Abstract
Background: In the assisted reproductive technique, cryopreserving in vitro-matured oocytes is a new strategy to extend the pool of total oocytes. However, oocyte cryopreservation technique is still unsatisfied. So the assessment of cyro-damage on meiotic spindle and mitochondrial function is necessary to evaluate and refine the current protocols. Material and Methods: The immature oocytes were donated from women undergoing ICSI cycles. Cytoskeleton was assessed by α-tubulin and mitochondria through the fluorescent ΔΨm reporter JC-1. Results: Relative inner membrane potential in MII oocytes from vIVM group sharply decreased, compared with the control (n=30) (1.397 vs. 1.019, P<0.05). 45.2% defective spindles were observed in fIVM group, compared with 48.0% in vIVM group (P>0.05). Oocytes in fIVM (35.5%, 11/31) and vIVM (40.0%, 10/25) displayed abnormal chromosome (P>0.05). Conclusion: In vitro maturation (IVM) has an adverse effect on the organization of spindle and chromosome, and no significantly effect on spindle and chromosome was discovered after vitrification-thaw cycle, while there was obvious damage of oocyte mitochondrial function of in vitro-matured oocyte detected after warming, which may be the reason of the low following developmental potential.
Keywords: Assisted reproduction, vitrification, human oocytes, mitochondrial function, spindle
Introduction
In the assisted reproductive technique (ART), 15-20% oocytes retrieved from patients is meiotically immature [1,2]. Since, there is the possibility of abnormal embryonic development and defective cytoplasmic maturation, those immature oocytes are often discarded [3,4]. However, the number of mature oocytes, retrieved from the patients who are low responders or who have an unsynchronized cohort of follicles, is often not enough, those immature oocytes are clinically essential to increase yield of total available oocytes. At the same time, cryopreservation of in vitro-mature oocytes, with no or minimal hormonal stimulation, could meet the demand of patients who want to preserve fertility in case of pathologies or therapies that damage ovarian reserves [5]. Oocytes storage may also circumvent the ethical and legal problems encountered in embryo cryopreservation and develop the oocyte banking [6,7]. Lately, progress in human oocyte cryopreservation has been achieved, based on the effective delivery rate [8]. Recent study showed that vitrification is a prospective cryopreservation technique with prominently improved the survival rate of oocytes [9-11].
The first baby from a vitrified in vitro matured oocyte was reported at 2009 [12]. In this case, 89% of oocytes extruded a polar body (16/18), 25% survived (4/16), 75% fertilized (3/4), and a singleton pregnancy was achieved from 3 embryos transfer [12]. Several studies have undertaken comparison of freezing at either the immature stage or metaphase II (MII) following IVM [7,13-20], cryopreserving of at MII stage following IVM is more efficient than at the immature stage prior to IVM process [5,20]. Nevertheless, the fertilization/blastocyst rate is unsatisfactorily after freezing-thawing cycle [14,17,19,21]. The previous research on the influence of cryopreserving human in vivo matured oocytes have found that surviving oocytes had normal spindles and karyotypes, but with cortical granule exocytosis, swelling of smooth endoplasmic reticulum vesicles, mitochondrial damage, and low fertilization/blastocyst rate [11,22]. Therefore, little is known about the nature of structure and function affected by vitrification of in vitro-matured oocytes.
So we hypothesize that the osmotic pressure change during dehydration-rehydration cycles may affect mitochondrial function and the meiotic spindle configuration. As a consequence, the potential for oocytes fertilization and development of embryo may be reduced, and even completely ruined. In our study, the influence of vitrification will be studied on human in vitro-matured oocytes by mitochondrial function with JC-1 and cytoskeleton through α-tubulin.
