Abstract
Lemur tyrosine kinase-3 (LMTK3) belongs to the family of serine-threonine-tyrosine kinases and the aberrant expression of LMTK3 was observed in several human malignancies. However, the association of LMTK3 with clinical outcomes in colorectal cancer patients is unclear. Thus, this present study was to evaluate the association of LMTK3 expression level with clinicopathologic factors and prognosis of patients with colorectal cancer (CRC). The expression level of LMTK3 in 69 archival paraffin-embedded colorectal tumor tissue specimens was examined by immunohistochemistry (IHC). As a result, we found that the LMTK3 expression level was significantly elevated in CRC tissues as compared with Crohn’s disease or colorectal polyp tissues (P<0.0001, P<0.0001, respectively). Positive LMTK3 signals in the colorectal cancer cells were observed in about 89.9% (62 of 69) CRC tissue specimens. Additionally, LMTK3 expression was significantly correlated with lymph node metastasis and tumor-node-metastasis (TNM) classification (P=0.003, and P=0.008, respectively), but not with sex, age, tumor location, histological differentiation, tumor size, or depth of tumor invasion (all P>0.05). Kaplan-Meier survival curves showed that the overall survival rate was significantly higher in the patients with low expression of LMTK3 when compared with those patients with high LMTK3 (P=0.010). Moreover, multivariate analysis revealed that LMTK3 expression was an independent prognostic factor for CRC patients (P=0.047). These results suggest that LMTK3 protein could serve as a prognostic marker for CRC patients.
Keywords: Colorectal cancer, lemur tyrosine kinase-3 (lmtk3), immunohistochemistry (ihc), prognosis
Introduction
Colorectal cancer (CRC) is a common type of gastrointestinal malignancies and the second leading cause of cancer-related death in Western countries [1,2]. Despite recent advances in treatment strategies including surgery, chemotherapy and radiotherapy, the overall 5-year survival rate for patients with advanced CRC remains poor [1]. The incidence of CRC in China is increasing in the last several decades [3]. Therefore, it is necessary to explore novel cancer-related genes for predicting the progression and prognosis of CRC and developing targeted therapy.
Lemur tyrosine kinase-3 (LMTK3) is a member of the serine-threonine-tyrosine kinases family [4-7], and was recently identified as a new ERα regulator that plays a central role in endocrine resistance for breast cancer [8,9]. However, LMTK3 may also have another role in breast cancer or other cancers, not related to ERα. For instance, Zhao et al. recently demonstrated that LMTK3 may be a target of miR-34a and overexpression of miR-34a could inhibit cell proliferation, S phase ratio, and tumor formation in an E2-dependent manner in breast cancer cell line MCF-7 [10]. Meanwhile, Naik et al. reported that LMTK3 was associated with the Wnt/β-catenin signaling pathway that is known to play a pivotal role in the progression of colorectal cancer [11,12]. However, the clinical significance of LMTK3 in CRC has not been well investigated.
In this study, we first examined the expression level of LMTK3 in CRC tissue specimens by immunohistochemical analysis (IHC), and determined the correlation between tumor LMTK3 expression and various clinicopathological parameters as well as patients’ prognosis.
Materials and methods
Patients and tissue samples collection
We obtained archived formalin-fixed and paraffin-embedded tumor tissues from 69 CRC patients (39 men and 30 women; age range, 26-87 years old; average age, 62.77 ± 12.26 years old) who underwent surgery at the Third Affiliated Hospital of Soochow University (Jiangsu Province, China) between Jan 2009 and Dec 2011. Patients who underwent any forms of preoperative chemotherapy and/or radiation therapy were excluded. Furthermore, none of patients enrolled in this study suffered from other cancers. Each patient with CRC was classified on the basis of the tumor-node-metastasis classification (TNM) of the International Union against Cancer (UICC) [13]. The remaining clinical and pathological features are shown in Table 1. The samples from five cases of intestinal polyp (3 men and 2 women; age range, 44-67 years old; average age, 54.20 ± 10.01 years old) and five cases of Crohn’s disease (3 men and 2 women; age range, 28-56 years old; average age, 38.40 ± 11.06 years old) who had never received a diagnosis of malignancy were chosen as the control group. This study protocol was approved by the ethics committee of the Third Affiliated Hospital of Soochow University. A written informed consent was obtained from all subjects involved in this study.
Table 1.
