Abstract
STAT3 is constitutively activated in many human cancers including gastric cancer and plays crucial roles in modulating cancer cell proliferation, survival, metastasis as well as the microenvironment of pre-metastatic niches. Accumulating evidence has implicated STAT3 as a promising target for cancer therapy and it has been well established that tumor cell metastasized to lymph node is associated with poor prognosis. However, little is known about the relation between STAT3 activation in tumor cell-free lymph nodes and patient clinical outcomes. The objective of the current study was to investigate the role of STAT3 activity in tumor cell-free lymph nodes in tumor progression and prognosis for gastric cancer patients. Immunohistochemical analyses for p-STAT3, Ki-67, CD68 and Bcl-xL were performed in tumor cell-free lymph nodes from 60 gastric cancer patients. Survival analysis was conducted by using the Kaplan-Meier method. Immunohistochemical analyses showed that hyperactivity of STAT3 in tumor cell-free lymph nodes was significantly associated with tumor recurrence, and STAT3 activation pattern coincides with expression Ki-67, CD68, Bcl-xL. Survival analysis revealed that persistent STAT3 activation in uninvolved lymph nodes was positively associated with poor overall survival (P<0.05). These findings suggest that STAT3 activation in tumor-free lymph nodes is involved in the pathogenesis and metastasis of gastric cancer and that elevated STAT3 activity in lymph nodes prior to tumor cell arrival may indicate a poorer prognosis. These clinical studies support our findings in mouse tumor models showing that STAT3 activation is crucial for pre-metastatic niche formation and metastasis.
Keywords: STAT3, gastric cancer, tumor cell-free lymph node, recurrence, prognosis
Introduction
Gastric cancer remains one of the most common causes of cancer-related deaths worldwide, especially in Eastern Asia area, which is 28.1% for males and 13.0% for females respectively [1]. Even according to the latest cancer statistic of United States, the incidence and death rate of gastric cancer in Asia ethnic group are still higher than other ethnic groups despite of the advances in treatment and subsequent improvement in prognosis [2]. Among several factors, tumor stage, such as depth of tumor invasion and lymph node metastasis, has generally been shown to be the main risk factor for recurrence in gastric cancer patients [3,4] and it has been reported that the relatively higher death rate of gastric cancer was partially associated with lymph duct and venous invasion and lymph node metastasis [5]. However, the underlying molecular mechanisms responsible for lymphoid metastasis and tumor recurrence have not been fully elucidated.
Recent advances in cancer biology study have revealed that signal transducer and activator of transcription (STAT) proteins, especially STAT3, was a vital factor in carcinogenesis and tumor metastasis [6-8]. STAT proteins belong to a family of transcription factors that are normally inactive within the cytoplasm of cells and become activated by tyrosine phosphorylation in response to cytokines and growth factors. In normal cells, STAT activation is transient, whereas in a large number of primary tumors and cancer-derived cell lines, STAT proteins (in particular STAT3) remain activated by persistently activated tyrosine kinases and/or a decrease in the negative regulators of STAT dephosphorylation [9,10]. STAT3 was first identified as a DNA-binding factor that selectively binds to the interleukin 6 (IL-6)-responsive element in the promoter of acute phase genes from IL-6-stimulated hepatocytes. Activated STAT3 (p-STAT3) dimerizes and translocates into the nucleus, where its occupation of specific DNA-binding sites results in the increased transcription of several molecules that are involved directly in cell proliferation, survival, and metastasis, which include Bcl-2, Survivin, MMP-2 and many others [11-14].
It has been widely documented that dysregulation of STAT3 by constitutive activation in tumor cells acts an important role as a crucial oncogenic mediator and potent transcriptional factor [15-22], and clinical study shows that STAT3 activation in gastric cancer cells significantly associated with regional lymph node invasion and tumor recurrence [23]. Moreover, numerous studies have also demonstrated persistent activation of STAT3 in myeloid cells and T cells at primary tumor sites, facilitating immunosuppression and promoting tumor angiogenesis, tumor growth, and metastasis [24-28]. Notably, our recent study [29] reveals that STAT3 even can be activated in myeloid cells in pre-metastatic niches in mice and contributes to tumor cell metastasis, and blocking STAT3 activity in myeloid cells disrupts existing pre-metastatic niches. Activated STAT3 in the future metastatic sites promotes colonization of myeloid cells and therefore formation of pre-metastatic niches. Analyzing uninvolved lymph nodes from high-risk prostate cancer and melanoma patients revealed that elevated STAT3 activity in CD68+ myeloid cells coincides with increased proliferation and survival signals at pre-metastatic sites.
