Abstract
Fibroblast growth factor receptor 2 (FGFR2) is activated in many cancers and considered as a potential therapeutic molecular target including for endometrial endometrioid carcinoma (EEC). Overexpression of FGFR2 isoform IIIc (FGFR2IIIc) has been shown to be associated with carcinogenesis in various cancers, but its expression in EEC has not been reported yet to the best of our knowledge. In this study, we identified roles for FGFR2IIIc in EEC carcinogenesis and demonstrated its diagnostic and prognostic values in EEC. FGFR2IIIc expression was compared between 10 normal endometrium, 10 atypical endometrial hyperplasias, and 47 EEC specimens using immunohistochemistry and quantitative real-time PCR. Atypical hyperplasia, Grade 1 (G1), and Grade 2 (G2) differentiated EEC tissues showed significantly higher FGFR2IIIc expression than normal endometrium tissue. However, as compared to G1 and G2 EECs, Grade 3 (G3) differentiated EEC tissue showed lower FGFR2IIIc expression (P<0.05). There was no significant association between FGFR2IIIc expression and patient age, lymph node metastasis, and EEC stage. These results suggest that altered FGFR2IIIc expression plays an important role in EEC carcinogenesis and may occur in precancerous tissues. However, FGFR2IIIc appears to be not related to EEC progression. Some G3 EECs may develop through different carcinogenic processes than G1 and G2 EECs.
Keywords: Endometrial carcinoma (EC), endometrial endometrioid carcinoma (EEC), fibroblast growth factor receptor 2 (FGFR2), IIIc, carcinogenesis, differentiation
Introduction
Endometrial carcinoma (EC) is one of the most common cancers among women worldwide. About 80% of EC are endometrial endometrioid carcinomas (EECs). EEC may be preceded by precancerous lesions such as hyperplasia and atypical hyperplasia [1,2]. Atypical hyperplasia always shows similar findings to G1 EEC in not only histological appearance but also molecular features [3]. EECs are divided into 3 grades according to histological differentiation: grade 1 (G1), well differentiated; grade 2 (G2), moderately differentiated; and grade 3 (G3), poorly differentiated. Histological grade determines prognosis. As compared to patients with G1 and G2 EECs, patients with G3 EEC present with significantly worse prognosis [4,5].
Despite being a serious public health problem, EC has not drawn enough attention. Moreover, only a few potential molecular therapeutic targets have been identified, the most recent being fibroblast growth factor receptor 2 (FGFR2). FGFR2 and its signalling pathway are reported to be activated in many cancers due to gene amplification and point mutations. In addition, FGFR2 is a potential therapeutic molecular target for patients with FGFR2 activation-associated cancer such as EC [6-12].
Alternative splicing of FGFR2 produces 2 isoforms, FGFR2IIIb and FGFR2IIIc. In normal tissue, FGFR2IIIb is mainly expressed in epithelial cells, while FGFR2IIIc is mainly expressed in mesenchymal or stromal cells [13]. FGFR2IIIc expression has been reported in various cancers including bladder cancer and ovarian cancers [14-19]. However, to our knowledge, the role of FGFR2IIIc in EEC has not yet been clarified. The purpose of the present study was to identify the role of FGFR2IIIc expression in EEC carcinogenesis and to investigate association of FGFR2IIIc expression with clinicopathological features in EEC.
Methods and materials
Case selection
We reviewed the pathology data for 47 EEC patients who underwent surgery at Nippon Medical School hospital. Normal and atypical hyperplasia tissue samples were also obtained from 10 patients respectively. Two certified pathologists (WX Peng and Z Naito) reviewed all cases in order to verify the original histopathological diagnosis, grading, and EEC stage according to the World Health Organization (WHO) classification system and 2008 International Federation of Gynecology and Obstetrics (FIGO) grading of EC. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Informed consent for the usage of tissues was obtained from all patients.
