Abstract
Uterine tumors with sex cord-like elements are divided in two groups; uterine tumors resembling ovarian sex cord tumors (UTROSCT), and endometrial stromal tumors with sex cord-like elements (ESTSCLE). UTROSCT is currently defined as the neoplasm predominantly or exclusively composed of sex cord-like elements, and generally behaves in a benign fashion. We studied two unusual cases of UTROSCT with metastasis. One case was a 38-year-old multiparous woman presented with hypermenorrhea. The tumor grew as an intramural mass, and metastasized to a pelvic lymph node. Another case was a 57-year-old woman presented with genital bleeding. The tumor grew as a submucosal exophytic mass, and metastasized to the epiploic appendix. Microscopic examination of the 2 cases revealed that they were composed of sex cord-like cells, epithelioid cells and spindle cells. They exhibited solid pattern in predominance. Both solid and sex cord-like elements showed similar immunoreactivities for more than 3 sex cord markers, but simultaneously showed different staining patterns for some other markers. Characteristic features of endometrial stroma such as tongue-like infiltration and spiral arteries-like arterioles were not observed. RT-PCR analysis confirmed the absence of JAZF1-SUZ12 gene fusion, supporting the histopathological diagnosis of UTROSCT rather than ESTSCLE. The current cases warned the potential risk of UTROSCT whose biological behavior is still uncertain. We discuss histopathological, immunohistochemical and molecular findings of UTROSCT with metastasis.
Keywords: Uterine tumors resembling ovarian sex cord tumors (UTROSCT), endometrial stromal tumors with sex cord like elements (ESTSCLE), metastasis
Introduction
Uterine tumors infrequently demonstrate sex cord-like features. In the landmark publication by Clement and Scully in 1976 [1], 14 cases of uterine tumors resembling ovarian sex cord tumors (UTROSCT) were described as follows: group I tumors represented well-recognized endometrial stromal tumor with focal sex cord-like features, and group II tumors consisted mainly of sex cord-like elements. The current World Health Organization (WHO) classification defines group II tumors as miscellaneous tumors. Group I tumors are conventionally called endometrial stromal tumors with sex cord-like elements (ESTSCLE).
There have been around 50 cases of UTROSCT reported in the literature [2,3]. According to the previous studies and reviews, key features for differential diagnosis between group I and group II UTROSCT are as follows: (1) endometrial stromal tumors with foci of sex cord-like elements are essentially classified into group I; (2) the tumors predominantly or exclusively showing sex cord-like features are classified into group II; and (3) the majority of group II UTROSCT behaves in a benign fashion, whereas group I behaves similarly to low-grade endometrial stromal sarcoma. Pathologists face difficulties in differential diagnosis when the uterine tumors show atypical features that do not conform to the characteristics listed above.
In this study, we investigated 2 cases of UTROSCT that had extrauterine metastasis. Both tumors were composed mainly of CD10-negative epithelioid cells growing in a solid pattern, and typical cord-like structure was often embedded within a solid area. Solid and sex cord-like elements synchronously expressed several epithelial and mesenchymal markers including cytokeratin AE1/AE3, calretinin and CD56. Molecular analysis denied the gene fusion which is common in low grade endometrial stromal sarcoma.
Materials and methods
Immunohistochemistry
The resected tissues were fixed with 10% formalin and embedded in paraffin. Several 4-μm sections were cut from each paraffin block and were stained with hematoxylin and eosin (HE). Immunohistochemistry was done using ENVISION kit (DAKO, Carpinteria, CA) and autoclave antigen retrieval technique according to the manufacturer’s protocol. The source and dilution of antibodies are shown in Table 1. The intensity of the immunostaining was graded as follows; - indicates negative, + indicates focal/weak staining, ++ indicates diffuse/strong staining.
Table 1.