Material and methods
Source of oocytes
The study was approved by the Ethics Committee of Tongji Hospital, and patients who conducted intracytoplasmic sperm injection were included with written consent at the Reproductive Center of Tongji Hospital, Wuhan, China, between November 2012 and May 2013. The immature oocytes included were collected from patients (age 20-35 years) whose infertility was only due to male infertility or combined with oviduct factors. The oocytes were divided into two groups: (i) germinal vesicle (GV) oocytes matured in vitro (fIVM), (ii) GV oocytes matured in vitro first, then vitrificated (vIVM).
Stimulation protocol and oocyte recovery procedures
The patients were given standard ovarian stimulation using a gonadotropin-releasing hormone agonist long protocol [23]. After down-regulation by subcutaneous injection of 0.1 mg triptorelin acetate (Decapeptyl; Ferring), the patients were stimulated with 150-300 IU/d recombinant FSH (Gonal-F, Serono). The oocyte retrieval was performed through vaginal puncture under ultrasound guidance. After ovum pick-up, oocytes were denuded of cumulus cells with Hyaluronidase solution (Sigma, USA) in order to assess nuclear maturity. The GVs, which are also called failed-matured oocytes, were divided into either fIVM or vIVM groups through a random computer-generated list. Prior to cryopreservation, the oocytes were evaluated microscopically based on morphology to identify high-quality oocytes with an appropriate size, normal zona pellucida, and integral membrane.
In vitro maturation (IVM)
The immature oocytes were cultured in 1 mL of maturation medium (Cooper Surgical/SAGE, Trumbull, CT) supplemented with the final concentrations of 75 mIU/mL of FSH and LH at 37°C in an atmosphere of 5% CO2 and 95% air with high humidity for maturation in culture. The maturity of oocytes was assessed 24-28 hours after IVM culture, and the oocytes with a polar body were regarded as mature.
Vitrification and thawing
Mature oocytes were cryopreserved using a vitrification method previously described [21]. Briefly, the oocytes were suspended in equilibration medium containing 7.5% (v/v) ethylene glycol and 7.5% (v/v) dimethyl sulphoxide (DMSO) for 15 minutes at room temperature, and were then transferred to vitrification medium containing 15% (v/v) ethylene glycol, 15% (v/v) DMSO, and 0.5 mol/l sucrose at room temperature for 45 to 60 seconds. They were then loaded onto a specially designed vitrification device, the McGill Cryoleaf (Medicult, Jyllinge, Denmark), and were plunged immediately into liquid nitrogen for storage. For thawing, the McGill Cryoleaf was directly inserted into thawing medium of 1.0 mol/L sucrose in HEPES-buffered human tubal fluid (HTF) for 1 minute at 37°C. Warmed oocytes were transferred to diluent medium-I (0.5 mol/L sucrose in HEPES-buffered HTF) and diluent medium-II (0.25 mol/L sucrose), respectively, for 3 minutes each; then they were washed twice in washing medium (HEPES-buffered HTF containing 10% human serum albumin). Oocyte survival was evaluated based on the integrity of oocyte membrane and zona pellucida and culturing for 2 hours.
Measurement mitochondrial function
For mitochondrial staining, oocytes were cultured in M2 medium containing 2 uM JC-1 (Beyotime Institute of Biotechnology) for 30 min at 37.5°C CO2 in air. After washes, oocytes were still incubated in HEPES-buffered HTF and analyzed by fluorescence microscopy (Zeiss LSM510 META). An image was taken at 400 fold magnification in an optical cross section through the center of the oocyte at its largest nuclear diameter.
Statistical analysis
Image processing was performed using a laser scanning microscopes Image Browser. Data (mean ± SEM) were from at least three replicates per experiment and analyzed by analysis of variance using SPSS software (SPSS Inc., Chicago, IL, USA) followed by the Fisher’s least significant difference test. The number of oocytes observed was denoted in parentheses as (n). Difference at P<0.05 was considered to be statistically significant and different superscripts indicate the statistical difference.
