Abstract
We present two rare cases of in situ mantle cell lymphoma (“in situ MCL”) and three cases of MCL with mantle zone growth pattern (MCL-MZGP). The patients include four males and one female, with a median age of 66 years (range, 52 to 86 years). Two present with isolated lymphadenopathy and three with multiple lymphadenopathy. At presentation, the complete blood count (CBC) and serum lactate dehydrogenase (LDH) are normal in all cases. Histologic examination reveals an in situ pattern in two cases and a mantle zone growth pattern in three cases. The staging bone marrow biopsies show minimal involvement by lymphoma in one case and no morphologic evidence of lymphoma in four cases. All cases are positive for cyclin D1, including two with typical MCL phenotype and three with CD5-negativity. Four out of five cases express kappa light chain. FISH study for t(11;14) is performed in three cases, of which one is positive and two are inconclusive. For four patients with a median follow-up of 38 months, three are in clinical remission and one has persistent disease. In conclusion, the “in situ MCL” is associated with incidental finding, indolent clinical course and lower tumor burden. The predominant usage of kappa light chain and frequent CD5-negativity observed in our cases are unusual. We review the clinical and laboratory features of “in situ MCL” cases in the literature.
Keywords: Mantle cell lymphoma, in situ mantle cell lymphoma, t(11;14)
Introduction
Mantle cell lymphoma (MCL) comprises 3 to 10% of non-Hodgkin lymphomas and has an aggressive clinical course with a median survival of 3 to 5 years [1]. MCL is characterized by t(11;14)(q13;q32) translocation involving the CCND1 and immunoglobulin heavy chain genes. This translocation results in overexpression of cyclin D1, which leads to dysregulation of the cell cycle and, combined with chromosomal instability and activation of cell survival mechanisms, contributes to the pathogenesis of MCL [2,4]. MCL typically co-expresses B-cell antigens (CD19 and CD20) and CD5 and lacks CD23, with lambda light chain being more commonly used than kappa light chain. Three histological patterns have been recognized in lymphoid tissues: mantle zone, nodular and diffuse. It is believed that the histological patterns reflect the natural process of disease progression. A larger minority of MCL shows mantle zone pattern that appears to be associated with a better prognosis [3]. On very rare occasions, cyclin D1-positive cells are restricted to the mantle zones in a morphologically inconspicuous lymph node. This histologic pattern has been designated as “in situ MCL”, which is often an incidental finding and associated with indolent clinical behavior [12]. “In situ MCL” is considered as a very early stage of MCL or even a preneoplastic state. Due to its low frequency, the biological behaviors and clinical implications of “in situ MCL” remain unclear. Here, we present the clinicopathological features of five cases of MCL with in situ or MZGP pattern and also review the literature on “in situ MCL”.
Materials and methods
Case selection
We identify five cases of MCL with either “in situ MCL” or MCL-MZGP diagnosed in two tertiary hospitals of the North Shore-Long Island Jewish Health System from 2008 to 2013. All cases are identified on the basis of the histologic and immunophenotypic features.
Histological and immunohistochemical studies
Paraffin-embedded tissue is available from the diagnostic biopsies. Immunohistochemical studies are performed with a panel of pre-diluted antibodies including CD20 (Ventana), CD3 (Ventana), Cyclin D1 (Dako), CD5 (Ventana), CD10 (Ventana), CD23 (Ventana), BCL-6 (Dako) and BCL-2 (Zymed). Staining is performed using automated immunostainer (Ventana Medical System, Tucson, AZ, USA).
Flow cytometric analysis
Cells are stained with a panel of fluorescence labeled monoclonal antibodies including CD3, CD4, CD5, CD7, CD8, CD10, CD19, CD20, CD23, FMC7, kappa and lambda. The cells are washed with PBS for three times and fixed in 4% paraformadehyde PBS solution before analysis. Routine three-color flow cytometric analysis is performed using FACSDiva software (Becton-Dickenson Biosciences, San Jose, California).
Fluorescence in situ hybridization study
Fluorescence in situ hybridization (FISH) analysis is performed on tissue imprints using both LSI IGH/CCND1 dual-color, dual-fusion translocation probe and LSI IGH dual-color, break-apart probe (Abbott Molecular, Des Plains, IL) following the standard protocol.