Source and conditions of antibodies
| Antibody | Source | Working dilution |
|---|---|---|
| CK (AE1/AE3) | Progen | 1:600 |
| CD10 | Roche | Prediluted |
| Desmin | Nichirei | Prediluted |
| αSMA | Nichirei | Prediluted |
| HHF35 | Enzo | 1:50 |
| h-caldesmon | DAKO | 1:100 |
| Calretinin | Nichirei | 1:50 |
| Vimentin | DAKO | 1:100 |
| CD56 | Nichirei | Prediluted |
| WT1 | Nichirei | Prediluted |
| CD99 (MIC2) | DAKO | 1:100 |
| FOXL2 | IMGENEX | 1:250 |
| ER | Roche | Prediluted |
| PgR | Roche | Prediluted |
| Inhibin | DAKO | 1:25 |
| Melan A | Novo | 1:20 |
| HMB45 | DAKO | 1:100 |
| EMA | Nichirei | Prediluted |
| CK7 | DAKO | 1:100 |
| CK 19 | DAKO | 1:100 |
| CD34 | Nichirei | Prediluted |
| S100 | Nichirei | Prediluted |
| Bcl-2 | DAKO | 1:50 |
Gene fusion and somatic mutation analyses
RNA and DNA were extracted from the two cases of uterine tumors, respectively, using QIAGEN RNeasy FFPE kit and QIAamp DNA Mini kit (QIAGEN, Hidden, Germany) according to the manufacturer’s instructions. JAZF1-SUZ12/JJAZ1 and SYT-SSX gene fusion products were amplified by RT-PCR, and the exon 1 region of FOXL2 was amplified by PCR, using the primers described previously [4-6]. PCR condition was as follows; at 95°C for 5 min, 35 cycles at 96°C for 5 sec, 60°C for 5 sec, 68°C for 3 sec, with an extension step of 1 min at 72°C at the end of the last cycle. For FOXL2, after purification, DNA was labeled with Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Cleveland OH) and DNA direct sequencing was done using a sequencer ABI Prism 3100 Genetic Analyzer (Applied Biosystems).
The study design was approved by Institutional Review Board of Yokohama City University.
Results
Case 1
A 38-year-old Japanese woman, gravida 2, para 2, had suffered from hypermenorrhea for 4 years. With clinical diagnosis of submucosal leiomyoma, she received GnRH analogue therapy. Although the symptom remitted temporarily, hypermenorrhea relapsed within 3 years. She underwent transvaginal myomectomy at a local hospital. The provisional pathological diagnosis was mixed epithelial and mesenchymal tumor of uncertain malignant potential. She was referred to our hospital for confirming the pathological diagnosis and additional treatment. Preoperative imaging tests indicated a residual myometrial uterine tumor and metastasis to a left internal iliac lymph node. Total hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy were performed.
On macroscopic examination, the tumor mass was located at middle to left side of the uterine body (Figure 1A, left). The sagittal cut surface showed yellowish-white mass measuring 4.5 x 4.0 x 3.5 cm in myometrium (Figure 1A, right). The tumor border was irregular. Necrosis and hemorrhage were not found.
Figure 1.
