Abstract
Background: HER-2 overexpression is an independent predictor for poor prognosis of breast cancer patients. Recently, extracellular domain of HER-2 (ECD) was found detectable in the serum of breast cancer patients. In this prospective study, we wonder whether ECD levels predict the clinical outcome of metastatic breast cancer patients. Methods: ECD were measured in 190 women with metastatic breast cancer. Chi-square test was performed to determine the relationship between ECD status and clinical outcomes. Kaplan-Meier curves were applied for survival analysis. Results: Elevated ECD levels were significantly associated with short-term response to Herceptin treatment. The median PFS was significantly longer in ECD-Low patients. The patients who remained low ECD levels or achieved low ECD levels after treatments have significantly longer PFS than those whose levels remained high or converted from low to high. Conclusions: Overall, our results support the clinical utility of measuring serum HER2 ECD levels in patients with advanced breast cancer. Baseline and serial measurements of serum ECD levels are reliably predictive of clinical outcome of breast cancer patients.
Keywords: Epidermal growth factor receptor-2 (EGFR-2), extracellular domain, metastatic breast cancer
Introduction
The human epidermal growth factor receptor-2 (HER-2) proto-oncogene, also known as HER-2/neu and c-erbB-2, encodes a growth factor receptor which has been found to play a significant role in breast cancer [1]. The HER-2 gene is amplified or overexpressed in up to 30% of breast cancer patients. HER-2 overexpression is an independent predictor of shorter progression-free survival (PFS) and overall survival (OS) of breast cancer patients [2,3].
HER-2 is composed of an extracellular domain (ECD) that includes the binding site for its ligands, a transmembrane domain, and the intracellular kinase domain. HER-2 ECD can be cleaved from the surface of breast cancer cells by matrix metalloproteases and released into the serum, where it is detectable using an enzyme-linked immunosorbent assay (ELISA) [4,5]. Increased levels of HER-2 ECD (≥15 ng/mL) can be detected in 30%-90% of patients with metastatic breast cancer (MBC) [6,7]. As an easily accessible serum protein, HER-2 ECD is thus generating an increasing interest as a potential and clinically relevant new biomarker for breast cancer. Increased serum ECD concentrations was found to be an indicator of poor prognosis and poor response to chemotherapeutic and hormonal treatment regimens [6-8]. However, a pooled analysis of four trials of Trastuzumab in metastatic breast cancer showed that there was no clear relationship between baseline ECD values and tumor response, and HER-2 ECD levels declined irrespective of treatment received and tumor response after initiating combination therapy [9]. Therefore, the utility of serum ECD values as a potential marker of tumor response or progression is currently a matter of debate, and it is a general opinion that this issue deserves further study in large prospective trials [10-12].
In this prospective analysis of a cohort of metastatic breast cancer patients, we investigated whether HER-2 ECD measurements would be useful for clinical decision making. Specifically, we wonder whether baseline (pretreatment) ECD values can predict the clinical outcome of HER2-positive metastatic breast cancer patients and whether changes in HER2 ECD levels during treatment correlate with PFS.
Methods
Patient selection
A total of 190 patients with metastatic breast carcinoma referred to Zhejiang Cancer Hospital were recruited. Histological diagnosis was made at the Department of Pathology, Zhejiang Cancer Hospital. Before treatment, a 5-ml blood sample was drawn and centrifuged at 1000×g for 5 min. Serum was aliquoted in two parts and stored in polypropylene cryotubes at -80°C. All patients were provided written informed consent according to guidelines of the ethics committee of Zhejiang Cancer Hospital.
HER2 ECD testing
Serum samples were prospectively collected from breast cancer patients before treatment (n=190) and at the time of evaluation (n=46). Serum HER2 ECD levels were determined with the ADVIA Centaur HER2/neu assay (Bayer Corporation, Tarrytown, NY) according to the manufacturer’s instructions. Levels of HER-2 ECD >15 ng/mL are considered high ECD levels [5,13,14].