Immunoglobulin variable gene analysis
Genomic DNA is extracted from formalin-fixed and paraffin embedded tissue sections (QIAGEN GmbH, Hilden, Germany). PCR is performed on the genomic DNAs using 5’ Ig heavy chain variable region leader primer and 3’ constant region primer. The PCR products are used for IGHV gene mutation analysis.
Results
Clinical features
Table 1 summarizes the clinical and laboratory features of the five cases. The patients include four males and one female, with a median age of 66 years (range, 52 to 86 years). Clinical history is remarkable for carcinoma in two patients and non-caseating granuloma in one patient. The remaining two patients have no significant past history. Two patients present with isolated lymphadenopathy and three with multiple lymphadenopathy. At presentation, the CBC and serum LDH are normal in all cases. There is no evidence of splenic or gastrointestinal system involvement. The staging bone marrow biopsies show minimal involvement in one case, whereas no evidence of lymphoma is observed by morphology and cyclin D1 staining in the remaining cases. However, a minute monoclonal B-cell population is identified by flow cytometry in three of the last four cases. Four patients receive chemotherapy and one patient is newly diagnosed. The four patients who received treatment are still alive after a median follow-up of 38 months (range, 34 to 48 months), with three in clinical remission and one with persistent disease.
Table 1.
Clinical and laboratory features of five cases of MCL
| Cases | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 |
|---|---|---|---|---|---|
| Age | 65 | 52 | 86 | 67 | 46 |
| Sex | Female | Male | Male | Male | Male |
| Clinical history | Granuloma | Vocal SCC | Cancer | No | No |
| CBC | |||||
| WBC (X109/L) | 6.3 | 9.0 | 5.6 | 6.9 | 6.4 |
| Hb (g/dL) | 13.5 | 15.5 | 15.3 | 14.5 | 15.2 |
| Plt(X109/L) | 219 | 188 | 248 | 247 | 198 |
| LDH | 146 | 200 | 189 | 196 | 214 |
| Lymphadenopathy | Single | Single | Multiple | Multiple | Multiple |
| Splenomegaly | No | No | No | No | No |
| Site of Bx | Cervical LN | Cervical LN | Cervical LN | Axillary LN | Axillary LN |
| Histology | |||||
| In situ MCL | Yes | Yes | Yes | ||
| MCL-MZGP | Yes | Yes | Yes | ||
| BM involvement | Minimal | No* | No | No* | No* |
| Immunophenotype | |||||
| Surface Igs | kappa | Kappa | Kappa | Kappa | lambda |
| CD19 | + | + | + | + | + |
| CD20 | + | + | + | + | + |
| CD5 | - | - | + | - | + |
| CD23 | + (partial) | - | - | - | - |
| CD38 | + | N/A | + | + | + |
| FMC-7 | + (partial) | + | + | N/A | N/A |
| cyclin D1 | + | + | + | + | + |
| FISH for t(11;14) | # | # | N/A | + | N/A |
| IGHV mutation | Yes | N/A | N/A | N/A | N/A |
| Treatment | Yes | Yes | No | Yes | Yes |
| Follow-up | 38 | 38 | 2 | 48 | 34 |
| Status | AND | AND | Alive | AWD | AND |
AND, alive without disease; AWD, alive with disease; Bx, biopsy; BM, bone marrow; FISH, fluorescence in situ hybridization; Hb, hemoglobin; Ig, immunoglobulin; IGHV, Ig heavy chain variable region; LN, lymph node; N/A, not applicable; Plt, platelet; SCC, squamous cell carcinoma; WBC, white blood cell.
No involvement by morphology and cyclin D1 staining, but flow cytometry shows a minute population of monoclonal B-cells.
In conclusive due to limited number of cells for analysis.
Histopathology and immunohistochemical studies
“In situ MCL”
In case 1 the lymph node is mostly replaced by non-necrotizing granulomas and only scattered lymphoid follicles, some containing reactive germinal centers, and small areas of T-zones are identified between the granulomas (Figure 1A). In case 2 the lymph node biopsy shows architecture preservation with prominent paracortical hyperplasia, some follicles with reactive germinal centers, and presence of sinuses (Figure 1B). In both cases, the mantle zones appear normal or only mildly expanded (Figure 1C and 1D). There is no definitive morphologic evidence of lymphoproliferative disorder. However, flow cytometry performed as part of routine lymphoma work up identify a monoclonal B-cell population in both cases.
Figure 1.