Correlation between LMTK3 expression and patients’clinical characteristics
| Clinicopathological features | Cases | LMTK3 expression | X2 | P | |
|---|---|---|---|---|---|
|
| |||||
| High, n (%) | Low, n (%) | ||||
| Sex | 1.033 | 0.309 | |||
| Male | 39 | 23 (59.0) | 16 (41.0) | ||
| Female | 30 | 14 (46.7) | 16 (53.3) | ||
| Age (years) | 0.198 | 0.657 | |||
| ≥60 | 30 | 17 (56.7) | 13 (43.3) | ||
| <60 | 39 | 20 (51.3) | 19 (48.7) | ||
| Tumor location | 1.331 | 0.249 | |||
| Colon | 38 | 18 (47.4) | 20 (52.6) | ||
| Rectum | 31 | 19 (61.3) | 12 (38.7) | ||
| Histological differentiation | 0.278 | 0.598 | |||
| Well, moderate | 43 | 22 (51.2) | 21 (48.8) | ||
| Poor, mucinous | 26 | 15 (57.7) | 11 (42.3) | ||
| Tumor size | 2.339 | 0.126 | |||
| <5 cm | 32 | 14 (43.8) | 18 (56.2) | ||
| ≥5 cm | 37 | 23 (62.2) | 14 (37.8) | ||
| Depth of tumor invasion | 1.430 | 0.232 | |||
| T1, 2 | 15 | 6 (40.0) | 9 (60.0) | ||
| T3, 4 | 54 | 31 (57.4) | 23 (42.6) | ||
| Lymph node metastasis | 9.108 | 0.003 | |||
| Negative | 43 | 17 (39.5) | 26 (60.5) | ||
| Positive | 26 | 20 (76.9) | 6 (23.1) | ||
| Stage | 7.103 | 0.008 | |||
| I+II | 40 | 16 (40.0) | 24 (60.0) | ||
| III+IV | 29 | 21 (72.4) | 8 (27.6) | ||
Immunohistochemistry
Paraffin sections (3 μm) were dewaxed in xylene, rehydrated in graded ethanol solutions. Antigen retrieval was done in citrate solution (10 mmol/L, pH 6.0) at 100°C for 30 min. Then sections were cooled down and incubated in 0.3% H2O2 solution for 15 min to block the endogenous peroxidase activity, followed by rinsing three times with PBS (pH 7.4) for 5 min each, and then incubated with LMTK3 antibody (1:350 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in humid chamber at 4°C overnight. Negative controls were performed without primary antibody. After rinsing three times with PBS (pH 7.4) for 5 min each, sections were incubated with secondary antibody (Maixin Biotechnology Co. Ltd, Fuzhou, China) at room temperature for 30 min. After washing with PBS, the sections were stained with DAB, counterstained with hematoxylin and differentiated with 0.1% hydrochloric acid alcohol. Sections were then dehydrated, cleared and mounted.
Evaluation of LMTK3 positive staining
To quantify LMTK3 protein expression, the extent and intensity of immuno-reactivity were assessed and scored independently by two pathologists who were blinded to patients’ clinical data by light microscope (Leica DM2500) at magnification (x200) with a computer-based interface. According to the percentage of cells with positive staining in each microscopic field of view, the extent of staining was categorized to: 0 (<5% positive cells); 1 (5-25% positive cells); 2 (25-50% positive cells); 3 (>50% positive cells). The staining intensity in the nucleus and cytoplasm was also evaluated on a scale of 0-3 as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive) and 3 (strongly positive). By multiplying the scores for extent and intensity, a total score ranging from 0 to 9 was achieved [14]. In the study, the expression level of LMTK3 was considered high when score was equal to or more than 4 and low when score was less than 4.
Statistical analysis
Quantitative data and qualitative data were expressed as mean ± SD or rate. Student’s t-test and Chi-square test were used to compare the differences for mean or rate between two groups. Survival time was calculated from the first day of diagnosis to the date of last follow-up or death. Survival curves were analyzed using Kaplan-Meier curves, and the difference in survival rate was examined using the log-rank test. Multivariate COX model was performed to evaluate the prognosis factors for CRC. All statistical analyses were performed using the Statistical Package for the Social Sciences, version 13.0 (SPSS, Chicago, IL). A statistically significant difference was considered at P value less than 0.05.
Results
Analysis of LMTK3 expression in CRC tissues, Crohn’s disease and intestinal polyp tissues
LMTK3 expression was observed in 62 of 69 (the positive rate was 89.8%) CRC tissue specimens (Figure 1A and 1B) by IHC, whereas there was no or very weak LMTK3 staining in Crohn’s disease (Figure 1C) or intestinal polyp tissue specimens (Figure 1D). LMTK3 protein was localized at the nucleus and/or cytoplasm of tumor cells. As shown in Figure 2, the mean LMTK3 staining scores in CRC tissues were significantly higher than that in Crohn’s disease or intestinal polyp tissues (P<0.0001, P<0.0001, respectively). These data suggest that LMTK3 is highly expressed in CRC cells.