Immunohistochemical analysis
Paraffin-embedded tumors were cut into 3.5-μm thick sections and placed on silane-coated glass slides. The sections were de-waxed in xylene and rehydrated in a series of graded ethanol solutions, and the endogenous peroxidase activity was blocked with a 0.3% H2O2 methanol solution. Before application of the primary antibody, the slides were subjected to antigen retrieval by heating in 10 mM citrate buffer (pH 6.0) for 15 min at 121°C in an autoclave oven. Then, anti-FGFR2IIIc antibody (in house; 1:200 dilution) [20] was applied to the slides, which were incubated overnight at 4°C. The slides were rinsed in 0.01 mol/L phosphate- buffered saline, and bound antibodies were detected with the Simple Stain MAX PO (R) (Nichirei Corp. Tokyo, Japan) using 3,3’-diaminobenzidine tetrahydrochloride as the substrate. The peroxidase reaction was visualized with 0.02% 3,3’-diaminobenzidine tetrahydrochloride containing 0.005% H2O2 in 0.01 M Tris-phosphate buffer (pH 7.4). Finally, the sections were lightly counterstained with hematoxylin.
Blinded immunohistochemical evaluation of each case was carried out independently by the above pathologists.
The immunohistochemical expression of FGFR2IIIc was graded using the immunostain intensity score (IS) (0, completely negative; 1, weekly stained; and 2, moderately to strongly stained), and the graded percentage score (PS) of positive cells (0, less than 5%; 1, 5-50%; and 2, more than 50%). The total score (TS) was then calculated using the following formula: TS=IS×PS. The cases with TS of 4 were classified into high and the others into low expression groups. Subsequently, we analyzed the level of FGFR2IIIc expression according to patient age, tumor differentiation grade, lymph node metastasis, and EEC stage.
Quantitative real-time polymerase chain reaction (qRT-PCR)
To confirm FGFR2IIIc expression in the collected tissues, qRT-PCR was conducted using tissue isolated from formalin-fixed paraffin-embedded (FFPE) specimens. RNA samples were then extracted using RNeasy FFPE kit (Qiagen, Crawley, UK), according to the manufacturer’s instructions. 18S was used as internal control. Then, cDNA was synthesized from total RNA using SuperScript®VILOTM cDNA Synthesis Kit following the manufacturer’s protocol (Invitrogen by Life Technologies, Carlsbad, CA). The corresponding cDNA was amplified using specific primers for FGFR2IIIc, 18S rRNA, and a TaqMan probe (all from Applied Biosystems), with denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15, and annealing at 60°C for 60 seconds.
Statistical analysis
Fisher’s exact test was used for statistical analyses of the results of the immunohistochemical studies. The qRT-PCR findings were analyzed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Differences among means were evaluated by a 2×2 contingency table using Fisher’s exact test or ANOVA followed by Dunnett’s post-hoc test. P<0.05 was considered significant. All results shown represent mean ± SEM.
Results
In normal endometrium tissue, although all 10 cases showed FGFR2IIIc expression, the level in 9 (90%) of them was low (Table 1). In contrast, 80% of atypical hyperplasia and 49% of EEC cases showed high expression of FGFR2IIIc. As compared to normal tissue, FGFR2IIIc expression in both atypical hyperplasia and ECC showed significantly higher expression (P<0.01 and P<0.05, respectively) (Table 1) (Figure 1). However, there was no significant difference between hyperplasia and ECC cases. Similar results were also observed using IS assessment (Table 2).
Table 1.
Association of FGFR2IIIc expression as evaluated by total score (TS) with different histological types
| Normal endometrium n=10 | Hyperplasia n=10 | ECC n=47 | |
|---|---|---|---|
| High TS | 1 (10%) | 8 (80%) | 23 (49%) |
| Low TS | 9 (90%) | 2 (20%) | 24 (51%) |
| P-value | p<0.01 | p<0.05 |
Figure 1.