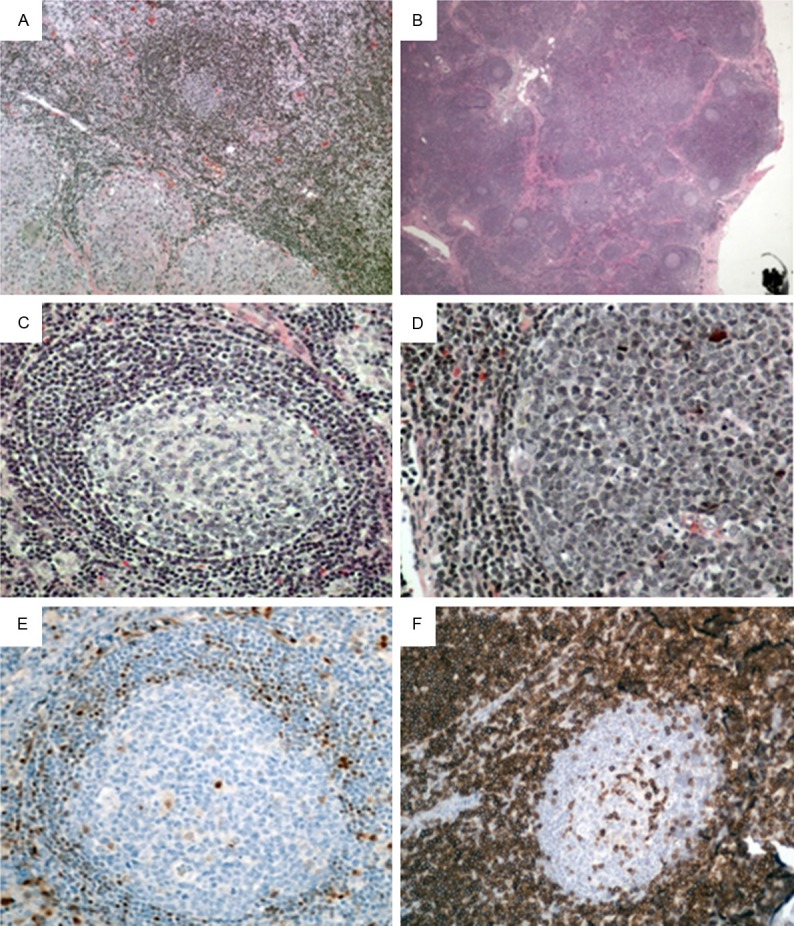
“In situ MCL” in lymph nodes. (A) H&E staining in case 1 reveals a reactive-appearing follicle with germinal center in a background of non-caseating granulomas (X100). (B) H&E staining in case 2 reveals a lymph node with multiple reactive-appearing follicles and prominent paracortical hyperplasia (X20). H&E higher power view of two reactive-appearing follicles with germinal centers and normal mantle zones (X200, C) and (X400, D). (E) Immunohistochemical staining for cyclin D1 highlights scattered positive cells in mantle zones (X200). (F) CD5 (case 2) reveals negative staining in mantle zone cells and numerous T-cells in paracortical regions (X200).
Immunohistochemical studies are subsequently performed on paraffin-embedded tissue sections. The germinal center cells show positive staining for CD20, BCL-6, and are negative for BCL-2. The mantle zone cells show positive staining for CD20, BCL-2 and cyclin D1 (Figure 1E), and are negative for CD5 (Figure 1F). The cyclin D1-positive cells are confined to the mantle zones without mantle zone expansion.
MCL-MZGP
In case 4 and 5, the lymph nodes are partially effaced by crowded follicles containing reactive germinal centers. The mantle zones are markedly expanded and focally fused between adjacent follicles. Occasional primary follicles are also noted. T-zones are present and sinuses are intact in some areas (Figure 2A, 2B and 2E).
Figure 2.