Figure 1.

The immunohistochemical staining of LMTK3 protein is shown in colorectal cancer (A, high expression; B, low expression), Crohn’s disease (C) and intestinal polyp (D) tissue samples (x200; Leica DM2500).
Figure 2.
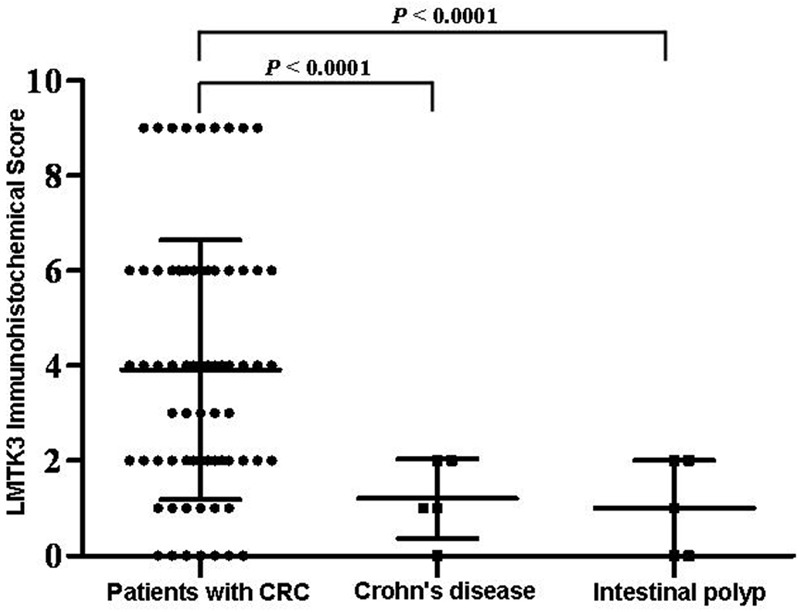
Comparison of LMTK3 expression levels between CRC tissues and Crohn’s disease or intestinal polyp tissues. P-value was calculated by the unpaired t test.
Correlations between LMTK3 expression and patients’ clinical characteristics
To determine the clinical significance of LMTK3, we evaluated the associations between LMTK3 expression and patients’ clinicopathological parameters (Table 1). High LMTK3 expression was positively associated with lymph node metastasis (P=0.003) and higher TNM stage (P=0.008). However, there were no correlations between LMTK3 expression and sex, age, tumor location, histological differentiation, tumor size, or depth of tumor invasion (P=0.309, P=0.657, P=0.249, P=0.598, P=0.126 and P=0.232, respectively).
Prognostic value of LMTK3 expression in CRC patients
Kaplan-Meier survival curves showed that the overall survival rate was significantly higher in the patients with low expression of LMTK3 than in those with high LMTK3 (Figure 3) (87.5% vs. 59.5%, X2=6.582, P=0.010). As shown in Table 2, multivariate analysis demonstrates that high LMTK3 expression was significantly associated with increased hazard risk of death for CRC patients compared with the low expression group (HR=3.239, 95% CI=1.015-10.339).
Figure 3.
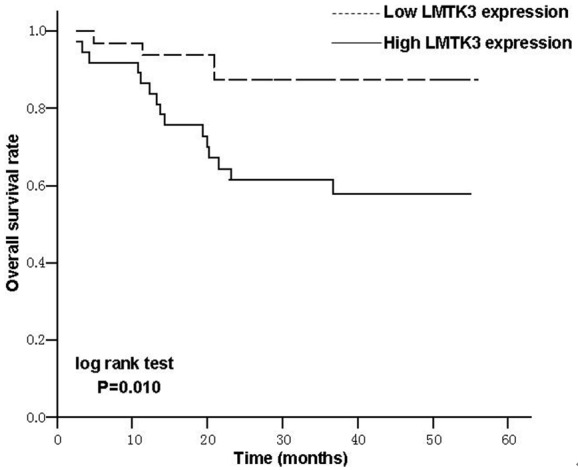
Kaplan-Meier survival curves of CRC patients based on the LMTK3 expression level. Patients with high LMTK3 expression level had a significantly poorer survival than those with low LMTK3 expression level (P=0.010, log-rank test).
Table 2.
