Abstract
Background: Protein phosphatase-2A (PP2A) is one of the major cellular serine-threonine phosphatases. It positively regulates apoptosis and negatively regulates the mitogenic pathway, suggesting that loss of it might be involved in cancer development. Recent studies found its association with breast, lung and colorectal cancer; however, its expression profile and its prognostic value in clear cell renal cell carcinoma (ccRCC) have not been investigated. Methods: Real-time quantitative PCR (qRT-PCR) and Western blot were used to explore PP2A expression in ccRCC and normal renal tissues. Moreover immunohistochemistry (ICH) was used to detect the expression of PP2A in ccRCC. Spearman’s rank correlation, Kaplan-Meier plots and Cox proportional hazards regression model were used to analyze the data. Results: Down-regulated expression of PP2A mRNA and protein was observed in the majority of ccRCC by qRT-PCR and Western blot when compared with their paired normal renal tissues. Clinic pathological analysis was showed a significant correlation existed between the lower expression of PP2A protein with the histological grade, lymph node metastasis and tumor distant metastasis (P<0.05); Survival analysis by Kaplan-Meier survival curve and log-rank test demonstrated that reduced PP2A expression in cancer tissue predicted poorer overall survival (OS) compared with group in higher expression. Notably, multivariate analyses by Cox’s proportional hazard model revealed that expression of PP2A was an independent prognostic factor in ccRCC. Conclusions: These results suggest that the aberrant expression of PP2A in human ccRCC is possibly involved with tumorigenesis and development, and the PP2A protein could act as a potential biomarker for prognosis assessment of renal cancer. Further studies on the cellular functions of PP2A need to address these issues.
Keywords: Phosphatase-2A, PP2A, serine-threonine phosphatases, clear cell renal cell carcinoma, biomarker, prognosis
Introduction
Renal cell carcinoma (RCC) is the most lethal urologic tumor and the sixth leading cause of cancer deaths in Western countries. Each year, around 200,000 patients are diagnosed with this malignancy resulting in approximately 100,000 deaths, and its incidence is increasing steadily in recent years [1,2]. RCC is represented by 80% by clear cell RCC (ccRCC), originating from the renal proximal tubule [3]. Nearly 25-30% of patients with RCC have evidence of metastases at initial presentation [4,5]. Although radical nephrectomy is effective to cure early and local RCCs, 30% of patients develop metastatic disease after surgery [6]. Patients with metastatic RCC face a dismal prognosis and have limited therapeutic options. Median survival in a recent cohort was only 1.5 years with fewer than 10% of patients surviving to 5 years [7]. Therefore, it is of paramount importance to better understand the pathogenesis of aggressive RCC in order to develop effective strategies for the prevention and treatment of RCC.
Protein phosphatase 2A (PP2A) is a member of phosphoprotein phosphatase (PPP) family which belongs to the super-family of protein serine/threonine phosphatases that reverse the actions of protein kinases by cleaving phosphate from serine and threonine residues of proteins [8]. PP2A is highly conserved across a variety of eukaryotic species and accounts for as much as 1% of the total cellular protein [9]. The structure of PP2A is composed of three subunits: catalytic (C), scaffold (A), and regulatory (B) subunit, with functional variability [10]. The PP2A holoenzyme exists in soluble form and gets compartmentalized in the cytosol, nucleus, mitochondria, cytoskeleton, and organelle membranes [11]. It has been proven that PP2A regulates various cellular processes, including protein synthesis, cellular signaling, and cell cycle determination, and apoptosis, metabolism, and stress responses [12,13]. In various malignancies, such as lung, breast, colon, gastric, and leukemia, it has been shown that PP2A is down-regulated and its role in transformation clearly defines PP2A as a tumor suppressor gene [14]. The tumor suppressing function of PP2A makes it a possible target in anticancer therapy. But there have been no reports about PP2A in renal cancer and the role of PP2A in renal cancer is still unknown.