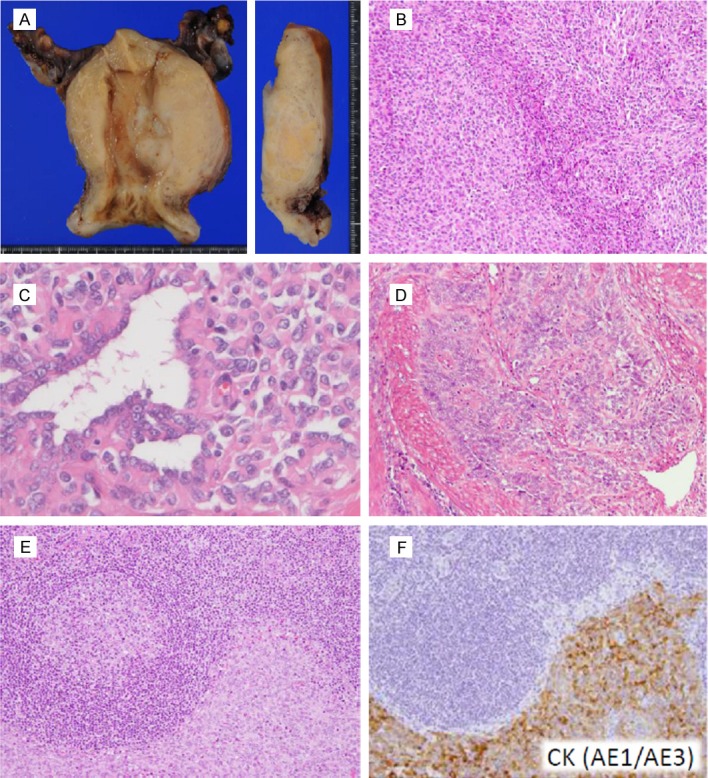
Macroscopic and microscopic features of Case 1. (A) Section of the resected uterus showing infiltrative yellowish-white mass protruding into the lumen is presented (left). The cut surface exhibits irregular tumor border (right). (B, C) The neoplasm shows solid growth pattern predominantly (B) with focal tubular formation (C). (D) Trabecular and cord-like tumor cells deeply infiltrate the myometrium. (E, F) A left iliac internal lymph node shows metastatic tumor with solid pattern, infiltrating adjacent to a germinal center (E). Cytokeratin (AE1/AE3) immunostaining of the serial section highlights metastatic tumor cells (F).
On microscopic examination, the resected uterine tumor grew predominantly in solid pattern composed of epithelioid cells. Some of them had round to oval nuclei with eosinophilic cytoplasm, whereas others had spindle nuclei with scant cytoplasm (Figure 1B and 1C). The majority of tumor cells looked like mesenchymal in origin, but were not alike endometrial stroma. The tumor vasculature did not have spiral artery-like arterioles. Typical cord-like and tubular components were also distributed in the tumor mass (Figure 1D). These sex cord-like components often assimilated into epithelioid or spindle cells. Cellular atypia was mild and mitoses were scarcely detected, 0-1/10 high power field (HPF); however, tumor nests deeply infiltrated smooth muscle bundles of the myometrium with lymphatic invasion. A left internal iliac lymph node had metastasized tumor cells that were positively stained for some epithelial and sex cord markers (Figure 1E and 1F).
After hysterectomy and bilateral salpingo-oophorectomy, the patient has received high-dose progesterone therapy, and no tumor recurrence has been found by clinico-radiological workup for 11 months after total hysterectomy.
Case 2
A 57-year-old Japanese woman, whose reproductive history was unknown, visited our hospital due to vaginal bleeding. Pelvic imaging tests showed a polypoid submucosal mass in the uterus. Total hysterectomy and bilateral salpingo-oophorectomy were performed. A 1 cm-sized nodular lesion was detected in the epiploic appendix, which was also resected.
On macroscopic examination, the polypoid yellowish-white mass measuring 4.5 x 4.0 x 6.4 cm located at the fundus of uterus, protruding into the uterine cavity. The tumor border was demarcated. Necrosis and hemorrhage were not found. The tumor was limited to submucosa, and myometrium was not involved.
On microscopic examination, the polypoid tumor was composed predominantly of monotonous mesenchymal cells (Figure 2A). These neoplastic cells were not alike endometrial stromal cells or leiomyoma cells in shape. They had oval or angular nuclei (Figure 2B), and were often aligned in lacework. Mitosis was not detectable. Spiral arteries-like arterioles were not observed. There was an area composed predominantly of cord-like and tubular components (Figure 2C) containing many foamy cell nests (Figure 2D). The sex cord-like elements were often surrounded by thin fibrotic stroma. Although the tumor grew limitedly in the submucosa without myometrial invasion, tumor cells involved endometrial glands and small veins, suggesting infiltrative nature (Figure 2E and 2F). A nodule in the epiploic appendix was 13 x 11 mm in size, and it was composed of epithelioid cells with oval nuclei identical to the uterine tumor.