Indeed, many previous studies have demonstrated that constitutive activation of STAT3 in a wide variety of human malignancies’ primary tumor cells has been recognized as a predictor for poorer survival, including gastric cancer [30-34], but the impact of activated STAT3 in tumor cell-free lymph nodes in gastric cancer on patients’ prognosis remains to be identified. Our current study evaluates the expression of p-STAT3 (Tyr705) in uninvolved lymph nodes from high-risk human gastric cancer patients, with the aim to investigate whether STAT3 is persistently activated at tumor cell-free lymph nodes and whether STAT3 signaling is correlated with myeloid cell infiltration as well as patients’ prognosis.
Patients and methods
Patients and tissue specimens
Gastric cancer patients’ specimens were obtained from Department of Pathology, Huashan Hospital. Briefly, samples from 60 gastric cancer patients (all of whom were confirmed by pathological examination that had both kinds of tumor cell-involved and tumor cell-free lymph nodes), who received surgical treatment at Huashan Hospital in 2005, were collected and confirmed as gastric adenocarcinoma, and then made available for this study. Paraffin-embedded tissue from benign lymph nodes were obtained and prepared as 4 mm sections on unstained slides for subsequent analyses. Retrospective follow-up was successfully conducted on 48 patients at July 2011. The use of all tissue blocks for this study was approved by the Institutional Ethics Review Board of Huashan Hospital.
Immunohistochemistry staining
The immunostaining was performed using the Dako EnVisionTMtKit (Dako, Denmark), and detected by Dako Liquid DABt, which is based on horseradish peroxidase (HRP)-labeled polymer conjugated with secondary antibodies. Briefly, paraffin-embedded specimens were cut into 4-mm sections, mounted onto polylysine-coated slides, dewaxed in xylene, rehydrated in alcohol, and treated with 3% H2O2 in methanol for blocking endogenous peroxidase activity. For antigen retrieval, the sections were autoclaved for 15 min 95°C at in EDTA (PH=8.0). After treatment with 1% PBS for 5 min 3 times, the consecutive tissue sections were incubated for 60 min at 37°C with anti-STAT3 (Tyr705) monoclonal antibodies (1:200 dilution, Cell Signaling), or anti-CD68 monoclonal antibody (diluted 1:80; AbDseroTec), or anti-Ki67 monoclonal antibody (diluted 1:100; Abcam), or anti-Bcl-xL monoclonal antibody (diluted 1:200; Cell Signaling), and then were incubated overnight at 4°C in moist chambers. The primary antibody was detected using the Dako EnVisionTM Kit. Reaction products were visualized by Dako Liquid DAB Substrate-Chromagen System and then counterstained with hematoxylin. Negative controls were samples that were incubated with normal mouse serum.
Evaluation of immunostaining
All immunostained sections were blindly evaluated by two independent investigators. The scoring of p-STAT3 expression was based on both intensity and extensity. The percentage of positive cells were determined quantitatively by assessing the lymphatic sinus section, and each sample was scored on a scale of 0 to 4:0, negative; 1, positive staining in 1-25% cells; 2, 25-50% cells; 3, 50-75% cells; and 4, 75-100% cells. Intensity was graded as follows: 0, no signals; 1, weak; 2, moderate; and 3, strong staining. A total score of 0-12 was finally calculated and graded as: I, score 0-1; II, 2-4; III, 5-8; IV, 9-12. Grades I and II represented low staining, and grades III and IV represented high staining. Ki-67 and Bcl-xL scores were counted on a minimum of 10 randomly selected ×40 high-power fields containing representative sections of lymphatic sinus, and CD68 score was evaluated by assessing the representative sections of lymphatic sinus, by calculating the percentage of positively stained cells to total cells, and positive staining in 1-25% cells represented low staining, and >25% represented high staining.