Results
Patient and oocyte characteristics
There was no significant difference in the characteristics of age, Day 3 FSH, and BMI between the fIVM and vIVM groups (Table 1). A total of 60 (76.3%) oocytes matured from fIVM group, while the rate of maturation in vIVM group was 74.4% (n=64) (P>0.05; Table 1). After warming, a total of 49 of 64 from vIVM group (76.6%) survived from vIVM group.
Table 1.
Patient and oocyte characteristics
| Variables | fIVM (n=80) | vIVM (n=86) | P value |
|---|---|---|---|
| Age (years) | 28.5 ± 3.4 | 28.4 ± 3.6 | NS |
| BMI (Kg/m2) | 22.9 ± 2.6 | 23.0 ± 2.5 | NS |
| Day 3 FSH (IU/L) | 6.6 ± 1.2 | 6.6 ± 1.3 | NS |
| M11 oocytes (matured) | 61 (76.3%) | 64 (74.4%) | NS |
Values are expressed as mean ± SD; NS=not significant (p>0.05).
The change of mitochondrial function after warming
At an inner mitochondrial membrane potential (ΔΨm)<100 mV, JC-1 remain a monomer and emits green fluorescence in the FITC channel (low polarized mitochondria), whereas at ΔΨm>140 mV, it forms J-aggregates and emits red fluorescence (high polarized mitochondria) [24]. We investigated the high polarization of mitochondria in MII oocytes by examining the relative level of red to green fluorescence emission. Relative inner membrane potential in MII oocytes from vIVM (n=24) sharply decreased, compared with the control (n=30) (1.019 vs. 1.397, P<0.05), (Figure 1A and 1B), indicating a decreased ΔΨm.
Figure 1.
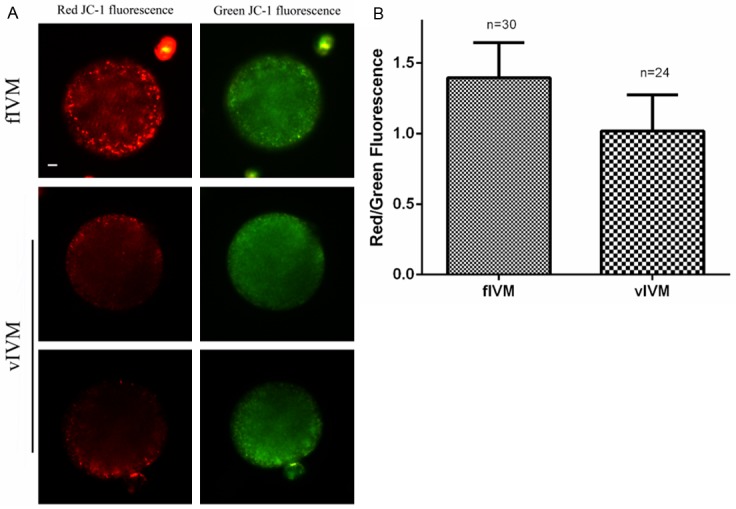
Effects of vitrification on the inner mitochondrial membrane potential measured by JC-1 fluorescence. A: Relative inner membrane potential (ΔΨm) in MII oocytes from vIVM and fIVM groups (bar=10 um). B: Red/green fluorescence intensity in both groups, data are mean + SEM of at least three independent experiments, n shows number of oocytes (P<0.05).
Assessment of spindle and chromosome configuration
Spindle with a barrel-shaped structure was regarded as normal, however, spindle configuration included partial or total disorganization of microtubules was abnormal [25]. Chromosome configuration was regarded as morphologically normal when chromosomes as a compact metaphase plate were located at the equatorial region, otherwise, chromosome was abnormal, showing lagging and irregularly scattered structure [25]. We examined the spindle and chromosome by confocal scanning. As showed in Figure 1A, most of oocytes in fIVM group exhibited barrel-shaped spindles and well-aligned chromosomes. However, 45.2% (14/31) defective spindles were observed in fIVM group, compared with 48.0% (12/25) in vIVM group (P>0.05) (Figure 2B). Oocytes in fIVM (35.5%, 11/31) and vIVM (40.0%, 10/25) displayed abnormal chromosome (P>0.05) (Figure 2B).