Cox model survival analysis of patients’ clinicopathological parameters and LMTK3 expression in CRC
| Clinicopathological parameters | Comparison/reference | HR (95% CI) | P Value |
|---|---|---|---|
| LMTK3 expression | Higher/Lower | 3.239 (1.015-10.339) | 0.047 |
| Gender | Female/Male | 1.386 (0.546-3.514) | 0.492 |
| Age | <60/≥60 | 1.043 (0.407-2.670) | 0.930 |
| Lymph node metastasis | Positive/Negative | 0.935 (0.108-8.070) | 0.951 |
| TNM stage | III, IV/I, II | 2.108 (0.235-18.875) | 0.505 |
Discussion
It is well known that the conventional treatment strategies in CRC therapy mostly relied on surgery, radiation, hormones and chemotherapy within the last few decades. However, the prognosis and quality of life for CRC patients have been poorly improved. Therefore, in recent years, some novel anticancer therapies such as molecular-targeted drugs and antibodies or cancer vaccines were introduced [15,16]. Targeted therapies are expected to have a high specificity toward tumor cells and provide a broader therapeutic window with less toxicity. Tyrosine kinases are an especially important therapeutic target because they play a critical role in the modulation of growth factor signaling [17-19]. LMTK represents a group within the super-family of tyrosine kinases, and is composed of LMTK1, LMTK2 and LMTK3 [4,5]. Recent studies found that LMTK3 was expressed in gastric cancer and breast cancer tissue specimens, suggesting a possible target and a new reliable biomarker [20,21]. In the present study, our IHC analysis showed that LMTK3 expression was higher in CRC tissues than in Crohn’s disease or intestinal polyp tissues. These results indicate that LMTK3 protein might involve in tumor progression and serve as a potential target and biomarker for various malignancies.
Importantly, our results suggested that high LMTK3 expression was associated with poor clinicopathological parameters such as lymph node metastasis and advanced TNM stage, indicating that LMTK3 protein may be a novel biomarker for predicting tumor progression in CRC patients. In fact, in breast cancer cells, Stebbing et al. have found that LMTK3 protein was expressed in nuclear and/or cytoplasmic, and demonstrated that the LMTK3 expression level was associated with tumor grade in the European cohort and TNM stage as well as overall and disease-free survival in the Asian cohort [20]. More recently, Wakatsuki et al. reported that LMTK3 polymorphisms were significantly associated with prognosis of patients with gastric cancer [21]. Similar to these findings, our present study exhibited a correlation between LMTK3 expression and survival rate of CRC patients. Moreover, the COX model analysis indicated that high expression level of LMTK3 was a significant prognostic factor for a poor overall survival rate of CRC patients. These results indicate that LMTK3 protein might be a very good prognostic marker for CRC patients and might help for establishing the treatment strategy.
Recent studies found that the inhibition of LMTK3 might result in reduced β-catenin-dependent transcription [11]. The β-catenin is a key mediator of the canonical Wnt/β-catenin signaling pathway that has been considered to be a major driving force in colorectal carcinogenesis [22-24]. When the Wnt signal is present, GSK3β activity is inactivated, so that β-catenin accumulates in the cytoplasmic and translocates subsequently into the nucleus [25,26]. The nuclear β-catenin interacts with members of the TCF/LEF transcription co-factor family to activate downstream target genes such as cell cycle-regulating genes (c-myc and cyclin D1) and genes related to metastasis and invasion of cancer cells (MMP-7 and uPA) [27-30]. Our results suggest that the blockade of LMTK3 protein may be benefit for the prognosis of CRC patients.
In conclusion, our results demonstrated that LMTK3 protein is a potential novel biomarker that may help to improve CRC progression and prognostic assessment. Furthermore, LMTK3 may be a useful target for treating CRC. However, the precise functions of LMTK3 in CRC patients and the molecular mechanisms how LMTK3 protein exert their biological effect in CRC are rarely reported, further studies are needed to validate these critical issues.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (NSFC) (81171653, 30972703 and 81301960) and Natural Science Foundation of Jiangsu Province (BK2011246 and BK2011247).
Disclosure of conflict of interest
None.