Figure 2.
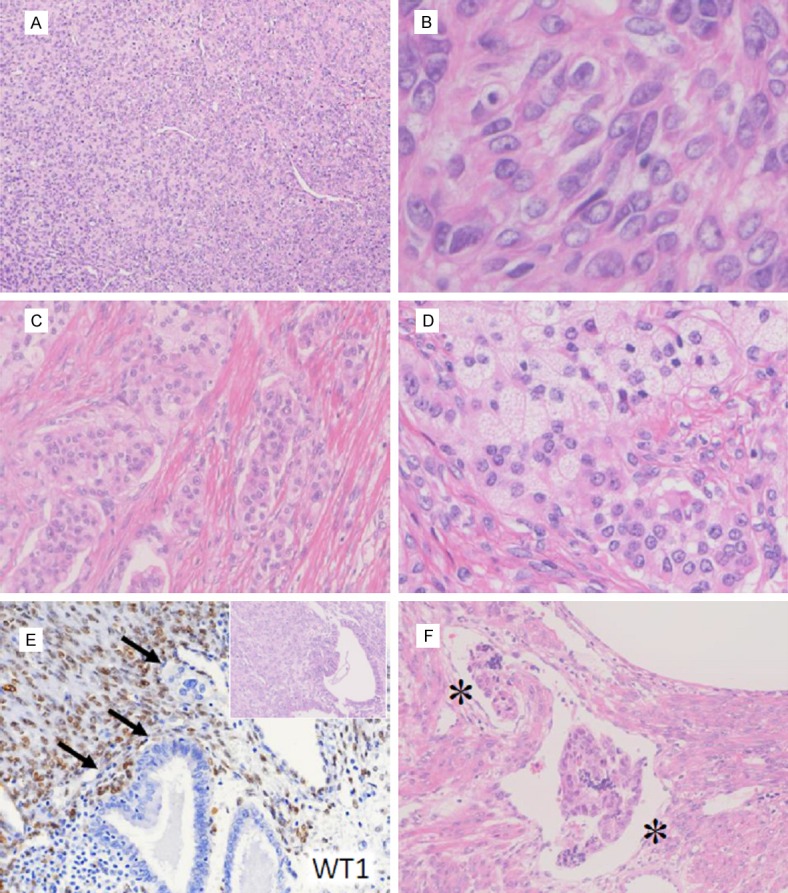
Microscopic features of Case 2. (A, B) The solid area composed of epithelioid cells (A). High-power field of diffusely proliferating tumor cells are shown (B). (C, D) Epitheliod cell nests with sex cord-like differentiation (C) with lipidized cells (D) are shown. (E) Endometrial glands are infiltrated by WT1-positive tumor cells (arrows). Inset: HE staining of the serial section. (F) Vascular space invasion is detected in the tumor periphery. The asterisks indicate the vessel lumen.
The patient did not receive additional therapy, and has been followed for 8 years without evidence of recurrence. The follow up is then ended.
Immunohistochemical and molecular findings
In Case 1, the tumor cells showed strong staining for calretinin in tubular components, and weakly positive staining in solid components (Figure 3A). The other sex cord markers CD99 and CD56 were strongly immunostained in solid parts and focally in tubular component (Figure 3B). An epithelial marker cytokeratin (CK) AE1/AE3 and sex cord markers WT1 and FOXL2 were diffusely positive (Figure 3C), whereas an endometrial stromal marker CD10 was completely negative (Figure 3D). Estrogen receptor (ER) and progesterone receptor (PR) were diffusely stained in both components. Metastatic tumor cells showed similar immunostaining patterns to those in the uterus. The MIB-1 index was 3% at most. The Immunohistochemical findings were summarized in Table 2.
Figure 3.