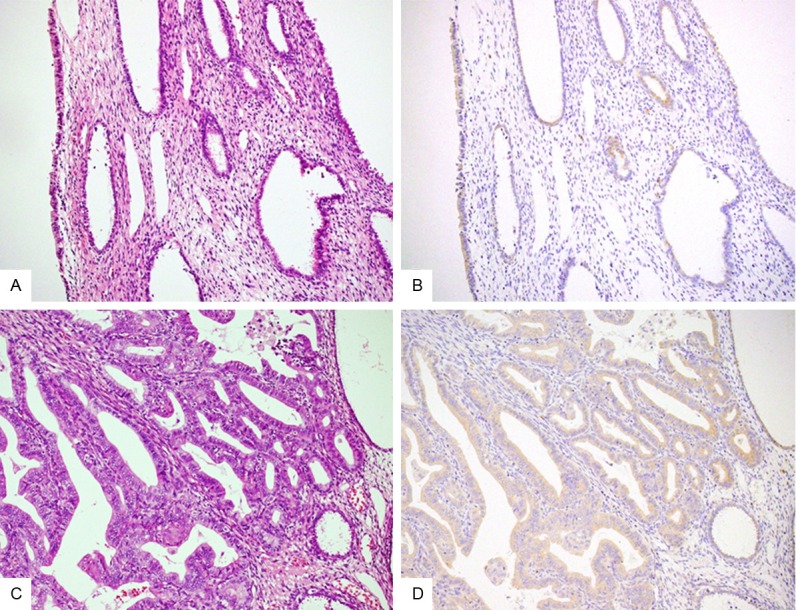
Immunohistochemical results of FGFR2IIIc in normal tissue and hyperplasia. As compared to normal endometrium (A: HE stain; B: immunostain; ×200), atypical hyperplasia cases (C: HE stain; D: immunostain; ×200) show significantly higher expression of FGFR2IIIc.
Table 2.
Association of FGFR2IIIc expression as evaluated by intensity score (IS) with different histological types
| Normal endometrium n=10 | Hyperplasia n=10 | ECC n=47 | |
|---|---|---|---|
| High IS | 1 (10%) | 10 (100%) | 32 (68%) |
| Low IS | 9 (90%) | 0 | 15 (32%) |
| P-value | p<0.001 | p<0.01 |
Clinicopathological features of each EEC case are listed in Table 3. The patients ranged in age from 32-84 years, with a median age of 57 years. Therefore, we divided the patients into 2 age groups: below 57 years (n=22) and 57 years or older (n=25). With regard to histological differentiation, 21 cases were G1, 15 cases were G2, and 11 cases were G3. Among them, 24 patients underwent lymphadenectomy, of whom 3 had lymph node involvement. Moreover, 31 patients had stage I tumors, 3 had stage II tumors, and 13 had stage III or higher stage tumors. In order to investigate the role of FGFR2IIIc in EEC, we analyzed the association between FGFR2IIIc expression level and clinicopathological features. In total, 23 patients (49%) showed high FGFR2IIIc expression (Table 4). Among them, (1) 10 patients were younger than 57 years and 13 were 57 years or older; (2) 14 patients had G1, 7 patients had G2, and 2 patients had G3 EEC; (3) 1 patient had lymph node metastasis while 8 were negative; and (4) 16 patients had stage I tumors and 7 had stage II or higher stage tumors. Although FGFR2IIIc expression was not statistically different between G1 and G2 EECs, G3 EEC showed significantly decreased FGFR2IIIc level as compared to G1 and G2 EECs (P<0.05) (Table 4) (Figure 2). Similar results were obtained using IS assessment (P<0.005) (Table 5). No association was detected between FGFR2IIIc expression and patient age, lymph node metastasis, or stage.
Table 3.
Clinicopathological features of patients
| Clinicopathological features | Cases |
|---|---|
| Age (yrs) | |
| Under 57 | 22 |
| 57 or older | 25 |
| Grade | |
| 1 | 21 |
| 2 | 15 |
| 3 | 11 |
| LN metastasis | |
| Positive | 3 |
| Negative | 21 |
| No data | 23 |
| Stage | |
| I | 31 |
| II | 3 |
| III or higher | 13 |
Table 4.
Association between clinicopathological features and TS
| Clinicopathological features | High TS | Low TS | P-value |
|---|---|---|---|
| Age (yrs) | NS* | ||
| Under 57 (n=22) | 10 (45%) | 12 (55%) | |
| 57 or older (n=25) | 13 (52%) | 12 (48%) | |
| Grade | p<0.05 | ||
| 1 and 2 (n=36) | 21 (78%) | 15 (22%) | |
| 3 (n=11) | 2 (18%) | 9 (82%) | |
| LN metastasis | NS* | ||
| Positive (n=3) | 1 (33%) | 2 (67%) | |
| Negative (n=21) | 8 (38%) | 13 (62%) | |
| No data (n=23) | 14 (61%) | 9 (39%) | |
| Stage | NS* | ||
| I (n=31) | 16 (52%) | 15 (48%) | |
| II or higher (n=16) | 7 (44%) | 9 (56%) |
not significant.