In the present study, we examined both PP2A mRNA and protein expression by Real-time quantitative PCR (qRT-PCR) and Western blot and investigate the expression of the human PP2A proteins by Immunohistochemistry (IHC) and identify their potential roles in tumor occurrence, development and prognosis for patients with renal cancer.
Materials and methods
Patients and specimens
For qRT-PCR and Western blot analysis, we collected 10 paired fresh ccRCC and normal tissue samples from patient are that underwent surgery between January 2012 and December 2012. One hundred and six patients with ccRCC were included in the study, which was approved by the local ethics committee. The patients underwent radical nephrectomy at the Department of Urology, Shanghai Tenth People’s Hospital of Tongji University (Shanghai, China), between 2006 and 2008. None of the patients had received chemotherapy or radiotherapy before surgery. The diagnosis was confirmed by histopathological examination of the specimens. After surgery, tumor specimens and normal renal tissues were collected and stored in liquid nitrogen until use. Parts of each sample were fixed in formalin, embedded in paraffin and stored in the Department of Pathology, Shanghai Tenth People’s Hospital of Tongji University (Shanghai, China). All the patients were staged according to the tumor node metastasis staging system [15] and nuclear grade was evaluated on the basis of the Fuhrman criteria [16]. Clinical data of all the patients were collected from hospitalization and subsequent records. Detailed information is listed in Table 1. All patients were followed up until September 2012 with a median observation time of 48 months.
Table 1.
Correlation between PP2A expression and clinic pathological characteristics of ccRCC patients
| Parameters | Group | Total | PP2A expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Gender | Male | 58 | 30 | 28 | 0.212 |
| Female | 48 | 19 | 29 | ||
| Age (years) | <65 | 57 | 25 | 32 | 0.917 |
| ≥65 | 49 | 21 | 28 | ||
| Histological grade | I-II | 41 | 26 | 15 | 0.005 |
| III-IV | 65 | 23 | 42 | ||
| Tumor size (cm) | <4 cm | 67 | 36 | 31 | 0.452 |
| ≥4 cm | 39 | 18 | 21 | ||
| Tumor stage | T1-T2 | 62 | 30 | 32 | 0.947 |
| T3-T4 | 44 | 21 | 23 | ||
| Lymph nodes metastasis | Absence | 88 | 42 | 46 | 0.015 |
| Presence | 18 | 3 | 15 | ||
| Distant metastasis | Absence | 92 | 41 | 51 | 0.008 |
| Presence | 14 | 1 | 13 | ||
Real-time quantitative PCR
Total RNA was isolated tissue using TRIZOL reagent according to the manufacturer’s protocol (Invitrogen). RNA was reverse transcribed using SuperScript First Strand cDNA System (Invitrogen) according to the manufacturer’s instructions. The PP2A sense primer was 5’-TCTCAGGCATACGCTGACTAC-3’, and the antisense primer was 5’-GGAGACTCTGTACTCGAAGGT-3’. For the GAPDH gene, the sense primer was 5’-TGCACCACCAACTGCTTAGC-3’, and the antisense primer was 5’-GGCATGGACTGTGGTCATGAG-3’. The PCR amplification were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, on a Applied Biosystems 7900HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (Takara). Data was collected and analyzed by SDS2.3 Software (Applied Biosystems). The expression level of each candidate gene was internally normalized against that of the GAPDH. The relative quantitative value was expressed by the 2-ΔΔCt method. Each experiment was performed in triplicates and repeated three times.
Western blot assay
Total proteins from tissues were lysed in lysis buffer containing protease inhibitor. Protein concentration was determined using a Bio-Rad protein assay system (Bio-Rad). Equivalent amounts of proteins were separated by SDS-PAGE, and then transferred to polyvinylidene difluoride membranes (Bio-Rad). After being blocked in Tris buffered saline (TBS) containing 5% non-fat milk, the membranes were incubated with specific primary antibodies (Epitomics) at 4°C for 12 hours and then with horseradish peroxidase conjugated anti-Rabbit antibody for 2 hours at room temperature. ECL detection reagent (Amersham LifeScience, Piscataway, NJ) was used to demonstrate the results.
