 11.3 | 0.009 |
| Histologic grade | 0.003 | 0.022 | 0.106 | |||||||
| I | 59 (23.0) | 59 (23.6) | 0 (0.0) | 59 (23.4) | 0 (0.0) | 55 (24.2) | 4 (13.3) | |||
| II | 122 (47.5) | 121 (48.4) | 1 (14.3) | 121 (48.0) | 1 (20.0) | 108 (47.6) | 14 (46.7) | |||
| III | 76 (29.6) | 70 (28.0) | 6 (85.7) | 72 (28.6) | 4 (80.0) | 64 (28.2) | 12 (40.0) | |||
| T stage | 0.299 | 0.316 | 0.516 | |||||||
| 1 | 162 (63.0) | 158 (63.2) | 4 (57.1) | 160 (63.5) | 2 (40.0) | 145 (63.9) | 17 (56.7) | |||
| 2 | 93 (36.2) | 91 (36.4) | 2 (28.6) | 90 (35.7) | 3 (60.0) | 80 (35.2) | 13 (43.3) | |||
| 3 | 2 (0.8) | 1 (0.4) | 1 (14.3) | 2 (0.8) | 0 (0.0) | 2 (0.9) | 0 (0.0) | |||
| Lymph node metastasis | 0.704 | 0.657 | 0.420 | |||||||
| Absent | 164 (64.1) | 160 (64.3) | 4 (57.1) | 160 (63.7) | 4 (80.0) | 147 (65.0) | 17 (56.7) | |||
| Present | 92 (35.9) | 89 (35.7) | 3 (42.9) | 91 (36.3) | 1 (20.0) | 79 (35.0) | 13 (43.3) | |||
| ER | 0.021 | 0.002 | 0.029 | |||||||
| Negative | 73 (28.4) | 68 (27.2) | 5 (71.4) | 68 (27.0) | 5 (100.0) | 59 (26.0) | 14 (46.7) | |||
| Positive | 184 (71.6) | 182 (72.8) | 2 (28.6) | 184 (73.0) | 0 (0.0) | 168 (74.0) | 16 (53.3) | |||
| PR | 0.103 | 0.006 | 0.111 | |||||||
| Negative | 94 (36.6) | 89 (35.6) | 5 (71.4) | 89 (35.3) | 5 (100.0) | 79 (34.8) | 15 (50.0) | |||
| Positive | 163 (63.4) | 161 (64.4) | 2 (28.6) | 163 (64.7) | 0 (0.0) | 148 (65.2) | 15 (50.0) | |||
| HER-2 | 1.000 | 0.587 | 0.639 | |||||||
| Negative | 203 (79.0) | 197 (78.8) | 6 (85.7) | 198 (78.6) | 5 (100.0) | 178 (78.4) | 25 (83.3) | |||
| Positive | 54 (21.0) | 53 (21.2) | 1 (14.3) | 54 (21.4) | 0 (0.0) | 49 (21.6) | 5 (16.7) | |||
| Molecular subtype | 0.049 | < 0.001 | 0.021 | |||||||
| Luminal A | 126 (49.0) | 125 (50.0) | 1 (14.3) | 126 (50.0) | 0 (0.0) | 116 (51.1) | 10 (33.3) | |||
| Luminal B | 63 (24.5) | 62 (24.8) | 1 (14.3) | 63 (25.0) | 0 (0.0) | 56 (24.7) | 7 (23.3) | |||
| HER-2 | 18 (7.0) | 17 (6.8) | 1 (14.3) | 18 (7.1) | 0 (0.0) | 17 (7.5) | 1 (3.3) | |||
| Triple negative | 50 (19.7) | 46 (18.4) | 4 (57.1) | 45 (17.9) | 5 (100.0) | 38 (16.7) | 12 (40.0) | |||
| Ki-67 LI (%, mean ± SD) | 15.6 ± 17.6 | 15.1 ± 17.1 | 30.0 ± 26.6 | 0.028 | 15.2 ± 17.3 | 35.0 ± 20.0 | 0.013 | 15.0 ± 17.3 | 20.0 ± 19.2 | 0.139 |
| Tumor recurrence | 12 (4.7) | 11 (4.4) | 1 (14.3) | 0.287 | 12 (4.8) | 0 (0.0) | 1.000 | 11 (4.8) | 1 (3.3) | 1.000 |
| Patient death | 19 (7.4) | 18 (7.2) | 1 (14.3) | 0.420 | 19 (7.5) | 0 (0.0) | 1.000 | 16 (7.0) | 3 (10.0) | 0.473 |
As a result, p63 positivity in cancer cell components was associated with higher histologic grade (P = 0.003), ER negativity (P = 0.021), triple negative type (P = 0.049), and higher Ki-67 LI (P = 0.028). This result is compatible with previous reports that p63 was more frequently expressed in IDCs of triple negative type and basal-like type [3,12]. p40 (DB) positivity in cancer cell component was associated with higher histologic grade (p = 0.022), ER negativity (P = 0.002), PR negativity (P = 0.006), triple negative type (P < 0.001), and higher Ki-67 LI (P = 0.013). Lastly, p40 (CB) positivity in cancer cell components was associated with older age (P = 0.009), ER negativity (P = 0.029), and triple negative type (P = 0.021). To assess whether each myoepithelial cell marker had a different expression status, we performed Mann-Whitney tests (Figure 3A). The results indicate that p63 and p40 (DB) have a similar tendency to be expressed in cancer cells; however, p40 (CB) expression was statistically different from both p63 and p40 (DB).