Figure 2.
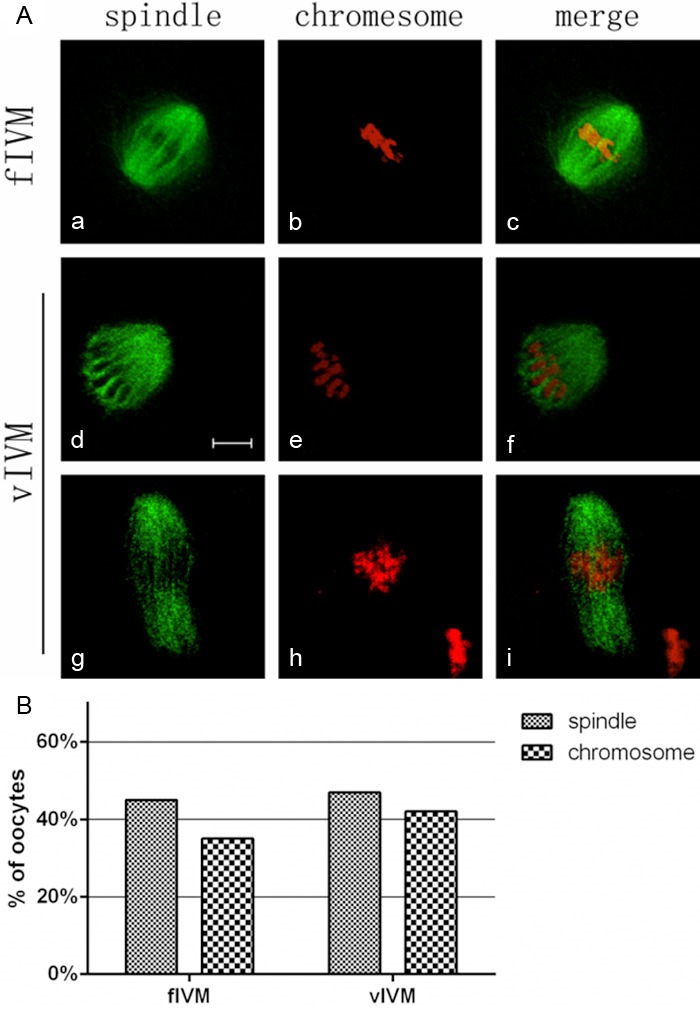
The change of spindles and chromosomes after vitrification. (A) Representative images of the spindles and chromosomes in both groups. Normal spindle and aligned chromosomes are seen in fIVM; misaligned chromosomes were detected in model groups. Figures (d) and (g) show the defective spindles; Figures (e) and (h) show lagging chromosomes and irregularly scattered chromosomes; Figures (c), (f) and (i) include chromosomes and spindles. α-Tubulin (green); DNA (red). (Bar=10 um); (B) Percentage of oocytes with abnormal spindle and misaligned chromosomes in MII oocytes, data are mean + SEM of at least three independent experiments.
Discussion
Cryopreserving IVM oocytes is a new strategy to extend the pool of total oocytes. However, the efficiency of in vitro-maturation procedure and oocyte cryopreservation methods is not satisfactory, as they reduced the developmental potential, the assessment of cryo-damage on cell structure and functions of organelles is necessary to evaluate and refine the current protocols. The main results in this study revealed that vitrification of in vitro-mature oocytes disturbed mitochondrial function, and no significant change was found at the meiotic spindle configuration. The immature oocytes were without surrounding granulosa cells because we need check the polar body during the ICSI cycle.