Statistical analysis
Continuous data were summarized using descriptive statistics. Student’s t test were used to analyze the difference. Chi-square test was performed to determine the relationship between ECD status and clinical parameters such as age, lymph node metastasis. Kaplan-Meier curves were produced for PFS and median PFS were calculated from these curves. For models of ECD levels over time, statistical comparison of PFS was conducted for patient with remained low ECD levels or achieved low ECD levels (ECD LOW) compared with those whose levels remained above 15 ng/mL or converted from low to high elevated ECD levels (ECD HIGH).
Effects were considered significant if p<0.05. All analysis were two sided tests conducted using SPSS16.0 software.
Results
bECD and overall treatment response
As previously reported, we set the cutpoint at 15 ng/mL as the upper limit of the normal bECD level to separate the cohort into two groups: bECD low and bECD high. Generally, bECD levels have no significant association with short-term treatment response (Figure 1A and Table 1). However, the long-term outcome was worse in bECD high patients. The 1-year survival, 2-year survival and 3-year survival were 50.5%, 25.3% and 23% in bECD low patients and 25.0%, 9.8% and 0% in bECD high patients. The median PFS was 12.3 months in bECD low patients (95% CI: 10.2-14.4, n=130) and 7.5 months in bECD high patients (95% CI: 7.5-9.4, n=60) (Figure 1B, Log Rank test, p=0.0004). This effect is irrespective of tissue HER-2 expression since the median PFS of bECD low patients were significantly longer than that of bECD high patients in both HER-2 negative and HER-2 positive groups (Figure 1C and 1D). In HER-2 negative group, the median PFS was 10.5 months in bECD low patients (95% CI: 7.7-13.9, n=69) and 5.5 months in bECD high patients (95% CI: 0-11.3, n=20) (Figure 1C, Log Rank test, p=0.012). In HER-2 positive group, the median PFS was 13.0 months in bECD low patients (95% CI: 10.3-15.8, n=61) and 7.6 months in bECD high patients (95% CI: 6.2-9.1, n=40) (Figure 1D, Log Rank test, p=0.0003).
Figure 1.

bECD and overall treatment response. A: bECD levels in patients with different treatment response (One Way ANOVA test, p=0.1365). B: Progression-free survival (PFS) in patients with different bECD status (bECD low: n=130; bECD high: n=60; Log Rank test, p=0.0004). C: Progression-free survival (PFS) in HER-2-negative patients with different bECD status (bECD low: n=69; bECD high: n=20; Log Rank test, p=0.012). D: Progression-free survival (PFS) in HER-2-positive patients with different bECD status (bECD low: n=61; bECD high: n=40; Log Rank test, p=0.0003).
Table 1.
Treatment responses according to the baseline level of soluble HER-2
| Characteristic | Low bECD (No.) | High bECD (No.) | Chi-Square p |
|---|---|---|---|
| Herceptin treatment | |||
| ORR | 30 | 14 | 0.0128 |
| SD | 6 | 8 | |
| PD | 0 | 4 | |
| Overall response | |||
| ORR | 76 | 29 | 0.1435 |
| SD | 41 | 19 | |
| PD | 13 | 12 |
bECD and Herceptin treatment response
In addition, bECD levels were significantly associated with short-term Herceptin response (One Way ANOVA test, p=0.0125; ORR: 19.6±2.4 ng/mL; SD: 28.5±3.5 ng/mL; PD: 34.6±5.2 ng/mL, Figure 2A). Patients with low bECD levels had a higher rate of ORR (Table 1). For patients without Herceptin treatment, the median PFS was 10.4 months (95% CI: 7.8-13.0, n=94) in bECD Low patients and 5.8 months (95% CI: 1.6-9.6, n=34) in bECD high patients (Figure 2B, Log Rank test, p=0.016). For patients received Herceptin treatment, the median PFS was 14.9 months (95% CI: 11.6-18.2, n=36) in bECD Low patients and 7.6 months (95% CI: 5.7-10.2, n=26) in bECD high patients (Figure 2C, Log Rank test, p=0.001).