Figure 2.
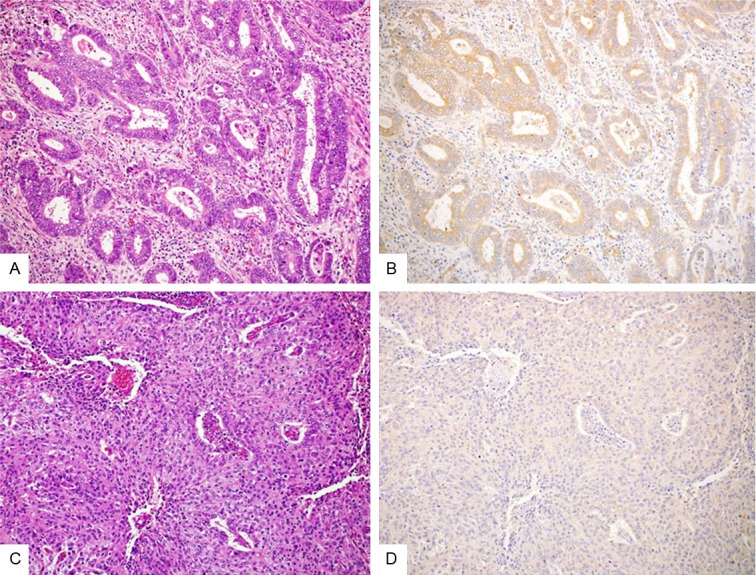
Immunohistochemical results of FGFR2IIIc in G1 and G3 EECs. G1 EEC (A: HE stain; B: immunostain; ×200) shows high FGFR2IIIc expression, but G3 EEC (C: HE stain; D: immunostain; ×200) shows significantly decreased expression of FGFR2IIIc.
Table 5.
Association between clinicopathological features and IS
| Clinicopathological features | High IS | Low IS | P-value |
|---|---|---|---|
| Age (yrs) | NS* | ||
| Under 57 (n=22) | 15 (65%) | 7 (35%) | |
| 57 or older (n=25) | 17 (68%) | 8 (32%) | |
| Grade | p<0.005 | ||
| 1 and 2 (n=36) | 29 (81%) | 7 (19%) | |
| 3 (n=11) | 3 (27%) | 8 (73%) | |
| LN metastasis | NS* | ||
| Positive (n=3) | 2 (67%) | 1 (33%) | |
| Negative (n=21) | 12 (57%) | 9 (43%) | |
| No data (n=23) | 18 (78%) | 5 (22%) | |
| Stage | NS* | ||
| I (n=31) | 20 (65%) | 11 (35%) | |
| II or higher (n=16) | 12 (75%) | 4 (25%) |
not significant.
To determine FGFR2IIIc mRNA level, we performed qRT-PCR using FFPE tissue, and found that FGFR2IIIc mRNA levels in atypical hyperplasia and G1 EEC were significantly higher than that in normal endometrium (Figure 3). However, the FGFR2IIIc mRNA level in G3 EEC was significantly lower than that in G1 EEC and atypical hyperplasia, results which validate the immunohistochemical analysis.
Figure 3.
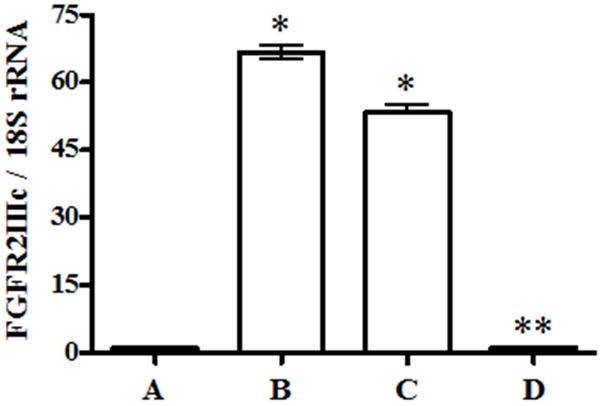
mRNA expression of FGFR2IIIc in tissues. Tissues from atypical hyperplasia (B) and G1 EEC (C) show higher FGFR2IIIc mRNA expression than normal tissue (A). However, G3 EEC (D) shows significantly decreased expression compared with G1 EEC. The data represent mean ± SEM (n=3). *P<0.05 versus A and D. **P<0.05 versus B and C.