Figure 3.
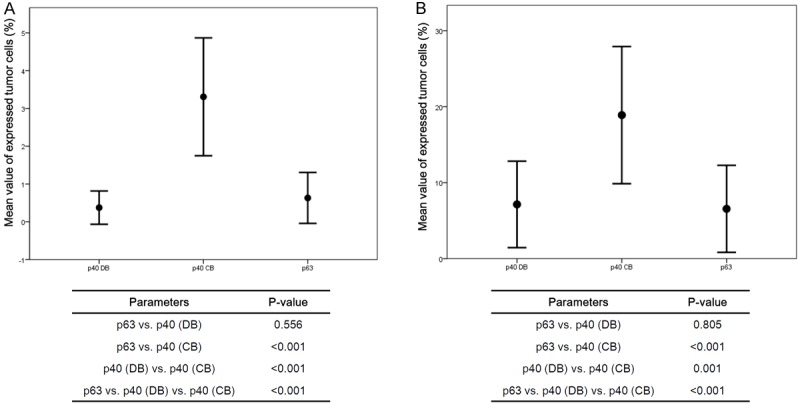
Proportion of tumor cells expressing p63, p40 (DB), and p40 (CB). Among the three myoepithelial markers, p40 (CB) was the most frequently expressed in the cancer cells of IDC (A) and metaplastic carcinoma (B).
Clinicopathologic features of metaplastic carcinoma according to the expression status of p63, p40 (DB), and p40 (CB)
Koker MM, et al. reported that p63 expression in breast cancer is highly sensitive and specific in metaplastic carcinoma [3]. Therefore, we examined whether there was any difference in the expression status between p63 and its isoform, p40, in metaplatic carcinomas. Table 5 shows the comparison of clinicopathologic features in 36 metaplastic carcinomas, according to the positivity of three myoepithelial markers. Interestingly, we found that p63 and p40 (DB) expression profiles were clearly distinct by histologic subtype; they were highly expressed in squamous subtype but rarely expressed in matrix-producing and spindle subtypes (Figure 2, middle and bottom panelss, P < 0.001). When we performed Mann-Whitney test with metaplastic cancers, the results indicated that p63 and p40 (DB) have similar expressions, however, p40 (CB) expression was statistically different from p63 and p40 (DB), much like the expression of IDCs (Figure 3B).
Table 5.
Clinicopathologic Features of Metaplastic Carcinoma, According to the Expression Status of p63, p40 (DB), and p40 (CB)
| Parameters | Total N = 36 | p63 | p40 (DB) | p40 (CB) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Negative n = 27 (%) | Positive n = 9 (%) | P-value | Negative n = 26 (%) | Positive n = 10 (%) | P-value | Negative n = 11 (%) | Positive n = 25 (%) | P-value | ||
| Age (years, mean ± SD) | 52.0 ± 10.1 | 51.3 ± 10.6 | 54.1 ± 8.5 | 0.478 | 51.4 ± 10.8 | 53.4 ± 8.3 | 0.614 | 50.5 ± 11.2 | 52.6 ± 9.7 | 0.575 |
| Histologic grade | 1.000 | 1.000 | 0.703 | |||||||
| II | 11 (30.6) | 8 (29.6) | 3 (33.3) | 8 (30.8) | 3 (30.0) | 4 (36.4) | 7 (28.0) | |||
| III | 25 (69.4) | 19 (70.4) | 6 (66.7) | 18 (69.2) | 7 (70.0) | 7 (63.6) | 18 (72.0) | |||
| T stage | 0.798 | 0.784 | 0.505 | |||||||
| 1 | 11 (30.6) | 8 (29.6) | 3 (33.3) | 8 (30.8) | 3 (30.0) | 5 (45.5) | 6 (24.0) | |||
| 2 | 16 (44.4) | 13 (48.1) | 3 (33.3) | 12 (46.2) | 4 (40.0) | 3 (27.3) | 13 (52.0) | |||
| 3 | 9 (25.0) | 6 (22.2) | 3 (33.3) | 6 (23.1) | 3 (30.0) | 3 (27.3) | 6 (24.0) | |||
| Lymph node metastasis | 0.648 | 1.000 | 0.678 | |||||||
| Absent | 28 (77.8) | 20 (74.1) | 8 (88.9) | 20 (76.9) | 8 (80.0) | 8 (72.7) | 20 (80.0) | |||
| Present | 8 (22.2) | 7 (25.6) | 1 (11.1) | 6 (23.1) | 2 (20.0) | 3 (27.3) | 5 (20.0) | |||
| ER | 1.000 | 1.000 | 0.216 | |||||||
| Negative | 33 (91.7) | 25 (92.6) | 8 (88.9) | 24 (92.3) | 9 (90.0) | 9 (81.8) | 24 (96.0) | |||
| Positive | 3 (8.3) | 2 (7.4) | 1 (11.1) | 2 (7.7) | 1 (10.0) | 2 (18.2) | 1 (4.0) | |||
| PR | 0.443 | 0.484 | 0.524 | |||||||