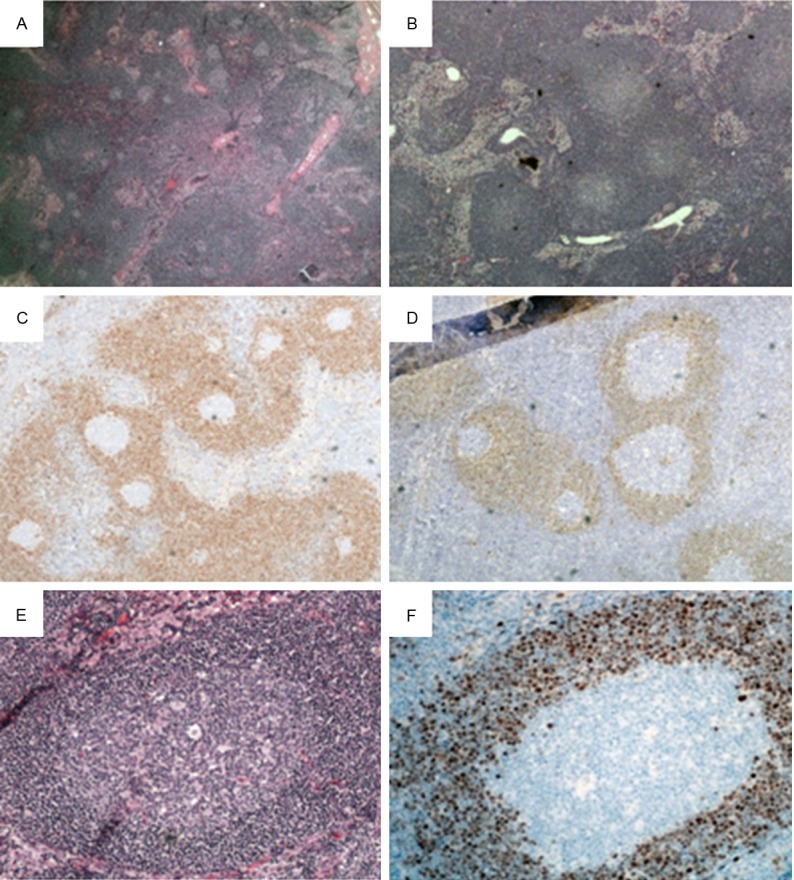
MCL-MZGP in lymph nodes. H&E staining in case 5 (A, X20) and case 4 (B, X40) reveals numerous reactive-appearing follicles with germinal centers and focally intact sinuses. Immunohistochemical staining for cyclin D1 highlight expanded mantle zones in case 5 (C) and case 4 (D) (X40). (E) Higher power view of one reactive-appearing follicle with germinal centers and expanded mantle zones (H&E, X200). (F) Higher power view of cyclin D1 staining in one follicle (X200).
Immunohistochemical studies are performed on paraffin-embedded tissue sections. The mantle zone cells reveal positive staining for CD20 and cyclin D1, and are negative for CD10 and CD23. The neoplastic cells appear to be CD5 positive in case 5, but are negative in case 4. The mantle zones are virtually replaced by cyclin D1 positive cells which focally extend into interfollicular regions (Figure 2C, 2D and 2F). The germinal centers appear positive for CD20, CD10 and bcl-6, and negative for bcl-2. The interfollicular regions comprise of predominantly T-cells.
Mixed “in situ” and MZGP pattern
In case 3, some follicles have normal mantle zones consistent with in situ pattern whereas others show mantle zone expansion consistent with MZGP pattern (Figure 3A). The mantle zone cells show positive staining for CD20, CD5 and cyclin D1 (Figure 3B-D), and are negative for CD10 and CD23.
Figure 3.
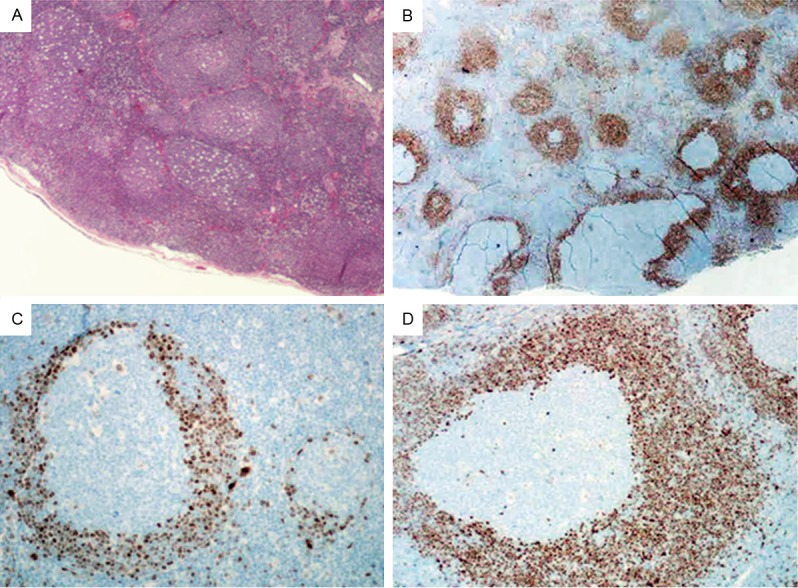
Histopathology and immunohistochemical studies in case 3. (A) H&E staining reveals numerous reactive-appearing follicles (X40). (B) immunohistochemical staining for cyclin D1 reveals a mixture of MCL-MZGP and “in situ MCL” (X40). Higher power view of Cyclin D1 staining for “in situ MCL” (C) and MCL-MZGP (D) (X200).