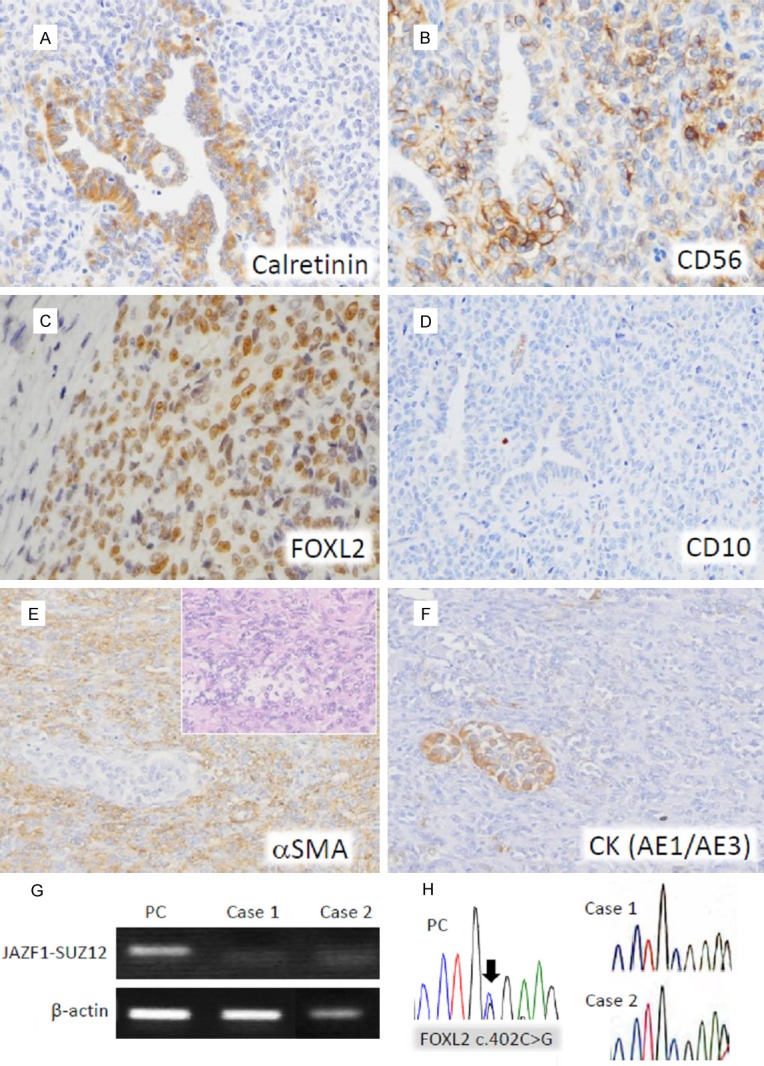
Immunohistochemical staining and molecular analyses. (A-D) Immunostaining in cord-like and solid elements of Case 1: calretinin is expressed preferentially in tubular elements (A), CD56 shows variable positivity in both areas (B), FOXL2 is diffusely positive in both areas (C), and CD10 is unstained (D). (E, F) Immunostaining in cord-like and solid elements of Case 2: α-smooth muscle actin is diffusely positive in the solid lesion (E), whereas cytokeratin (AE1/AE3) is expressed predominantly in the cord-like area. (F) Inset of (E): HE staining of the serial section. (G) RT-PCR analysis for JAZF1-SUZ12 gene fusion. The 93 bp RT-PCR products are detected in a positive control (PC; a case of endometrial stromal sarcoma, low grade), but not in the Cases 1 and 2. (H) Direct DNA sequencing analysis of FOXL2 exon 1 is shown. The FOXL2 c.402C>G somatic mutation is indicated by an arrow in a positive control (PC; a case of adult granulosa cell tumor of the ovary), but not in the Cases 1 and 2.
Table 2.