Discussion
In EC, different histological types may have different precancerous status and may be associated with distinct molecular and genetic alterations. EEC occurs most frequently in pre- and peri-menopausal women and is strongly associated with excessive estrogen exposure. EEC is usually found to coexist or succeed atypical endometrial hyperplasia, also known as precancerous lesion. The development of EEC is a multistep process, and each step involves accumulation of genetic aberrations. Genomic instability such as microsatellite instability and chromosomal aneuploidy, inactivation of tumor suppressor genes such as PTEN and p53, and activation of oncogenes such as K-Ras and β-catenin are important events in this process [21,22].
Recently, the FGF/FGFR signalling pathway has attracted attention as an important mechanism of carcinogenesis and tumor development. According to the study of Soufla et al, FGF2 up-regulation was found to be strongly related to endometrial carcinogenesis [23]. FGFR2 is one of the most important receptors of the FGF/FGFR pathway. FGFR2 single-nucleotide polymorphisms are strongly related to the oncogenesis of breast cancer and EC [10,24-27]. According to the recent report by Byron et al, activating mutations in FGFR2 were found in 16% of EC cases, and up-regulated FGFR2 mRNA expression was observed in these EC specimens [7]. In EC cells with activated FGFR2, knockdown of FGFR2 induces cell death, suggesting that FGFR2 is important to EC cell proliferation [7]. FGFR2IIIc activation also drives cell proliferation. In prostate cancer, FGFR2IIIc expression correlates with tumor progression [28,29]. In rat bladder cancer cells, FGFR2IIIc expression is correlated with epithelial-to-mesenchymal transition, a phenomenon that is associated with tumor progression and invasion [30]. Furthermore, immunohistochemical analysis of uterine cervical tissue revealed a correlation between FGFR2IIIc expression and the progression of cervical dysplasia. The same result was obtained in an in-situ hybridization validation study [20], suggesting that abnormal FGFR2IIIc expression is an early event in uterine cervix carcinogenesis, and that immunohistochemical staining is a convenient and reliable method to evaluate FGFR2IIIc expression in human tumor tissue.
In this study, we examined the expression and localization of FGFR2IIIc in normal endometrial tissue, atypical hyperplasia, and EEC. Significantly higher FGFR2IIIc expression was observed in atypical hyperplasia and G1 EEC. This provides evidence that FGFR2IIIc expression increases in endometrial tissue as it progresses from normalcy to atypical hyperplasia, and maintains a high level of expression in G1 EEC. This indicates that FGFR2IIIc plays an important role in the carcinogenesis of EEC, and that alteration in its expression may occur during the atypical hyperplasia stage. In the early stage of EEC, high FGFR2IIIc expression is important for tumor growth and maintenance. However, G3 EEC showed significantly lower FGFR2IIIc expression than G1 and G2 EECs, suggesting that FGFR2IIIc may not be related to tumor progression. This result is different from that in prostate and bladder cancers [28-30], suggesting that FGFR2IIIc may play distinct roles in different tissues. Our data are supported by investigations of Voss et al. as well as Kuwabara et al. who found that G3 EEC have similar clinical, immunohistochemical, and prognostic characteristics with other endometrial cancers such as papillary serous carcinoma and clear cell carcinoma rather than G1 and G2 ECCs [4,5]. More genetic aberration accumulations are needed to drive EEC progression and some G3 EEC cases may develop through different carcinogenic processes than G1 and G2 EECs.
G3 differentiation is considered one of the strongest predictors of recurrence and progression in EEC [31,32]. Patients with G3 EEC are recommended for more aggressive therapy. Therefore, correct assessment of grade differentiation is important. It is very common that grade differentiation is often misinterpreted using curettage biopsy specimens because of the limited amount of the sample. Our results show that high FGFR2IIIc expression correlates with G1 and G2 EECs, especially using the immunostain intensity evaluation, but not with G3 ECC. Using limited biopsy specimens, immunohistochemical results for FGFR2IIIc may not only aid in predicting the risk for recurrence and prognosis of patients, but also in deciding the treatment plan.