Statistical analysis
All statistical analysis and graphs were performed using the GraphPad Prism 5 statistical software package for Windows. Correlation between p-STAT3 expression and clinicopathological was conducted by the two tailed Mann-Whitney U-test or Kruskal-Wallis test. Fisher’s exact test was used for testing relationship between p-STAT3 expressions in benign lymph node with other three markers. Survival time was calculated as the date of resection until date of death or date of the latest follow-up. Survival curves were plotted by the Kaplan-Meier method and statistical differences were analyzed using the log-rank test. P<0.05 denoted the presence of a statistically significant difference.
Results
Study population
The clinicopathological information about these 60 patients is shown in Table 1. There were 43 males and 17 females, whose ages were ranged from 37 to 90 years with an average age of 62 years. The primary tumor size was ranged from 1 cm to 20 cm with an average size of 6 cm. The TNM stage was calculated according to the AJCC criteria, and there were 37 patients (62%) in stage II, 19 patients (32%) in stage III and 4 patients (6%) in stage IV. After checking the patients’ medical documents carefully and conducting the follow-up by calling their families, 24 patients were confirmed with recurrence after gastrostomy, 12 patients without and the other 24 patients not clear.
Table 1.
Clinicopathologic correlation of p-STAT3 expression in 60 tumor cell-free lymph node tissues from gastric cancer patients
| Characteristic | n | p-STAT3 | P Value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| I | II | III | IV | |||
| Sex | 0.993 | |||||
| Male | 43 | 13 | 12 | 14 | 4 | |
| Female | 17 | 5 | 4 | 8 | 0 | |
| Age (years) | 0.136 | |||||
| ≤55 | 22 | 10 | 4 | 7 | 1 | |
| >55 | 38 | 8 | 12 | 15 | 3 | |
| Tumor size (cm) | 0.153 | |||||
| ≤5 | 37 | 13 | 10 | 13 | 1 | |
| >5 | 23 | 5 | 6 | 9 | 3 | |
| Stage | 0.364 | |||||
| 2 | 37 | 12 | 10 | 13 | 2 | |
| 3 | 19 | 4 | 5 | 8 | 2 | |
| 4 | 4 | 2 | 1 | 1 | 0 | |
| Recurrence | 0.012* | |||||
| With | 24 | 0 | 12 | 9 | 3 | |
| Without | 12 | 5 | 0 | 7 | 0 | |
| NA | 24 | 13 | 4 | 6 | 1 | |
p<0.05 was considered statistically significant.
NA: data not available.
p-STAT3 expression and correlation with clinicopathologic variables
Among 60 tumor cell-free lymph nodes, we observed grade I p-STAT3 expression in 18 tissue (30%), grade II p-STAT3 expression in 16 tissue (27%), grade III p-STAT3 expression in 22 tissue (37%), and grade IV p-STAT3 expression in 4 tissue (6%) by immunohistochemistry. Thus, strong p-STAT3 expression was found in 26 (43%) lymph node tissues. Table 1 shows p-STAT3 expression status in relation to various clinical and pathologic features. Activated STAT3 was positively associated with tumor recurrence significantly (P=0.012), but no significant correlation was found with patients’ age, gender, tumor size, or TNM stage.
p-STAT3 expression and correlation with Ki-67, CD68 and Bcl-xL
Evaluation of p-STAT3, Ki-67, CD68, Bcl-xL expression in tumor cell-free lymph nodes was shown in Figure 1. In these lymph node tissues with strong p-STAT3 expression, Ki-67, Bcl-xL and CD68 are also highly expressed. By using Fisher exact test, we found that p-STAT3 expression was positively associated with expression levels of Ki-67 (proliferation), CD68 (myeloid-derived cells), and Bcl-xL (survival/ant-apoptosis) (P=0.024, 0.018, 0.019, respectively, Table 2). These findings indicated that high p-STAT3 expression may play a key role in cell proliferation, survival/anti-apoptosis, and myeloid cell cluster formation since their expression patterns coincide.
Figure 1.