Summary of immunohistochemical results
| Case 1 | Case 2 | |||
|---|---|---|---|---|
|
| ||||
| Solid pattern | Sex cord-like elements | Solid pattern | Sex cord-like elements | |
| CK (AE1/AE3) | ++ | ++ | - | ++ |
| CD10 | - | - | - | + |
| Desmin | + | - | + | - |
| αSMA | + | - | ++ | - |
| HHF35 | + | - | ++ | - |
| h-caldesmon | + | + | + | - |
| Calretinin | + | ++ | + | - |
| Vimentin | ++ | ++ | ++ | + |
| CD56 | ++ | + | + | + |
| WT1 | ++ | ++ | + | + |
| CD99 (MIC2) | ++ | + | + | + |
| FOXL2 | ++ | ++ | - | - |
| ER | ++ | ++ | ++ | ++ |
| PgR | ++ | ++ | ++ | ++ |
| Inhibin | - | - | - | ++ |
| Melan A | - | - | - | + |
| HMB45 | - | - | - | - |
| EMA | - | - | - | - |
| CK7 | - | - | - | - |
| CK 19 | + | + | - | - |
| CD34 | - | - | - | - |
| S100 | - | - | - | + |
| Bcl-2 | + | + | - | - |
-, negative; +, focal/weak positive; ++, diffuse/strong positive; N/A, not available.
In Case 2, solid areas demonstrated intensive staining for a-smooth muscle actin (αSMA) (Figure 3E) and variable positive staining for CD99, WT1, calretinin and CD56. Cord-like areas were intensively stained for CK AE1/AE3 (Figure 3F) and inhibin. CD10 was unstained in solid areas, but weakly stained in sex cord-like cells. ER and PR were diffusely stained in both areas. The MIB-1 index was at most 1%. The immunohistochemical findings were summarized in Table 2.
In both Case 1 and Case 2, gene fusions of JAZF1-SUZ12 (JJAZ1) (Figure 3G) and SYT-SSX were not detected. FOXL2 gene mutation was also not found (Figure 3H).
Discussion
Differential diagnoses of the present cases were UTROSCT group I and group II, epithelioid variant of leiomyosarcoma, adenosarcoma, perivascular epithelioid cell neoplams (PEComa) and synovial sarcoma. Morphologic features were inconsistent with high grade sarcoma. The possibilities of PEComa and synovial sarcoma were denied by negative staining for HMB45 and the absence of SYT-SSX gene fusion. In Case 1, the tumor cells showed only focal positivity for αSMA and h-caldesmon, thus epithelioid leiomyosarcoma was not probable. In Case 2, solid areas were immunoreactive in part for αSMA and h-caldesmon, but also variably stained for calretinin, CD56 and some other sex cord markers, indicating that they were bipotential or pulripotent. The possibility of CD10-negative endometrial stromal sarcoma had to be carefully investigated in Case 1, because this case showed infiltrative growth pattern that is often seen in endometrial stromal sarcoma. Morphologically, proliferating tumor cells were not alike endometrial stromal cells. In infiltrative fronts, tumor cells often formed trabecular or wormlike pattern, but not “tongue-like” pattern. A previously reported cases of ESTSCLE include CD10-negative tumor [7]. The case was described as high grade ESTSCLE with abundant pleomorphism, which was not seen in Case 1. In Case 2, typical cord-like and trabecular lesions showed patchy CD10 staining. The result should not be used as the evidence of endometrial stromal tumor, because focal CD10-positive staining can occur in ovarian sex cord-stromal tumors [8]. More importantly, uniform distribution of spiral artery-like vessels, which is the key feature of endometrial stromal tumors, was lacked in our cases. All the above-mentioned morphologic and immunohistochemical features of the present cases supported diverse phenotypic profiles of UTROSCT with solid pattern predominance.