Recent reports revealed that endometrial cancer cell lines with activating FGFR2 mutations are selectively sensitive to the pan-FGFR inhibitor, PD173074 [32]. Targeted therapy for ECs with overexpression of FGFR2 is under consideration. FGFR2IIIc is an important isoform of FGFR2 and may serve as another good candidate for targeted therapy with pan-FGFR inhibitors in EEC. Additional studies are needed to determine the association between high FGFR2IIIc expression in EEC and susceptibility to FGFR2 inhibitors.
Acknowledgements
This work was supported in part by Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (No. 24791725 to WX Peng) and Grants-in-Aid for Clinical Rebiopsy Bank Project for Comprehensive Cancer Therapy Development to Z.N. from Ministry of Education, Culture, Sport, Science, and Technology, Japan (S1311022).
Disclosure of conflict of interest
There are no conflicts of interest to declare.
References
- 1.Mutter GL. Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol. 2000;19:301–309. doi: 10.1097/00004347-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Boruban MC, Altundag K, Kilic GS, Blankstein J. From endometrial hyperplasia to endometrial cancer: insight into the biology and possible medical preventive measures. Eur J Cancer Prev. 2008;17:133–138. doi: 10.1097/CEJ.0b013e32811080ce. [DOI] [PubMed] [Google Scholar]
- 3.Zakharov V, Lin HK, Azzarello J, McMeekin S, Moore KN, Penning TM, Fung KM. Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int J Clin Exp Pathol. 2010;3:608–617. [PMC free article] [PubMed] [Google Scholar]
- 4.Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124:15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Kuwabara Y, Susumu N, Banno K, Hirao T, Kawaguchi M, Yamagami W, Suzuki N, Aoki D, Nozawa S. Clinical characteristics of prognostic factors in poorly differentiated (G3) endometrioid adenocarcinoma in Japan. Jpn J Clin Oncol. 2005;35:23–27. doi: 10.1093/jjco/hyi003. [DOI] [PubMed] [Google Scholar]
- 6.Langheinrich MC, Schellerer V, Perrakis A, Lohmüller C, Schildberg C, Naschberger E, Stürzl M, Hohenberger W, Croner RS. Molecular mechanisms of lymphatic metastasis in solid tumors of the gastrointestinal tract. Int J Clin Exp Pathol. 2012;5:614–623. [PMC free article] [PubMed] [Google Scholar]
- 7.Byron SA, Gartside MG, Wellens CL, Mallon MA, Keenan JB, Powell MA, Goodfellow PJ, Pollock PM. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008;68:6902–6907. doi: 10.1158/0008-5472.CAN-08-0770. [DOI] [PubMed] [Google Scholar]
- 8.Heiskanen M, Kononen J, Bärlund M, Torhorst J, Sauter G, Kallioniemi A, Kallioniemi O. CGH, cDNA and tissue microarray analyses implicate FGFR2 amplification in a small subset of breast tumors. Anal Cell Pathol. 2001;22:229–234. doi: 10.1155/2001/981218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin EY, Lee BH, Yang JH, Shin KS, Lee GK, Yun HY, Song YJ, Park SC, Kim EG. Up-regulation and coexpression of fibroblast growth factor receptors in human gastric cancer. J Cancer Res Clin Oncol. 2000;126:519–528. doi: 10.1007/s004320000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong KK, Sellers WR, Meyerson M, Greulich H. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105:8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, Teague J, Butler A, Edkins S, Stevens C, Parker A, O’Meara S, Avis T, Barthorpe S, Brackenbury L, Buck G, Clements J, Cole J, Dicks E, Edwards K, Forbes S, Gorton M, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Kosmidou V, Laman R, Lugg R, Menzies A, Perry J, Petty R, Raine K, Shepherd R, Small A, Solomon H, Stephens Y, Tofts C, Varian J, Webb A, West S, Widaa S, Yates A, Brasseur F, Cooper CS, Flanagan AM, Green A, Knowles M, Leung SY, Looijenga LH, Malkowicz B, Pierotti MA, Teh BT, Yuen ST, Lakhani SR, Easton DF, Weber BL, Goldstraw P, Nicholson AG, Wooster R, Stratton MR, Futreal PA. Somaticmutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005;65:7591–7595. doi: 10.1158/0008-5472.CAN-05-1855. [DOI] [PubMed] [Google Scholar]