Flow cytometry findings
In all the cases flow cytometric analysis is performed in both lymph node and bone marrow specimens (Table 1). Two cases show classic MCL phenotype, whereas three cases are CD5-negative, including both “in situ MCL” cases. Interestingly, four out of five cases express kappa light chain. Both CD38 and FMC-7 are positive in all the cases with data available for analysis. CD23 is partially expressed in one case.
FISH study and IGHV gene mutation analysis
In three cases we study the t(11;14) translocation by FISH using both dual-color, dual-fusion translocation probe and dual-color break-apart probe, of which one is positive, and two are inconclusive due to limited number of cells for analysis and both are “in situ MCL”. In addition, we have sufficient material to perform IGHV study using paraffin embedded sections in case 1, which shows a clonal B-cell population with evidence of somatic mutation of IGHV gene.
Discussion
“In situ MCL” is very rare and only a small number of cases have been reported in literature [4-12]. A recent study that evaluates 1292 consecutive reactive lymph nodes from 131 patients reveals no single case of “in situ MCL” [13]. Given its rarity, the biological behaviors and clinical implication of “in situ MCL” remain unclear. Two cases reported here show an in situ pattern in an otherwise reactive lymph node and are both incidental findings and have indolent clinical course, which fulfill the diagnostic criteria for “in situ MCL”. On the other hand, MCL-MZGP appears to have higher tumor burden at presentation than “in situ MCL”. However, we do not observe any difference between the two groups with regard to overall survival. All four patients who receive treatment are still alive at the time of this submission, but one patient with MCL-MZGP has persistent disease. Whether “in situ MCL” represents a precursor lesion or early colonization by MCL is still being debated. Studies have shown that patients with an incidentally diagnosed “in situ MCL” do not progress to overt disease, irrespective of whether treatment has been administrated or not. Furthermore, in some cases “in situ MCL” is retrospectively identified years before the discovery of overt MCL [12,14]. Interestingly, our case 3 shows a mixture of in situ pattern and mantle zone growth pattern in a single lymph node, which illustrates the disease progression. These findings support the notion that “in situ MCL” may represent a precursor or even a preneoplastic lesion. On the other hand, in situ lesions have also been identified in some patients with a previous history of MCL who develop overt MCL soon after the discovery of in situ lesion. One study suggests that these cases should be considered as early colonization by MCL rather than a precursor lesion [13]. It is important to make such a distinction because the managements for these two groups are different.
In reviewing of the literature we identify twenty-one “in situ MCL” cases (Table 2) [4-12]. The clinical and laboratory features of the twenty-one cases plus two current cases are reviewed. The median age at presentation is 65 years (range, 29 to 84 years), with no significant sex predilection. Most patients are asymptomatic and present with a single enlarged lymph node. Extranodal involvement is observed in some patients. Splenic involvement is very rare though bone marrow or/and peripheral blood involvement is observed in a subset of patients. The in situ lesion is usually an incidental finding and most patients are managed with a “watch and wait” approach. The vast majority of patients are alive without disease progression after a median follow up of three years.
Table 2.