UTROSCT has been characterized as exclusively or predominantly sex cord-like in morphology. Irving et al. reported that all 5 UTROSCT they examined were composed of more than 70% of sex cord-like elements and suggested 50% of sex cord-like elements or more would be needed for histological diagnosis [7]. In our cases, typical sex cord-like appearance was often masked by diffuse proliferation of epithelioid or spindle cells. Recent microscopic observation of UTROSCT expanded the original histopathological spectrum manifested by cords, trabeculae, nests and tubules of epithelioid cells to encompass some related features such as retiform, solid, and glomeruloid patterns [9]. Our cases might be a variant with solid pattern predominance.
Although we diagnosed our cases as UTROSCT, there were arguments about compelling evidence for the pathological diagnosis of these tumors with metastasis. At least, immunohistochemical studies did not delineate differential staining patterns of the sex cord-like elements between group I and II tumors [9,10]. Overlapped immunoreactivities for epithelial, mesenchymal, and sex cord markers rather suggest that the tumor composed of such elements may potentially develop and differentiate into more than one lineage that can be characterized by disease-specific chromosomal abnormalities. The genetic study by Staats et al. demonstrated that JAZF1-SUZ12 gene fusion was detectable in a case of ESTSCLE but not in any of 24 cases of UTROSCT [11]. The current 2 cases did not have JAZF1-SUZ12 gene fusion, which we cited as genetic evidence against ESTSCLE. Wang et al. revealed 2 balanced chromosomal translocations: t(X;6) (p22.3;q23.1) and t(4;18)(q21.1;q21.3) in a case of UTROSCT [12]. They also showed intensive immunostaining for bcl-2 mapped on chromosome 18q21.3, indicating the role of bcl-2 in the development of UTROSCT [12]. Bcl-2, however, is also positive in endometrial stromal sarcomas [13]. In the current study, we investigated possible single-base mutation in the FOXL2 gene, mapped on chromosome 3q23 that is frequently observed in adult granulosa cell tumors (AGCT) of the ovary [14]. Histological studies have suggested the similarities of sex cord-like elements between UTROSCT and ovarian sex cord-stromal tumors. Although the tumor cells in Case 1 were diffusely immunostained for FOXL2, direct sequence analysis did not support common genetic abnormality between UTROSCT and AGCT. A recent study revealed that ESTSCLE had PHF1 gene rearrangements with JAZF1 or unknown partners [15]. Further cytogenetic analyses are needed for characterization of uterine tumors with sex cord-like elements and possible differences in genetic rearrangement patterns between group I and group II tumors.
In the literature, there were 3 cases of UTROSCT with metastasis or recurrences. The first case described that metastatic tumor formed tubules with central lumina in omentum [16]. The second case showed solid and tubular patterns in the metastasized small bowel tumor [17]. The third case presented Sertoli-like features in the subcutaneous metastatic lesion [18]. In the current 2 cases, metastatic tumor cells in the lymph node and epiploic appendix showed solid pattern, replicating the predominant morphology of the primary lesions. Although typical cord-like and trabecular patterns were obscure, these metastatic cells were positively immunostained for several sex cord and epithelial markers. Further studies are need to understand biological malignancy of UTROSCT.
In conclusion, we presented 2 cases of UTROSCT with extrauterine metastasis. Case 1 was the first case of lymph node metastasis. Case 2 was followed for 8 years without recurrence. To our knowledge, 8 year was the longest-term follow up of UTROSCT with metastasis.
Acknowledgements
We thank the members of the pathological laboratories in Yokohama City University and Asahikawa Kosei Hospital for excellent technical assistance. This work is partly supported by JSPS KAKENHI Grant Number 1123590406 (to MF).
Disclosure of conflict of interest
None.