| Negative | 34 (94.4) | 26 (96.3) | 8 (88.9) | 25 (96.1) | 9 (90.0) | 10 (90.9) | 24 (96.0) | |||
| Positive | 2 (5.6) | 1 (3.7) | 1 (11.1) | 1 (3.8) | 1 (10.0) | 1 (9.1) | 1 (4.0) | |||
| HER-2 | 1.000 | 1.000 | 0.306 | |||||||
| Negative (0, 1+) | 35 (97.2) | 26 (96.3) | 9 (100.0) | 25 (96.2) | 10 (100.0) | 10 (90.9) | 25 (100.0) | |||
| Equivocal (2+) | 1 (2.8) | 1 (3.7) | 0 (0.0) | 1 (3.8) | 0 (0.0) | 1 (9.1) | 0 (0.0) | |||
| Histologic Subtype | < 0.001 | < 0.001 | 0.210 | |||||||
| Matrix-producing | 23 (63.9) | 22 (81.5) | 1 (11.1) | 21 (80.8) | 2 (20.0) | 9 (81.8) | 14 (56.0) | |||
| Rhabdoid | 1 (2.8) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (4.0) | |||
| Spindle | 5 (13.9) | 5 (18.5) | 0 (0.0) | 5 (19.2) | 0 (0.0) | 2 (18.2) | 3 (12.0) | |||
| Squamous | 7 (19.4) | 0 (0.0) | 7 (77.8) | 0 (0.0) | 7 (70.0) | 0 (0.0) | 7 (28.0) | |||
| Ki-67 LI (%, mean ± SD) | 42.5 ± 21.0 | 46.5 ± 21.0 | 31.1 ± 17.4 | 0.057 | 45.2 ± 20.2 | 36.0 ± 22.5 | 0.249 | 40.9 ± 16.8 | 43.3 ± 23.0 | 0.757 |
| Distant metastasis | 5 (13.9) | 4 (14.8) | 1 (11.1) | 1.000 | 4 (15.4) | 1 (10.0) | 1.000 | 2 (18.2) | 3 (12.0) | 0.631 |
Discussion
Tumor protein p63, a member of the p53 family, has several isoforms. TAp63 and ΔNp63 (p40) are known as the two major isoforms of p63. TAp63’s utility in the diagnosis of cancer has been reported in tumors for several organs. The diagnostic utility of p40, however, is still not well understood, particularly in breast disease. Thus, we examined diverse aspects of expression status of these two p63 isoforms in the cell components of breast disease ranging from benign proliferative lesions to malignant tumors. In order to verify their utility in diagnosing breast disease, we used immunohistochemical analysis to compare the expression profiles of p63 to p40, using pan-p63 antibody and p40 antibody, respectively.
Previous studies reported that p63 is a highly sensitive myoepithelial marker, showing up in 100% of expression profiles in normal myoepithelial cells [1,2]. We confirmed that p63 is very sensitive in detecting myoepithelial cells of benign proliferative lesions, such as adenosis and intraductal papilloma. Further, we found that p40 (DB) has the same expression in these cells as p63. Although p40 (CB) showed the greatest sensitivity among these three myoepithelial markers in the two benign lesions and DCIS, it had the lowest specificity. It was expressed not only in the myoepithelial cells but also in the luminal ductal cells of adenosis and intraductal papilloma cases and in the tumor cells of DCIS, respectively. p40 (CB) expression was more frequently observed in tumor cells of DCIS than in luminal ductal cells of adenosis and intraductal papilloma. The expression intensity in tumor cells, however, was notably lower than that for myoepithelial cells. Therefore, we suggest that, in the practical diagnosis of DCIS, there is little chance for p40 (CB) to misdiagnose tumor cells as myoepithelial cells.