- 12.Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3543. [PubMed] [Google Scholar]
- 13.Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 15.Valve E, Martikainen P, Seppänen J, Oksjoki S, Hinkka S, Anttila L, Grenman S, Klemi P, Härkönen P. Expression of fibroblast growth factor (FGF)-8 isoforms and FGF receptors in human ovarian tumors. Int J Cancer. 2000;88:718–725. doi: 10.1002/1097-0215(20001201)88:5<718::aid-ijc6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Drugan CS, Paterson IC, Prime SS. Fibroblast growth factor receptor expression reflects cellular differentiation in human oral squamous carcinoma cell lines. Carcinogenesis. 1998;19:1153–1156. doi: 10.1093/carcin/19.6.1153. [DOI] [PubMed] [Google Scholar]
- 17.Cha JY, Lambert QT, Reuther GW, Der CJ. Involvement of fibroblast growth factor receptor 2 isoform switching in mammary oncogenesis. Mol Cancer Res. 2008;6:435–445. doi: 10.1158/1541-7786.MCR-07-0187. [DOI] [PubMed] [Google Scholar]
- 18.Kwabi-Addo B, Ropiquet F, Giri D, Ittmann M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate. 2001;46:163–172. doi: 10.1002/1097-0045(20010201)46:2<163::aid-pros1020>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, McDermott LA, Coldren CD, Nemenoff RA, Merrick DT, Helfrich BA, Bunn PA Jr, Heasley LE. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signalling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawase R, Ishiwata T, Matsuda Y, Onda M, Kudo M, Takeshita T, Naito Z. Expression of fibroblast growth factor receptor 2IIIc in human uterine cervical intraepithelial neoplasia and cervical cancer. Int J Oncol. 2010;36:331–340. [PubMed] [Google Scholar]
- 21.Kapucuoglu N, Aktepe F, Kaya H, Bircan S, Karahan N, Ciris M. Immunohistochemical expression of PTEN in normal, hyperplastic and malignant endometrium and its correlation with hormone receptors, bcl-2, bax, and apoptotic index. Pathol Res Pract. 2007;203:153–162. doi: 10.1016/j.prp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu FS. Molecular carcinogensis of endometrial cancer. Taiwan J Obstet Gynecol. 2007;46:26–32. doi: 10.1016/S1028-4559(08)60102-3. [DOI] [PubMed] [Google Scholar]
- 23.Soufla G, Sifakis S, Spandidos DA. FGF2 transcript levels are positively correlated with EGF and IGF-1 in the malignant endometrium. Cancer Lett. 2008;259:146–155. doi: 10.1016/j.canlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Katoh Y, Katoh M. FGFR2-related pathogenesis and FGFR2-targeted therapeutics. Int J Mol Med. 2009;23:307–311. doi: 10.3892/ijmm_00000132. [DOI] [PubMed] [Google Scholar]
- 26.Katoh M. Cancer genomics and genetics of FGFR2. Int J Oncol. 2008;33:233–237. [PubMed] [Google Scholar]
- 27.Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol oncol. 2009;115:325–328. [Google Scholar]
- 28.Oltean S, Sorg BS, Albrecht T, Bonano VI, Brazas RM, Dewhirst MW, Garcia-Blanco MA. Alternative inclusion of fibroblast growth factor receptor 2 exon IIIc in Dunning prostate tumors reveals unexpected epithelial mesenchymal plasticity. Proc Natl Acad Sci U S A. 2006;103:14116–14121. doi: 10.1073/pnas.0603090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stroma and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Lutman CV, Havrilesky LJ, Cragun JM, Secord AA, Calingaert B, Berchuck A, Clarke-Pearson DL, Soper JT. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol oncol. 2006;102:92–97. doi: 10.1016/j.ygyno.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Byron SA, Pollock PM. FGFR2 as a molecular target in endometrial cancer. Future Oncology. 2009;5:27–32. doi: 10.2217/14796694.5.1.27. [DOI] [PubMed] [Google Scholar]