Immunohistochemistry staining
All samples were fixed in 10% formaldehyde solution, embedded in paraffin blocks, cut in 4 μm thick sections, and mounted on glass slides. Each slide was dewaxed in xylene and rehydrated in grade alcohol, followed by boiling in 10 mmol/L of citrate buffer (PH 6.0) for antigen retrieval. After inhibition of endogenous peroxidase activities for 30 minutes with methanol containing 0.3% H2O2, the sections were blocked with 2% bovine serum albumin for 30 minutes and incubated overnight at 4°C with primary Rabbit monoclonal anti-PP2A antibody (Epitomics). After washing thrice with PBS, the slides were incubated with horseradish peroxidase-conjugated mouse-anti-rabbit IgG for 30 minutes, followed by reaction with diamin obenzidine and counterstaining with Mayer/hematoxylin. Negative control was done by omission of the primary antibody and substituting it with nonspecific rabbit IgG.
Evaluation of immunohistochemical staining
The evaluation of the immunohistochemical staining was performed independently by two authors without knowledge of the clinicpothological information. The immunoreactive scores besides PP2A were determined by the sum of extension and intensity as literature reported previously [17]. The intensity of the staining was scored using the following scale: 0, no staining of the tumor cells; +, mild staining; ++, moderate staining and +++, marked staining. The area of staining was evaluated and recorded as a percentage: 0, less than 5%; +, 5%-25%; ++, 26%-50%; 3+, 51%-75% and 4+, more than 75%. The combined scores were recorded and graded as follows: -, 0; +, 1-2; ++, 3-5; +++, 6-7. Additionally, for statistical analysis, the - and 1+ cases were pooled into the low-expression group, and the 2+ and 3+ cases were pooled into the high-expression group.
Statistical analysis
Computerized statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0. The t-test was used to analyze the data from qRT-PCR and Western blot in the tissues. Clinical and histopathologic information and the results from the ICH studies were entered into a database. The significance of PP2A expression for tumor was analyzed by the Kaplan-Meier method, and the differences were evaluated by the log-rank test. Multivariable recurrence-free survival analyses were performed with the Cox proportional hazards model. Differences were considered significant if the P-value from a two-tailed test was <0.05.
Results
Expression of PP2A mRNA by qRT-PCR and PP2A protein expression by Western blot in paired renal tissues
We examined PP2A mRNA expression in 10 paired fresh ccRCC and normal tissue samples by qRT-PCR. It showed that the decreasing PP2A mRNA expression could be detected in renal cancer samples in comparison with the normal renal samples (P<0.05, Figure 1A). To investigate whether PP2A was also reduced at the protein level, Western blot was performed. We found that the protein level of PP2A in tumor samples was significantly lower than that in normal renal samples (Figure 1B).
Figure 1.
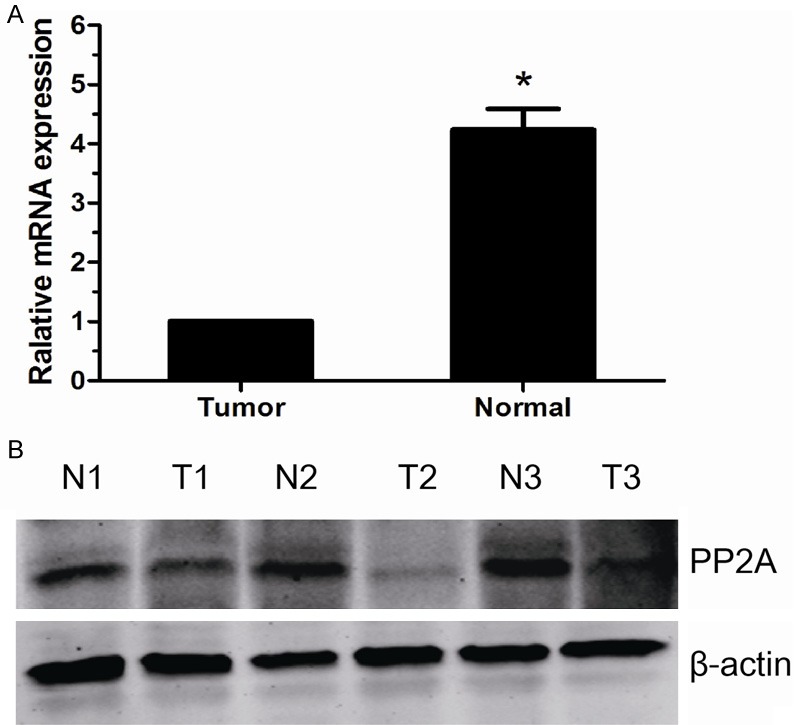
The expression of PP2A mRNA and protein in the human ccRCC surgical specimens. A. The relative mRNA expression of PP2A was lower in ccRCC tissues than in matched normal renal tissues (P<0.05). B. The PP2A protein expression was lower in the ccRCC tissues than in matched normal renal tissues. N, normal renal tissues; T, ccRCC tissues.