Clinical and laboratory features of twenty-one reported in situ MCL cases
| Case | Age/Sex | Site of Bx | Other sites | BM | PB | Management | F/U (yr) | Status | CD5 | Comosite lymphoma | Other pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29/F | Inguinal LN | LNs | Yes | Yes | W&W | 19.5 | AWD | - | - | - |
| 2 | 72/F | Submandibular LN | No | Yes | Yes | W&W | 12 | AWD | - | - | Granuloma |
| 3 | 42/F | Cervical LN | No | No | No | W&W | 1 | AND | - | - | Breast Ca |
| 4 | 84/F | Spleen | No | N/A | No | W&W | 1.4 | Died | - | FL | Colon Ca |
| 5 | 42/M | Supclaviculal LN | No | No | N/A | RT | 1.7 | AND | - | - | Castleman disease |
| 6 | 59/M | Cervical LN | No | No | No | W&W | 5 | AND | - | - | Thyroid ca |
| 7 | 65/F | LN | No | Yes | N/A | Chemo | 0.5 | AND | - | - | Granuloma |
| 8 | 40/M | Cervical LN | No | N/A | N/A | N/A | N/A | N/A | - | FL | - |
| 9 | 70/M | Cervical LN | No | N/A | No | W&W | 4 | MCL | - | - | - |
| 10 | 70/F | Nasopharynx | No | No | No | W&W | 3 | AND | + | - | - |
| 11 | 58/M | Intestine | No | Yes | No | Chemo | 1.4 | AND | + | - | - |
| 12 | 42/F | LNs, GIT | LNs | Yes | No | Chemo | 6 | AND | + | - | - |
| 13 | 78/F | Lacrimal gland | No | No | No | N/A | N/A | N/A | + | eMZL | - |
| 14 | 72/F | Cervical | No | N/A | No | RT | 2 | AND | + | NMZL | Breast Ca |
| 15 | 82/M | Oropharynx | LT | N/A | Yes | W&W | 3 | AWD | + | CLL/SLL | - |
| 16 | 82/M | LN | No | No | No | Chemo | 1.5 | AND | + | CLL/SLL | - |
| 17 | 80/M | Inguinal LN | LNs | Yes | Yes | Chemo | N/A | N/A | + | CLL/SLL | - |
| 18 | 80/M | Cervical LN | No | No | No | Chemo | 1.5 | Died | + | FL | - |
| 19 | 65/F | Intramammal LN | LNs | No | No | Chemo | 5 | AND | + | FL | - |
| 20 | 65/M | Appendix | No | N/A | N/A | W&W | 4 | MCL | + | - | - |
| 21 | 68/M | Mediastinal LN | No | N/A | N/A | W&W | 1 | AND | N/A | - | - |
AND, alive with no disease; AWD, alive with disease; Bx, biopsy; BM, bone marrow; Ca, carcinoma; Chemo, chemotherapy; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; eMZL, extranodal marginal zone lymphoma; F, female; FL, follicular lymphoma; F/U, follow up; GIT, gastrointestinal; LN, lymph node; LT, lymphoid tissue; M, male; N/A, not applicable; NMZL, nodal marginal zone lymphoma; PB, peripheral blood; RT, radiation therapy; W&W, watch and wait; yr, year.
MCL is characteristically CD5-positive, but a small subset has been reportedly CD5 negative, which appears to be associated with indolent clinical course and nodal involvement [15,16]. Based on CD5 expression, twenty-two of the twenty-three cases can be divided into two subgroups: (1) CD5 negative “in situ MCL”. There are eleven patients with a male to female ratio of 5 to 6. The median age is 59 years (range, 29 to 84 years). Most patients (8 of 11 cases) have a clinical history of cancer or inflammatory diseases. All but one patient have nodal involvement. Four patients receive either chemotherapy or radiation therapy. Three cases with IGHV mutation status available for analysis show evidence of somatic hypermutation. (2) CD5 positive “in situ MCL”. There are eleven cases in this group as well, with a median age of 72 years (range, 42 to 82 years). The male to female ratio is 6 to 5. Of the eleven patients, six have extranodal involvement, four have multiple lymphadenopathy, and seven are associated with other low grade B-cell lymphomas. Seven out of ten patients receive treatments. In summary, it appears that CD5 negativity in “in situ MCL” is associated with younger age, history of cancer or inflammatory diseases, nodal involvement, and lower chance to receive treatment, whereas CD5 expression is associated with older age, frequent extranodal involvement, frequent association with other low-grade B-cell lymphomas, and higher chance to receive treatment. However, the two groups do not show any difference in overall survival.