References
- 1.Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol. 1976;66:512–525. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- 2.Garuti G, Gonfiantini C, Mirra M, Galli C, Luerti M. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J Minim Invasive Gynecol. 2009;16:236–240. doi: 10.1016/j.jmig.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Giordano G, Lombardi M, Brigati F, Mancini C, Silini EM. Clinicopathologic features of 2 new cases of uterine tumors resembling ovarian sex cord tumors. Int J Gynecol Pathol. 2010;29:459–467. doi: 10.1097/PGP.0b013e3181dfcfdc. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T. Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol. 1998;153:1807–1812. doi: 10.1016/S0002-9440(10)65695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurihara S, Oda Y, Ohishi Y, Iwasa A, Takahira T, Kaneki E, Kobayashi H, Wake N, Tsuneyoshi M. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol. 2008;32:1228–1238. doi: 10.1097/PAS.0b013e31816a3b42. [DOI] [PubMed] [Google Scholar]
- 6.Kim MS, Hur SY, Yoo NJ, Lee SH. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J Pathol. 2010;221:147–152. doi: 10.1002/path.2688. [DOI] [PubMed] [Google Scholar]
- 7.Irving JA, Carinelli S, Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod Pathol. 2006;19:17–24. doi: 10.1038/modpathol.3800475. [DOI] [PubMed] [Google Scholar]
- 8.Oliva E, Garcia-Miralles N, Vu Q, Young RH. CD10 expression in pure stromal and sex cord-stromal tumors of the ovary: an immunohistochemical analysis of 101 cases. Int J Gynecol Pathol. 2007;26:359–367. doi: 10.1097/PGP.0b013e318064511c. [DOI] [PubMed] [Google Scholar]
- 9.Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int J Gynecol Pathol. 2008;27:229–235. doi: 10.1097/PGP.0b013e3181569a21. [DOI] [PubMed] [Google Scholar]
- 10.Baker RJ, Hildebrandt RH, Rouse RV, Hendrickson MR, Longacre TA. Inhibin and CD99 (MIC2) expression in uterine stromal neoplasms with sex-cord-like elements. Hum Pathol. 1999;30:671–679. doi: 10.1016/s0046-8177(99)90093-x. [DOI] [PubMed] [Google Scholar]
- 11.Staats PN, Garcia JJ, Dias-Santagata DC, Kuhlmann G, Stubbs H, McCluggage WG, De Nictolis M, Kommoss F, Soslow RA, Iafrate AJ, Oliva E. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol. 2009;33:1206–1212. doi: 10.1097/PAS.0b013e3181a7b9cf. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Blakey GL, Zhang L, Bane B, Torbenson M, Li S. Uterine tumor resembling ovarian sex cord tumor: report of a case with t(X;6)(p22.3;q23.1) and t(4;18)(q21.1;q21.3) Diagn Mol Pathol. 2003;12:174–180. doi: 10.1097/00019606-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava R, Shia J, Hummer AJ, Thaler HT, Tornos C, Soslow RA. Distinction of endometrial stromal sarcomas from ‘hemangiopericytomatous’ tumors using a panel of immunohistochemical stains. Mod Pathol. 2005;18:40–47. doi: 10.1038/modpathol.3800248. [DOI] [PubMed] [Google Scholar]
- 14.Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 15.D’Angelo E, Ali RH, Espinosa I, Lee CH, Huntsman DG, Gilks B, Prat J. Endometrial stromal sarcomas with sex cord differentiation are associated with PHF1 rearrangement. Am J Surg Pathol. 2013;37:514–521. doi: 10.1097/PAS.0b013e318272c612. [DOI] [PubMed] [Google Scholar]
- 16.Kantelip B, Cloup N, Dechelotte P. Uterine tumor resembling ovarian sex cord tumors: report of a case with ultrastructural study. Hum Pathol. 1986;17:91–94. doi: 10.1016/s0046-8177(86)80161-7. [DOI] [PubMed] [Google Scholar]
- 17.Biermann K, Heukamp LC, Buttner R, Zhou H. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int J Gynecol Pathol. 2008;27:58–60. doi: 10.1097/pgp.0b013e318057faf5. [DOI] [PubMed] [Google Scholar]
- 18.O’Meara AC, Giger OT, Kurrer M, Schaer G. Case report: Recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol Oncol. 2009;114:140–142. doi: 10.1016/j.ygyno.2009.03.021. [DOI] [PubMed] [Google Scholar]