Expression of PP2A in ccRCC as determined by ICH analysis
By using of ICH we investigated the protein expression of PP2A in ccRCC specimens and specimens of normal renal. The tumorous or non-tumor staining was semi-quantitatively scored by the intensity and the percentage of positive staining. ICH staining showed that the PP2A protein exhibited both cytoplasmic and nuclear staining patterns and immunohistological staining was stronger in benign tissues compared with malignant tissues (Figure 2A).
Figure 2.

PP2A protein expression in ccRCC surgical specimens and patient survival. A. ICH analysis of PP2A protein expression in 106 cases of ccRCC tissues. a. ICH expression of PP2A in normal renal tissues (×200). b. ICH expression of PP2A in normal renal tissues (×400). c. ICH expression of PP2A in ccRCC tissues (×200). d. ICH expression of PP2A in ccRCC tissues (×400). B. The survival analysis of PP2A. Patients with lower PP2A expression in tumor tissue were closely correlated with poorer overall survival than patients with tumor with higher expression (p<0.05, respectively).
Relationship between PP2A expression and ccRCC patients’ clinicopathologic variables
In our ccRCC cohort, the relationship between the expression of PP2A and patient clinical characteristics was shown in Table 1. Low expression of PP2A was found to significantly correlate with higher histological grade (P=0.005), lymph node metastasis (P=0.015) and tumor distant metastasis (P=0.008). No significant difference in PP2A expression was observed with gender, age, tumor size, tumor stage (P>0.05).
Relationship between clinicopathologic features, PP2A expression, and ccRCC patients’ survival: univariate survival analysis
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. Differences in survival times were assessed using the log-rank test. First, to confirm the representativeness of the ccRCC in our study, we analyzed established prognostic predictors of patient survival. Kaplan-Meier analysis demonstrated a significant impact of well-known clinical pathological prognostic parameters, such as histological grade, lymph node status and tumor distant metastasis on patient survival (P<0.05, Table 2). Assessment of survival in ccRCC patients revealed that lower expression of PP2A was correlated with adverse survival of ccRCC patients (P=0.007, Table 2, Figure 2B).
Table 2.
Prognostic factors in Cox proportional hazards model
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | 0.879 | 0.592-1.478 | 0.673 | |||
| Male vs. Female | ||||||
| Age (years) | 1.379 | 0.694-1.988 | 0.371 | |||
| ≥65 vs. <65 | ||||||
| Tumor stage | 2.962 | 2.174-3.886 | 0.108 | |||
| T3-4 vs. T1-2 | ||||||
| Histological grade | 3.984 | 2.833-5.827 | <0.001 | 2.637 | 1.849-4.126 | 0.012 |
| I-II vs. III-IV | ||||||
| Lymph node | 4.467 | 3.294-7.196 | 0.005 | 3.364 | 2.109-4.158 | 0.021 |
| Presence vs. Absence | ||||||
| Distant metastasis | 6.724 | 3.896-12.492 | <0.001 | 4.437 | 3.386-7.964 | 0.019 |
| Presence vs. Absence | ||||||
| PP2A | 3.964 | 2.579-5.947 | 0.007 | 3.427 | 2.147-5.764 | <0.001 |
| low vs. high | ||||||
Independent prognostic factors for ccRCC: multivariate cox regression analysis
Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of PP2A and those clinical pathological parameters that were significant in univariate analysis (histological grade, lymph node status and tumor distant metastasis) were further examined in multivariate analysis. The results showed that the expression of PP2A was an independent prognostic factor for overall patient survival (relative risk: 3.427, CI: 2.147-5.764, P<0.001, Table 2). With regard to other parameters, histological grade, lymph node status and tumor distant metastasis status were also shown to be an independent prognostic factor for overall survival (P<0.05, Table 2).