Proposed cells of origin for MCL include memory and naïve splenic marginal zone cells, peripheral memory B-cells, and lymphoid follicle mantle cells [17]. “In situ MCL” is believed to be related to naïve cells of primary follicle cells and of the inner mantle zone of the secondary follicles. Gene expression analysis reveals that MCL with high mutation rates in IGHV gene demonstrates a gene signature similar to memory B-cells while MCL with low mutation rates resembles naïve B-cells [18]. Three of the CD5-negative MCL cases show somatic hypermutation, suggesting that at least a subset of “in situ MCL” may originate from memory B-cells. Further study may help our understanding of the cell origin and pathogenesis of “in situ MCL”.
MCL exhibits predominant expression of lambda light chain, which is different from other lymphomas. The IGHV gene repertoire in MCL is remarkably biased, with four genes (IGHV3-21, IGHV4-34, IGHV1-8, and IGHV3-23) collectively accounting for almost half of the cases [19]. Immunogenetic studies have demonstrated the following features in MCL: (i) remarkable biases in the IGHV gene repertoire; (ii) restricted associations of certain V, D and J genes, leading to the formation of stereotyped B-cell receptors (BCRs); and (iii) very precisely targeted somatic hypermutation (SHM). The bias is very pronounced among IGHV3-21 MCL cases that almost exclusively express lambda light chains and display highly restricted use of the IGLV3-19 gene. These findings suggest that an antigendriven process may be implicated in the pathogenesis of MCL, at least for subsets of cases [20]. As opposed to lambda predominance seen in MCL, our cases show predominant usage of kappa light chain, which is also observed in a small cohort of indolent leukemic MCL [21]. All these cases appear to have a relatively indolent clinical course. Whether they represent a distinct functional group is still unclear at this time. Additional study is needed to address this issue.
In summary, we add two cases to a rare collection of “in situ MCL” that is often incidental finding and associated with indolent clinical course. The kappa predominance and frequent CD5-negativity observed in our cases are unusual for MCL, which suggests a possible antigen-driven origin in these cases. In reviewing of the literature, we have shown that “in situ MCL” appears to be heterogeneous.
Disclosure of conflict of interest
None.
References
- 1.Weisenburger DD, Armitage JO. Mantle cell lymphoma-an entity comes of age. Blood. 1996;87:4483–4494. [PubMed] [Google Scholar]
- 2.Argatoff LH, Connors JM, Klaso RJ. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89:2067–2078. [PubMed] [Google Scholar]
- 3.Majlis A, Pugh WC, Rodriguez MA, Benedict WF, Cabanillas F. Mantle cell lymphoma: correlation of clinical outcome and biologic features with three histologic variants. J. Clin. Oncol. 1997;15:1664–1671. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- 4.Nodit L, Bahler DW, Jacobs SA, Locker J, Swerdlow SH. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003;34:1030–1034. doi: 10.1053/s0046-8177(03)00410-6. [DOI] [PubMed] [Google Scholar]
- 5.Espinet B, Sole F, Pedro C, Garcia M, Bellosillo B, Salidoa M, Florensa L, Camacho F, Baro T, Lloreta J, Serrano S. Clonal proliferation of cyclin D1-positive mantle lymphocytes in an asymptomatic patient: an early-stage event in the development or an indolent form of a mantle cell lymphoma? Hum Pathol. 2005;36:1232–1237. doi: 10.1016/j.humpath.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Aqel N, Barker F, Patel K, Naresh KN. In-situ mantle cell lymphoma: a report of two cases. Histopathology. 2008;52:256–260. doi: 10.1111/j.1365-2559.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodig SJ, Healey BM, Pinkus GS, Kuo FC, Dal Cin P, Kutok JL. Mantle cell lymphoma arising within primary nodal marginal zone lymphoma: a unique presentation of two uncommon B-cell lymphoproliferative disorders. Cancer Genet Cytogenet. 2006;171:44–51. doi: 10.1016/j.cancergencyto.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Roullet MR, Martinez D, Ma L, Fowler MH, Mcphail ED, Judkins A, Arber DA, Bagg A. Coexisting follicular and mantle cell lymphoma with each having an in situ component: A novel, curious, and complex consultation case of coincidental, composite, colonizing lymphoma. Am J Clin Pathol. 2010;133:584–591. doi: 10.1309/AJCP5RT4MRSDGKSX. [DOI] [PubMed] [Google Scholar]