During IVM, immature oocytes are cultured in special conditions to continue developmental process to the MII stage. The coordination of nuclear and cytoplasmic maturation determined the developmental competence of oocytes [26]. In our study, immature oocytes were without surrounding granulosa cells, however, the rate of maturation was 75.3% (125/166), similar to previous studies [21,27]. Buckett had demonstrated that babies born after IVM do not have any increased fetal abnormality compared with regular IVF or spontaneous conceptions in fertile women [28]. However, some studies found that developmental potential of embryo reduced significantly after cryopreserving IVM oocytes compared with cryopreserving in vivo-matured oocytes or directly fertilizing after in vitro maturing [21,27,29]. Possibly, in vivo-matured oocytes are prone to cryo-damage.
As described in the results section, cryopreservation of in vitro-maturation oocytes significantly affects the inner mitochondrial membrane potential. Mitochondria are one of the most important organelles in the cytoplasm, and their dysfunction or abnormalities may injure embryo developmental potential [22]. And we usually assess mitochondrial function by the inner membrane potential probe JC-1. Similar to previous study [30], higher polarized mitochondrial clusters are localized within the pericortical region in maturing oocytes. Meanwhile, our studies showed that the relative ΔΨ in MII oocytes from vIVM group sharply decreased compared with fIVM group. Previous experiments also have suggested that dysfunction of mitochondrial potential can affect embryo development capacity [31] and that decline of relative ΔΨ is an early apoptotic event in cells [32]. Taken together, we concluded that the mitochondrial function affected by vitrification may be resulted from the decreasing follow-up fertilization and embryo development.
Data presented in this study demonstrated that 45.2% defective spindle and 35.5% abnormal chromosome were detected after in vitro maturation, which was higher than oocytes matured in vivo based on previous study [5,25]. However, the defective rate was not significantly elevated by vitrification procedure, 48.0% defective spindle and 40.0% abnormal chromosome in vIVM group. In previous studies, compared with maturation in vivo, oocytes matured in vitro had significantly decreased development competence, by deleterious effects on the organization of spindle and chromosome [25], the change in mitochondrial distribution pattern [33], low blastocyst rate [34]. Chang had found that the oocytes chromosome alignment was maintained through vitrification-thaw cycle and the spindle was able to recover immediately after warming in mouse oocytes [35]. Taken together, IVM could have deleterious effects on the organization of spindle and chromosome. And no significantly difference of the oocyte spindle and chromosome organization were detected after warming.
In summary, immature oocyte retrieval from IVF patients followed by IVM and oocyte vitrification which can increase the yield of total available oocytes, develop the oocyte bank, and circumvent the ethical and legal problems. We have provided proof of principle evidence that vitrification process affects oocyte mitochondrial function of in vitro-matured oocytes, which may be the reason of low embryo developmental potential. While no significant effect at the spindle and chromosome configuration was discovered. This will be a new insight for us to develop an advanced protocol of in vitro-maturation and vitrification.
Acknowledgements
Supported by the Fund of Health Department of Hubei Province (No. JX5A02).
Disclosure of conflict of interest
None.