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, Liu JH, Lou QY, Gan AH. Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol. 2010;16:960–965. doi: 10.3748/wjg.v16.i8.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 5.Tomomura M, Morita N, Yoshikawa F, Konishi A, Akiyama H, Furuichi T, Kamiguchi H. Structural and functional analysis of the apoptosis-associated tyrosine kinase (AATYK) family. Neuroscience. 2007;148:510–521. doi: 10.1016/j.neuroscience.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Kon T, Ohkura R, Yamakawa H, Ohara O, Yokota J, Sutoh K. BREK/LMTK2 is a myosin VI-binding protein involved in endosomal membrane trafficking. Genes Cells. 2008;13:483–495. doi: 10.1111/j.1365-2443.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- 7.Tyner JW, Deininger MW, Loriaux MM, Chang BH, Gotlib JR, Willis SG, Erickson H, Kovacsovics T, O’Hare T, Heinrich MC, Druker BJ. RNAi screen for rapid therapeutic target identification in leukemia patients. Proc Natl Acad Sci U S A. 2009;106:8695–8700. doi: 10.1073/pnas.0903233106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamas G, Filipovic A, Jacob J, Messier W, Zhang H, Yang D, Zhang W, Shifa BA, Photiou A, Tralau-Stewart C, Castellano L, Green AR, Coombes RC, Ellis IO, Ali S, Lenz HJ, Stebbing J. Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nat Med. 2011;17:715–719. doi: 10.1038/nm.2351. [DOI] [PubMed] [Google Scholar]
- 9.Stebbing J, Filipovic A, Lit LC, Blighe K, Grothey A, Xu Y, Miki Y, Chow LW, Coombes RC, Sasano H, Shaw JA, Giamas G. LMTK3 is implicated in endocrine resistance via multiple signaling pathways. Oncogene. 2012;32:3371–3380. doi: 10.1038/onc.2012.343. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Guo J, Li D, Jia C, Yin W, Sun R, Lv Z, Cong X. MicroRNA-34a Suppresses Cell Proliferation by Targeting LMTK3 in Human Breast Cancer MCF-7 Cell Line. DNA Cell Biol. 2013;32:699–707. doi: 10.1089/dna.2013.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik S, Dothager RS, Marasa J, Lewis CL, Piwnica-Worms D. Vascular Endothelial Growth Factor Receptor-1 Is Synthetic Lethal to Aberrant {beta}-Catenin Activation in Colon Cancer. Clin Cancer Res. 2009;15:7529–7537. doi: 10.1158/1078-0432.CCR-09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH, Wittekind CH, editors. International Union Against Cancer. TNM Classification of Malignant Tumors. 6th edn. New York: John Wiley-Liss; 2002. [Google Scholar]
- 14.Zhao Q, Shen WH, Chen ZW, Zhou ZS, Ji HX. High expression level of BLCA-4 correlates with poor prognosis in human bladder cancer. Int J Clin Exp Pathol. 2012;5:422–427. [PMC free article] [PubMed] [Google Scholar]
- 15.Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol. 2004;5:341–353. doi: 10.1016/S1470-2045(04)01490-1. [DOI] [PubMed] [Google Scholar]
- 16.Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF, Israel VK, Livingston RB, Gandara DR. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J. Clin. Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 17.Giamas G, Man YL, Hirner H, Bischof J, Kramer K, Khan K, Ahmed SS, Stebbing J, Knippschild U. Kinases as targets in the treatment of solid tumors. Cell Signal. 2010;22:984–1002. doi: 10.1016/j.cellsig.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Giamas G, Stebbing J, Vorgias CE, Knippschild U. Protein kinases as targets for cancer treatment. Pharmacogenomics. 2007;8:1005–1016. doi: 10.2217/14622416.8.8.1005. [DOI] [PubMed] [Google Scholar]
- 19.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing J, Filipovic A, Ellis IO, Green AR, D’Silva TR, Lenz HJ, Coombes RC, Wang T, Lee SC, Giamas G. LMTK3 expression in breast cancer: association with tumor phenotype and clinical outcome. Breast Cancer Res Treat. 2011;132:537–544. doi: 10.1007/s10549-011-1622-z. [DOI] [PubMed] [Google Scholar]
- 21.Wakatsuki T, Labonte MJ, Bohanes PO, Zhang W, Yang D, Azuma M, Brazi A, Ning Y, Loupakis F, Saadat S, Volz N, Stintzing S, El-Khoueiry R, Koizumi W, Watanabe M, Shah M, Stebbing J, Giamas G, Lenz HJ. Prognostic Role of Lemur Tyrosine Kinase-3 germline polymorphisms in Adjuvant Gastric Cancer in Japan and the United States. Mol Cancer Ther. 2013;12:2261–2272. doi: 10.1158/1535-7163.MCT-12-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 23.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 24.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 25.Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci U S A. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bienz M. The subcellular destinations of APC proteins. Nat Rev Mol Cell Biol. 2002;3:328–338. doi: 10.1038/nrm806. [DOI] [PubMed] [Google Scholar]
- 27.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 28.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]