Discussion
It has been postulated that phosphorylation and dephosphorylation are coordinated events that are governed and balanced by the activity of kinases and phosphatases [18]. Protein phosphatase type 2A is a ubiquitously expressed serine/threonine phosphatase, which acts as key dephosphorylating enzyme for various signal-transduction pathways in eukaryotic cells [19]. Although numerous studies have shown the role of PP2A as a tumor suppressor, the regulatory role of individual subunits, which is deregulated in cancer, remains to be defined. Recent evidence from the genomic and proteomic data clearly defines the critical role of PP2A subunits under both normal physiological and oncogenic conditions by regulating particular substrates [20]. The study showed decrease activity of any of PP2A subunits might contribute to the proliferation of cancer cells which focuses on the differential expression of various PP2A subunits in benign and malignant prostatic tissues [21]. Previous studies have reported the altered expression of PP2A in kinds of cancers such as lung carcinoma, breast carcinoma, colon carcinoma, gastric carcinoma and leukemia [14].
Renal carcinogenesis is characterized by distinct morphological, genetic and cellular events. Development and progression of renal cancer to metastasis and lethal state are believed to be driven by multiple genetic alterations, the nature of which has remained poorly understood. In the present study, we sought to determine whether there was any difference in PP2A expression between ccRCC and normal tissue samples which had not been studied previously. This study demonstrated that PP2A proteins were down-regulated in the ccRCC, and explored available evidence of close correlation of PP2A expression and the total patients’ survival during a five-year follow-up survey.
To directly address the potential roles for PP2A protein in the occurrence and development of renal cancer, an elaborate experiment was conducted and a rigorous analysis was performed of human PP2A mRNA and proteins on a renal cancer samples. Our results revealed that the PP2A expression in renal cancer tissues was remarkably lower than that in normal renal tissues (P<0.05). Pandey’s study also indicated that the abnormal expression of PP2A might be correlated with prostate oncogenic event [22].
In the present study, we found the expression level of PP2A in cytoplasmic and nuclear was significantly associated with histological grade (p=0.005), lymph node metastasis (p=0.015) and distant metastasis (p=0.008). It is suggested that PP2A are associated with tumor development and progression and may inhibit tumor invasion. We further imagined that the PP2A proteins may affect the activation of cellular signal transduction pathway, cell division cycle and tumor angiogenesis to influence biological behavior of tumor, and this had just been unraveled in the latest relevant researches. Recent study showed that PP2A can inhibit metastatic potential; in the study, the author showed PP2A is likely to play an integral role in tumor suppressor network, which is partially regulated by AKT/FAK/β-catenin in promoting AI growth of PCa [22].
Ultimately, a total of 106 patients histological proven renal cancer with follow-up information were conducted a systematically analysis to confirm the relationship of the PP2A proteins and outcome of patient initially. Our finding demonstrated that patients with higher expression of PP2A in tumor tissue had a better overall survival than patients with lower expression (P<0.05, respectively), providing an evidence that elevated expression of PP2A in renal cancer might facilitate a decreased malignant and better prognostic phenotype. It is noteworthy that by multivariate Cox analysis combining expression of PP2A proteins with other parameters, PP2A was found as an independent prognostic factor (p<0.001) for patient survival. The aberrant expression of PP2A protein linked to a poor prognosis of patients has never been investigated in renal cancer before.