Figure 2.
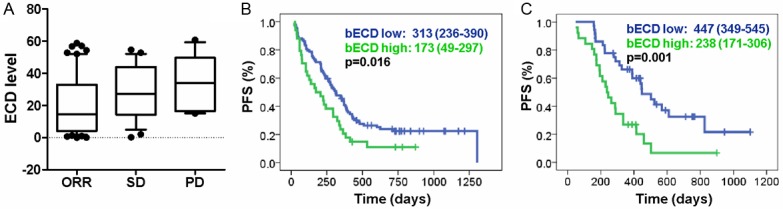
bECD and Herceptin treatment response. A: bECD levels in patients with different response to Herceptin treatment (One Way ANOVA test, p=0.0125). B: Progression-free survival (PFS) in Herceptin-non-treated patients with different bECD status (bECD low: n=94; bECD high: n=34; Log Rank test, p=0.016). C: Progression-free survival (PFS) in Herceptin-treated patients with different bECD status (bECD low: n=36; bECD high: n=26; Log Rank test, p=0.001).
Changes in ECD and long-term clinical outcomes
In a cohort of 46 patients whose HER2 ECD levels were monitored during treatment, 29 patients remains low ECD levels, 12 patients convert from high to low, 2 patients convert from low to high, and 3 patients remains high ECD levels. The changes in serum ECD levels are not related with the treatment response (Figure 3A). Although the number of patients with ECD levels was small, the patients who remained low ECD levels or achieved low ECD levels after treatments have significantly longer PFS than those whose levels remained above 15 ng/mL or converted from low to high regardless of treatment given (Log Rank test, p=0.019, Figure 3B). The median PFS was 14.7 months (95% CI: 7.8-21.5) and 6.4 months (95% CI: 5.0-7.8), respectively.
Figure 3.
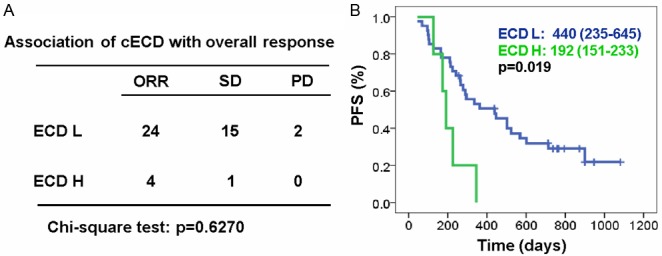
Clinical relevance of changes in ECD levels (cECD). A: Association of ECD changes with treatment responses (Chi-Square test, p=0.6270). ECD L means patients with ECD levels persistently low or converted from high to low. ECD H means patients with ECD levels converted from low to high. B: Progression-free survival (PFS) in patients with different cECD status (ECD L: n=41; ECD H: n=5; Log Rank test, p=0.019).
Discussion
The last two decades have witnessed the improvement in the management of breast cancer and persistent efforts have been underway to develop more tailored therapeutics. The success utilization of HER-2 amplification or overexpression as the therapeutic target and prognostic marker has spurred increasing interests in identifying novel tissue or serum biomarkers that would guide the individualized therapy.
Shortly after discovering the presence of HER-2 ECD in the serum of breast cancer patients, it has been proposed as a novel biomarker to monitor treatment response and disease progression [15,16]. In the present study, we found that baseline ECD levels significantly correlate with clinical outcome. Patients with low HER-2 ECD levels have significantly longer PFS. This finding is supported by data from the N9831 trial and other studies that patients with high ECD levels had a shorter survival [17-20]. However, these studies are controversial on the correlation of bECD levels with short-term treatment response. We found that patients with objective response to various treatments generally have low bECD levels, indicating that bECD can be used for a biomarker to monitor treatment response in addition to prediction long-term clinical outcome. Therefore, elevated HER-2 ECD might represent a more progressive subtype of breast cancer. However, the biological relevance and how ECD was released from HER-2-expressing breast cancer cells remain to be characterized. Clarifying its biological relevance may be helpful to define more subtypes of breast cancer for personalized therapy. Although the molecular mechanism is unknown, elevated bECD levels was found in patients with weak or negative HER-2 expression. It is possible that the release of ECD from the surface of tumor cells may convert high HER-2 expression to low HER-2 expression although the commonly used antibody for IHC was raised against the ICD of HER-2. It have been well recognized that EGFR ICD can translocate into the nucleus where it can modulate gene transcription to promote tumor progression. If this is true, HER-2 ECD may represent a unique subtype of breast cancer that need more specific molecular typing and target therapy.