- 9.Bassarova A, Tierens A, Lauritzsen GF, Fossa A, Delabie J. Mantle cell lymphoma with partial involvement of the mantle zone: an early infiltration pattern of mantle cell lymphoma? Virchows Arch. 2008;453:407–411. doi: 10.1007/s00428-008-0621-x. [DOI] [PubMed] [Google Scholar]
- 10.Koletsa T, Markou K, Ouzounidou S, Tsiompanou F, Karkavelas G, Kostopoulos I. In situ mantle cell lymphoma in the nasopharynx. Head Neck. 2013;35:E333–337. doi: 10.1002/hed.23206. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Tzankov A, Xu-Monette ZY, Hoeller S, Wang SA, Richards KL, Zhang S, Said JW, Medeiros LJ, Young KH. Clonally related composite follicular lymphoma and mantle cell lymphoma with clinicopathologic features and biological implications. Hum Pathol. 2013;44:2658–2667. doi: 10.1016/j.humpath.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Carvajal-Cuenca A, Sua LF, Silva NM, Pittaluga S, Royo C, Song JY, Sargent RL, Espinet B, Climent F, Jacobs SA, Delabie J, Naresh KN, Bagg A, Brousset P, Warnke RA, Serrano S, Harris NL, Swerdlow SH, Jaffe ES, Campo E. In situ mantle cell lymphoma: clinical implications of an incidental finding with indolent clinical behavior. Haematologica. 2012;97:270–278. doi: 10.3324/haematol.2011.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam P, Schiefer AI, Prill S, Henopp T, Quintanilla- Martínez L, Bösmüller HC, Chott A, Fend F. Incidence of preclinical manifestations of mantle cell lymphoma and mantle cell lymphoma in situ in reactive lymphoid tissues. Mod Pathol. 2012;25:1629–1636. doi: 10.1038/modpathol.2012.117. [DOI] [PubMed] [Google Scholar]
- 14.Racke F, Simpson S, Christian B, Blum K, Hasserjian R, Zhao W. American Society of Hematology. Evidence of long latency periods prior to development of mantle cell lymphoma. [Abstract]; Dec 6, 2010; San Diego, CA. 2010. [Google Scholar]
- 15.Liu Z, Dong HY, Gorczyca W, Tsang P, Cohen P, Stephenson CF, Berger CS, Wu CD, Weisberger J. CD5-mantle cell lymphoma. Am J Clin Pathol. 2002;118:216–224. doi: 10.1309/TE56-A43X-29TT-5H8G. [DOI] [PubMed] [Google Scholar]
- 16.Orchard J, Garand R, Davis Z, Babbage G, Sahota S, Matutes E, Catovsky D, Thomas PW, Avet-Loiseau H, Oscier D. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101:4975–4981. doi: 10.1182/blood-2002-06-1864. [DOI] [PubMed] [Google Scholar]
- 17.Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–1753. doi: 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Kienle D, Kröber A, Katzenberger T, Ott G, Leupolt E, Barth TF, Möller P, Benner A, Habermann A, Müller-Hermelink HK, Bentz M, Lichter P, Dōhner H, Stilgenbauer S. VH mutation status and VDJ rearrangement structure in mantle cell lymphoma: correlation with genomic aberrations, clinical characteristics, and outcome. Blood. 2003;102:3003–3009. doi: 10.1182/blood-2003-05-1383. [DOI] [PubMed] [Google Scholar]
- 19.Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, Lopez AN, Dagklis A, Rombout P, Beldjord K, Kolstad A, Dreyling MH, Anagnostopoulos A, Tsaftaris A, Mavragani-Tsipidou P, Rosenwald A, Ponzoni M, Groenen P, Ghia P, Sander B, Papadaki T, Campo E, Geisler C, Rosenquist R, Davi F, Pott C, Stamatopoulos K. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:3088–3095. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 20.Agathangelidis A, Hadzidimitriou A, Rosenguist R, Staamatopoulos K. Unlocking the secrets of immunoglobulin receptors in mantle cell lymphoma: implications for the origin and selection of malignant cells. Semin Cancer Biol. 2011;21:299–307. doi: 10.1016/j.semcancer.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Ondrejka SL, Lai R, Smith SD, Hsi ED. Indolent mantle cell leukemia: a clinicopathological variant characterized by isolated lymphocytosis, interstitial bone marrow involvement, kappa light chain restriction, and good prognosis. Haematologica. 2011;96:1121–1127. doi: 10.3324/haematol.2010.036277. [DOI] [PMC free article] [PubMed] [Google Scholar]