Conclusion
In this study, we reported for the first time that PP2A expression was down-regulated in clinical ccRCC tissues, and low expression of PP2A was associated closely with a more malignant clinical feature and poor prognosis of ccRCC patients. Our results suggest that PP2A low-expression might be useful as a prognostic factor for ccRCC patients. Apparently, a further understanding of the molecular mechanism by PP2A in human ccRCC would help in the discovery of novel targeted agents and might also lead to the development of new approaches for effective therapy of human ccRCC.
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81270831).
Disclosure of conflict of interest
The authors had no conflicts of interest to declare.
References
- 1.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: The 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 2.Montironi R, Santinelli A, Pomante R, Mazzucchelli R, Colanzi P, Filho AL, Scarpelli M. Morphometric index of adult renal cell carcinoma. Virchows Archiv. 2000;437:82–89. doi: 10.1007/s004280000216. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura K, Nakada C, Mashio M, Narimatsu T, Yoshimoto T, Tanigawa M, Tsukamoto Y, Hijiya N, Takeuchi I, Nomura T, Sato F, Mimata H, Seto M, Moriyama M. Down-regulation of SAV1 plays a role in pathogenesis of high-grade clear cell renal cell carcinoma. BMC Cancer. 2011;11:523. doi: 10.1186/1471-2407-11-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B, Zigeuner RE, Artibani W, Guillé F, Abbou CC, Salzano L, Gallo C. Comparison of predictive accuracy of four prognostic models for non-metastatic renal cell carcinoma after nephrectomy. Cancer. 2005;104:1362–1371. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 5.Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, Cindolo L, De la Taille A, Tostain J, Mulders PF, Salomon L, Zigeuner R, Prayer-Galetti T, Chautard D, Valeri A, Lechevallier E, Descotes JL, Lang H, Mejean A, Patard JJ. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z, Chu PG, Woda BA, Liu Q, Balaji KC, Rock KL, Wu CL. Combination of quantitative IMP3 and tumor stage: a new system to predict metastasis for patients with localized renal cell carcinomas. Clin Cancer Res. 2008;14:5579–84. doi: 10.1158/1078-0432.CCR-08-0504. [DOI] [PubMed] [Google Scholar]
- 7.Patil S, Ishill N, Deluca J, Motzer RJ. Stage migration and increasing proportion of favorable-prognosis metastatic renal cell carcinoma patients. Cancer. 2010;116:347–354. doi: 10.1002/cncr.24713. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Wang Z, Jiang C, Ding Y. PP2A-Mediated Anticancer Therapy. Gastroenterol Res Pract. 2013;2013:675429. doi: 10.1155/2013/675429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wera S, Hemmings B. Serine/threonine protein phosphatases. Biochem J. 1995;311:17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Claret FX. Phosphatases: the new brakes for cancer development? Enzyme Res. 2012;2012:659649. doi: 10.1155/2012/659649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki N, Ito M, Nakano T, Inagaki M. Spatiotemporal distribution of protein kinase and phosphatase activities. Trends Biochem Sci. 1994;19:448–52. doi: 10.1016/0968-0004(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 12.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virshup DM. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 14.Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14:152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lynch HT, Smyrk TC. Identifying hereditary nonpolyposis colorectal cancer. N Engl J Med. 1998;338:1537–1538. doi: 10.1056/NEJM199805213382109. [DOI] [PubMed] [Google Scholar]
- 18.Bononi A, Agnoletto C, De Marchi E, Marchi S, Patergnani S, Bonora M, Giorgi C, Missiroli S, Poletti F, Rimessi A, Pinton P. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. doi: 10.4061/2011/329098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 20.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC. The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Pandey P, Seshacharyulu P, Das S, Rachagani S, Ponnusamy MP, Yan Y, Johansson SL, Datta K, Fong Lin M, Batra SK. Impaired expression of protein phosphatase 2A subunits enhances metastatic potential of human prostate cancer cells through activation of AKT pathway. Br J Cancer. 2013;108:2590–600. doi: 10.1038/bjc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]