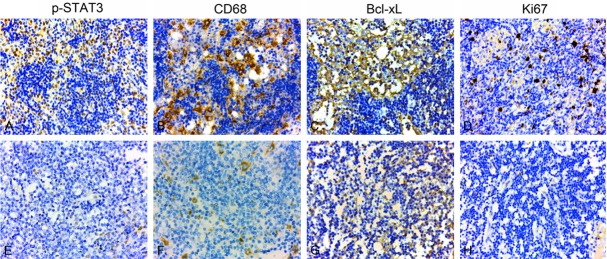
Evaluation of p-STAT3, Ki-67, CD68, Bcl-xL expression in tumor cell-free lymph nodes. A, E: p-STAT3 was detected in nuclei of lymphatic sinus cells, et al (×100). B, F: CD68 was detected in nuclei of lymphatic sinus cells (×100). C, G: Bcl-xL was detected in cytoplasm of lymphatic sinus cells (×100). D, H: Ki-67 was detected in nuclei of lymphatic sinus cells (×100). A-D: High expression; E-H: Low expression.
Table 2.
Relationship between p-STAT3 and Ki-67, CD68, Bcl-xL expression in 60 tumor cell-free lymph node tissues
| p-STAT3 expression | |||
|---|---|---|---|
|
|
|||
| Strong | Weak | P value | |
| Ki-67 expression | 0.024* | ||
| Strong | 12 | 6 | |
| Weak | 14 | 28 | |
| CD68 expression | 0.018* | ||
| Strong | 16 | 10 | |
| Weak | 10 | 24 | |
| Bcl-xL expression | 0.019* | ||
| Strong | 19 | 14 | |
| Weak | 7 | 20 | |
p<0.05 was considered statistically significant.
Correlation between p-STAT3 expression and patients’ overall survival
There were 48 patients with median follow-up of 23 months (from 1 to 79 months) available for the survival test of our study, and there were total 21 deaths. The overall survival rates were determined by using the Kaplan-Meier analysis. Among these 48 patients who had survival follow-up data, p-STAT3 over-expression influenced long-term patients’ overall survival time and rate after surgery. The mean overall survival time was 35.31±5.279 months when p-STAT3 lowly expressed in the tumor cell-free lymph nodes. However, when the lymph node p-STAT3 was highly expressed, the mean overall survival time was 28.58±6.802 months. Meanwhile, patients with heavy p-STAT3 expression in tumor cell-free lymph nodes have significant lower overall survival rate compared with those with light p-STAT3 expression signal (P=0.044, Figure 2). Taken together the overall survival rate and time were significantly lower and shorter in patients with high p-STAT3 expression in uninvolved lymph nodes than in those with low p-STAT3 expression.
Figure 2.
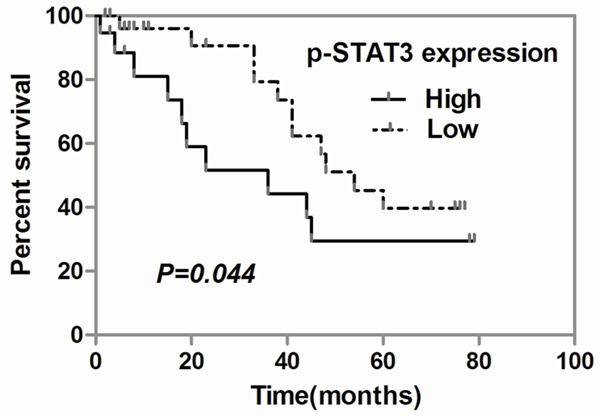
Kaplan-Meier curves for patients’ overall survival. The top survival curve shows the overall survival rate of 48 gastric cancer patients according to p-STAT3 expression in tumor cell-free lymph nodes (P<0.05). The bottom table shows the median survival time and OR (95% CI), and patient’s number calculating according to p-STAT3 expression.
Discussion
We conducted this study to examine the prognostic significance of p-STAT3 expression in the tumor cell-free lymph nodes from patients with gastric cancers. The results from this clinical study support our previous finding that consecutive activation of STAT3 can occur in distant organs before tumor cell arrival and that persistent STAT3 signaling in myeloid cells can increase their proliferation and survival. We show that STAT3 was strongly activated in 43.3% of tumor cell-free lymph nodes and the expression of activated STAT3 (p-STAT3) significantly correlated with worse prognosis by univariate analysis in the gastric cancer patients. Our observations also indicate that STAT3 is partially responsible for increased tumor recurrence after surgery treatment. Although overexpression of p-STAT3 in primary tumor sites has been recognized as a predictor of poorer survival in many malignancies, including gastric cancer [23,30-35], to our knowledge, this is the first time to report that STAT3 is also persistently activated in tumor cell-free lymph nodes in gastric cancer patients, which is positively associated with proliferation/survival signals in myeloid cell clusters. Hence, our findings suggest that STAT3 activation plays an important role in tumor metastasis in gastric adenocarcinoma. More importantly, p-STAT3 expression level in uninvolved lymph nodes can potentially be used as a prognostic factor for patients’ survival as well as tumor recurrence.