One of the drawbacks of using p63 is that it occasionally reveals positive activity in cancer cells of IDC [2,3,12,13]. We also observed that p40 positivity and p63 positivity of cancer cells (2.7% of p63, 1.9% of p40 [DB], and 11.7% of p40 [CB]) in IDC was within the range of p63 positive frequency, which had been reported in the previous studies. Interestingly, we discovered that IDCs which show p63 and p40 (DB) activity in cancer cells, showed resemblances in the expression rate and clinicopathologic features between the two. IDCs with p40 (CB) activity, however, were significantly different to those with p63 and p40 (DB) activity, not only in expression rate, but also in clinicopathologic features. We were not sure whether the high sensitivity and lower specificity of p40 (CB) caused these differences, or whether p40 (CB) might be more detectable in cancer cells with myoepithelial differentiation.
Next, we examined the expression status of myoepithelial cell markers in metaplastic carcinoma, since p63 is known as a very sensitive and specific marker for this type of breast cancer, showing over 90% expression frequency [3]. In our study, however, the expression rate for each myoepithelial cell marker was much lower than previously reported. We believe that lower expression profiles of p63 in our study compared with the previous series could be explained by limitation of the TMA ability to accurately reflect data about large and heterogenous tumor specimens of metaplastic carcinoma. When we histologically subdivided metaplastic carcinomas, according to the specific differentiations, both p63 and p40 (DB) expression status were different. Using these histologic subtypes, most cases with squamous differentiation showed positive activities; however, only minor cases with spindle cell differentiation or matrix-producing type showed positive activity. Unlike p63 and p40 (DB), p40 (CB) showed high expression in metaplastic carcinomas, regardless of histologic subtype.
Thus we found that there are distinctions in expression profile between p40 (CB) and p40 (DB) antibodies, which detect the same epitope of p63 protein. The difference is only that the former is monoclonal and the latter is polyclonal. The monoclonal antibody p40 (CB) had higher sensitivity and lower specificity than p40 (DB), a polyclonal antibody, which differs from the common knowledge. Because we performed the immunohistochemical assays under identical conditions, with the only variations in antibodies, we could not affirm why there were discrepancies in expression profiles among them. We considered p63 and p40 (DB) antibodies to be virtually interchangeable in the diagnosis of breast disease, but, our results highlight that we should be careful in using p40 (CB) positive cells for diagnosis, except when applied to myoepithelial cells.
In summary, we investigated the expression patterns of pan-p63 and p40 antibodies in various breast diseases, from benign proliferative diseases to malignant tumors, according to cellular components, myoepithelial cells, and cancer cells. We tested the validity of these myoepithelial markers and found that p63 and p40 (DB) expression status in myoepithelial cells were statistically similar in each other, while p40 (CB) positivity was more frequently observed in luminal cells, cancer cells, and myoepithelial cells. Therefore, we concluded that p40 antibodies as well as pan p63 antibody are very specific and sensitive myoepithelial cell markers; however, p40 positivity in the cancer cell component should be interpreted carefully, considering its relatively lower specificity.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012R1A1A1002886). This research has been supported by Korean Breast Cancer Foundation.
Disclosure of conflict of interest
None.
References
- 1.Barbareschi M, Pecciarini L, Cangi MG, Macri E, Rizzo A, Viale G, Doglioni C. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–1060. doi: 10.1097/00000478-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Werling RW, Hwang H, Yaziji H, Gown AM. Immunohistochemical distinction of invasive from noninvasive breast lesions: a comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol. 2003;27:82–90. doi: 10.1097/00000478-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol. 2004;28:1506–1512. doi: 10.1097/01.pas.0000138183.97366.fd. [DOI] [PubMed] [Google Scholar]
- 4.Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, Krammer PH, Melino G. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6:274–285. doi: 10.4161/cc.6.3.3797. [DOI] [PubMed] [Google Scholar]
- 5.Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nonaka D. A study of DeltaNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol. 2012;36:895–899. doi: 10.1097/PAS.0b013e3182498f2b. [DOI] [PubMed] [Google Scholar]
- 7.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 8.Gaetan MacGrogan FM, Usha Raju. WHO Classification of Tumours of the Breast. 2012 [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro-Silva A, Ramalho LNZ, Garcia SB, Brandao DF, Chahud F, Zucoloto S. p63 correlates with both BRCA1 and cytokeratin 5 in invasive breast carcinomas: further evidence for the pathogenesis of the basal phenotype of breast cancer. Histopathology. 2005;47:458–466. doi: 10.1111/j.1365-2559.2005.02249.x. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa M, Moritani S, Murakumo Y, Sato T, Hagiwara S, Suzuki C, Mii S, Jijiwa M, Enomoto A, Asai N, Ichihara S, Takahashi M. CD109 expression in basal-like breast carcinoma. Pathol Int. 2008;58:288–294. doi: 10.1111/j.1440-1827.2008.02225.x. [DOI] [PubMed] [Google Scholar]