Interestingly, serum ECD levels change in the course of treatment. Despite a small scale, we observed that patients who remained low ECD levels or achieved low ECD levels have significantly longer PFS than those whose levels remained above 15 ng/mL or converted from low to high regardless of treatment given. Our results are consistent with previous reports measuring serum HER2 ECD in Trastuzumab-treated patients or non-Trastuzumab-based setting. The randomized (paclitaxel with placebo or lapatinib) phase III study EGF30001 (n=579) showed that changes in ECD status correlates with patient outcome regardless of treatment given. ECD conversion from low to high was associated with worse PFS and low ECD level or converting from high to low had better PFS than a consistently elevated ECD [16]. In a pooled analysis of seven medical institutions of first-line Trastuzumab therapy (with or without chemotherapy; n=307) in MBC, Ali et al reported that a 20% decrease of serum HER2 from baseline in the early weeks following therapy initiation predicted for significantly increased objective response rate, prolonged time to progression, and longer survival to first-line Trastuzumab therapy [21]. Lipton et al showed that a ≥20% decrease of serum HER2 from baseline to as early as 4 weeks was associated with prolonged PFS and higher ORR in patients (n=138) receiving lapatinib monotherapy in the EGF20009 study [22]. Besides, an analysis of 158 patients with metastatic breast cancer suggested that increases over time of ECD/HER2 levels were strongly associated with poor survival [20]. Additionally, some other trials in HER2-overexpressing metastatic breast cancer (MBC) also indicated that early changes in elevated baseline serum ECD before Trastuzumab-based treatment was predictive of good tumor response [15,23-26].
Overall, our results support the clinical utility of measuring serum HER2 ECD levels in patients with advanced breast cancer. Baseline and serial measurements of serum ECD levels are reliably predictive of clinical outcome of breast cancer patients. Therefore, monitoring of serum HER-2 ECD levels in larger prospective clinical trials should be initiated to confirm its value in outcome prediction and individualized therapy.
Acknowledgements
This work was supported by Science and Technology Department of Zhejiang Province (2005C300001, 2012C13019-1, 2013C33205) and Program for Innovative Research Team in Zhejiang Province (No. 2010R50046).
Disclosure of conflict of interest
None.
References
- 1.Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59:1196–1201. [PubMed] [Google Scholar]
- 5.Payne RC, Allard JW, Anderson-Mauser L, Humphreys JD, Tenney DY, Morris DL. Automated assay for HER-2/neu in serum. Clin Chem. 2000;46:175–182. [PubMed] [Google Scholar]
- 6.Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, Price CP. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem. 2003;49:1579–1598. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 7.Carney WP, Leitzel K, Ali S, Neumann R, Lipton A. HER-2/neu diagnostics in breast cancer. Breast Cancer Res. 2007;9:207. doi: 10.1186/bcr1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney WP. Circulating oncoproteins HER2/neu, EGFR and CAIX (MN) as novel cancer biomarkers. Expert Rev Mol Diagn. 2007;7:309–319. doi: 10.1586/14737159.7.3.309. [DOI] [PubMed] [Google Scholar]
- 9.Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, Leyland-Jones B. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J. Clin. Oncol. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 10.Ali SM, Leitzel K, Lipton A, Carney WP, Kostler WJ. Value of serum human epidermal growth factor receptor 2 (HER2)/neu testing for early prediction of response to HER2/neu-directed therapies is still an open one and deserves further study in large prospective trials. J. Clin. Oncol. 2009;27:e273. doi: 10.1200/JCO.2009.23.4674. author reply e274-275. [DOI] [PubMed] [Google Scholar]