There are limitations in this study. For example, the sample size in this retrospective study in this study is relatively small, limiting the strength of the conclusion. We were not able to include more patients because it was difficult to contact the patient’s family through the phone numbers they left on medical documents about 5-6 years ago. Another limitation is that the data on cancer recurrences were not fully available in our current study, 24 out of 60 patients’ data were missing because we could not have the information whether these patients developed recurrence or not. The other limitation may refer to lack of mechanism research on gastric cancer cells or animal model. In the future, we need to enlarge the involved patient’s number as well as to explore the mechanism(s) by which STAT3 contributes to carcinogenesis, progress, metastasis and recurrence in gastric cancer.
In summary, our current small study has shown that individuals with STAT3-activated in tumor cell-free lymph nodes had more recurrence and poorer prognosis. As targeting pre-metastatic niches may be a new approach to prevent or reduce metastasis in cancer patients, our results suggest that activated STAT3 may be used to predict patient outcome and inhibition of STAT3 signaling might provide a therapeutic target for inhibiting gastric cancer recurrence and metastasis, and further prolong the survival of gastric cancer patients. In this respect, our findings may have substantial clinical implications.
Acknowledgements
We deeply thank the participants who have agreed to provide us with biological specimens; Pathology Department of Huashan Hospital for generously providing us with tissue specimens. This work was supported by grants from the National Natural Science Foundation of China (No. 81161120431); and by grants from the Ministry of Science and Technology of China (No. 2010CB732405).
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: A scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241:247–255. doi: 10.1097/01.sla.0000152019.14741.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10,783 patients with gastric cancer. Gastric Cancer. 1998;1:125–133. doi: 10.1007/s101200050006. [DOI] [PubMed] [Google Scholar]
- 5.Goseki N, Koike M, Yoshida M. Histopathologic characteristics of early stage esophageal carcinoma. A comparative study with gastric carcinoma. Cancer. 1992;69:1088–1093. doi: 10.1002/cncr.2820690503. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently-activated Stat3 maintains NF-κB constitutive activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role of Stat3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. The novel JAK2 inhibitor, AZD1480, potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darnell JE Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 12.Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 13.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 14.Han JC, Zhang KL, Chen XY, Jiang HF, Kong QY, Sun Y, Wu ML, Huang L, Li H, Liu J. Expression of seven gastric cancer-associated genes and its relevance for Wnt, NF-kappaB and Stat3 signaling. APMIS. 2007;115:1331–1343. doi: 10.1111/j.1600-0643.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 15.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 17.Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda A, Wang SC, Morris JP 4th, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 23.Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646–651. doi: 10.1111/j.1440-1746.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 24.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 25.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kujawski M, Kortylewski M, Lee HY, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid-cell-dependent tumor angiogenesis. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, Shen S, Priceman SJ, Kujawski M, Pal SK, Raubitschek A, Hoon DS, Forman S, Figlin RA, Liu J, Jove R, Yu H. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder M, Huang XY, Zhang JJ. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem. 2011;286:38886–388893. doi: 10.1074/jbc.M111.286245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Nagayasu T, Sekine I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833–838. doi: 10.1136/jcp.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemoto S, Ushijima K, Kawano K, Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M, Kakuma T, Kage M, Hori D, Kamura T. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101:967–972. doi: 10.1038/sj.bjc.6605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun M, Liu C, Nadiminty N, Lou W, Zhu Y, Yang J, Evans CP, Zhou Q, Gao AC. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72:82–89. doi: 10.1002/pros.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 35.Xiong H, Du W, Wang JL, Wang YC, Tang JT, Hong J, Fang JY. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012;90:1037–1046. doi: 10.1007/s00109-012-0869-0. [DOI] [PubMed] [Google Scholar]