- 11.Tse C, Lamy PJ. Clinical utility of serum human epidermal growth factor receptor 2 extracellular domain levels: stop the shilly-shally--it is time for a well-designed, large-scale prospective study. J. Clin. Oncol. 2009;27:e286–287. doi: 10.1200/JCO.2009.24.5100. [DOI] [PubMed] [Google Scholar]
- 12.Mathelin C, Croce S, Rault S, Gharbi M, Eichler F, Gairard B, Coumaros G, Koehl C. [Clinical usefulness of circulating ECD/HER-2 measurement for breast cancer patients’ management] . Presse Med. 2011;40:126–137. doi: 10.1016/j.lpm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 14.Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Luftner D, Yeung KK, Chan DW, Schwartz MK, Allard WJ. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001;21:1465–1470. [PubMed] [Google Scholar]
- 15.Kostler WJ, Schwab B, Singer CF, Neumann R, Rucklinger E, Brodowicz T, Tomek S, Niedermayr M, Hejna M, Steger GG, Krainer M, Wiltschke C, Zielinski CC. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:1618–1624. doi: 10.1158/1078-0432.ccr-0385-3. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Gagnon R, Di Leo A, Press MF, Arbushites M, Koehler M. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized phase III study. J. Clin. Oncol. 2009;27:5552–5558. doi: 10.1200/JCO.2008.21.1763. [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Aspitia A, Hillman DW, Dyar SH, Tenner KS, Gralow J, Kaufman PA, Davidson NE, Lafky JM, Reinholz MM, Lingle WL, Kutteh LA, Carney WP, Dueck AC, Perez EA. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: Results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer. 2013;119:2675–82. doi: 10.1002/cncr.28130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, Massuti B, Cortes-Funes H, Lloveras B. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6:2356–2362. [PubMed] [Google Scholar]
- 19.Fehm T, Maimonis P, Weitz S, Teramoto Y, Katalinic A, Jager W. Influence of circulating c-erbB-2 serum protein on response to adjuvant chemotherapy in node-positive breast cancer patients. Breast Cancer Res Treat. 1997;43:87–95. doi: 10.1023/a:1005700812422. [DOI] [PubMed] [Google Scholar]
- 20.Bramwell VH, Doig GS, Tuck AB, Wilson SM, Tonkin KS, Tomiak A, Perera F, Vandenberg TA, Chambers AF. Changes over time of extracellular domain of HER2 (ECD/HER2) serum levels have prognostic value in metastatic breast cancer. Breast Cancer Res Treat. 2009;114:503–511. doi: 10.1007/s10549-008-0033-2. [DOI] [PubMed] [Google Scholar]
- 21.Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Kostler WJ, Lotz JP, Luftner D, Pichon MF, Lipton A. Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113:1294–1301. doi: 10.1002/cncr.23689. [DOI] [PubMed] [Google Scholar]
- 22.Lipton A, Leitzel K, Ali SM, Carney W, Platek G, Steplewski K, Westlund R, Gagnon R, Martin AM, Maltzman J. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117:5013–5020. doi: 10.1002/cncr.26101. [DOI] [PubMed] [Google Scholar]
- 23.Esteva FJ, Cheli CD, Fritsche H, Fornier M, Slamon D, Thiel RP, Luftner D, Ghani F. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–443. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fornier MN, Seidman AD, Schwartz MK, Ghani F, Thiel R, Norton L, Hudis C. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol. 2005;16:234–239. doi: 10.1093/annonc/mdi059. [DOI] [PubMed] [Google Scholar]
- 25.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 26.Petersen ER, Sorensen PD, Jakobsen EH, Madsen JS, Brandslund I. Serum HER-2 predicts response and resistance to trastuzumab treatment in breast cancer. Clin Chem Lab Med. 2013;51:1483–92. doi: 10.1515/cclm-2012-0558. [DOI] [PubMed] [Google Scholar]