References
- 1.Mohsenzadeh M, Khaliliz MA, Nazari S, Jahromi VH, Agharahimi A, Halvaei I. Effect of vitrification on morphology and in-vitro maturation outcome of human immature oocytes. Ital J Anat Embryol. 2012;117:190–198. [PubMed] [Google Scholar]
- 2.Smitz JE, Cortvrindt RG. In vitro growth and maturation of oocytes in human and non-human primates. Gynecol Obstet Invest. 2004;57:18–21. [PubMed] [Google Scholar]
- 3.Smith GD. In vitro maturation of oocytes. Curr Womens Health Rep. 2001;1:143–151. [PubMed] [Google Scholar]
- 4.Racowsky C, Kaufman ML. Nuclear degeneration and meiotic aberrations observed in human oocytes matured in vitro: analysis by light microscopy. Fertil Steril. 1992;58:750–755. doi: 10.1016/s0015-0282(16)55323-0. [DOI] [PubMed] [Google Scholar]
- 5.Combelles CM, Chateau G. The use of immature oocytes in the fertility preservation of cancer patients: current promises and challenges. Int J Dev Biol. 2012;56:919–929. doi: 10.1387/ijdb.120132cc. [DOI] [PubMed] [Google Scholar]
- 6.Gosden RG. Fertility preservation: definition, history, and prospect. Semin Reprod Med. 2009;27:433–437. doi: 10.1055/s-0029-1241051. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Racowsky C, Combelles CM. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology. 2012;65:79–87. doi: 10.1016/j.cryobiol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, Remohi J, Ragni G, Ubaldi FM. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27:1606–1612. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 9.Garcia JI, Noriega-Portella L, Noriega-Hoces L. Efficacy of oocyte vitrification combined with blastocyst stage transfer in an egg donation program. Hum Reprod. 2011;26:782–790. doi: 10.1093/humrep/der008. [DOI] [PubMed] [Google Scholar]
- 10.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Burgos M, Herrero L, Megias D, Salvanes R, Montoya MC, Cobo AC, Garcia-Velasco JA. Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril. 2011;95:374–377. doi: 10.1016/j.fertnstert.2010.07.1089. [DOI] [PubMed] [Google Scholar]
- 12.Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, Buckett WM, Tulandi T, Tan SL. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91:372–376. doi: 10.1016/j.fertnstert.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 13.Baka SG, Toth TL, Veeck LL, Jones HJ, Muasher SJ, Lanzendorf SE. Evaluation of the spindle apparatus of in-vitro matured human oocytes following cryopreservation. Hum Reprod. 1995;10:1816–1820. doi: 10.1093/oxfordjournals.humrep.a136182. [DOI] [PubMed] [Google Scholar]
- 14.Chung HM, Hong SW, Lim JM, Lee SH, Cha WT, Ko JJ, Han SY, Choi DH, Cha KY. In vitro blastocyst formation of human oocytes obtained from unstimulated and stimulated cycles after vitrification at various maturational stages. Fertil Steril. 2000;73:545–551. doi: 10.1016/s0015-0282(99)00546-4. [DOI] [PubMed] [Google Scholar]
- 15.Goud A, Goud P, Qian C, Van der Elst J, Van Maele G, Dhont M. Cryopreservation of human germinal vesicle stage and in vitro matured M II oocytes: influence of cryopreservation media on the survival, fertilization, and early cleavage divisions. Fertil Steril. 2000;74:487–494. doi: 10.1016/s0015-0282(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 16.Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17:1885–1891. doi: 10.1093/humrep/17.7.1885. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Xing Q, Zhang ZG, Wei ZL, Zhou P, Cong L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod Biomed Online. 2009;19:369–373. doi: 10.1016/s1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 18.Versieren K, Heindryckx B, O’Leary T, De Croo I, Van den Abbeel E, Gerris J, De Sutter P. Slow controlled-rate freezing of human in vitro matured oocytes: effects on maturation rate and kinetics and parthenogenetic activation. Fertil Steril. 2011;96:624–628. doi: 10.1016/j.fertnstert.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 19.Fasano G, Demeestere I, Englert Y. In-vitro maturation of human oocytes: before or after vitrification? J Assist Reprod Genet. 2012;29:507–512. doi: 10.1007/s10815-012-9751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JA, Barritt J, Moschini RM, Slifkin RE, Copperman AB. Optimizing human oocyte cryopreservation for fertility preservation patients: should we mature then freeze or freeze then mature? Fertil Steril. 2013;99:1356–62. doi: 10.1016/j.fertnstert.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Shapiro DB, Bernal DP, Wright G, Kort HI, Nagy ZP. Human oocyte vitrification: in-vivo and in-vitro maturation outcomes. Reprod Biomed Online. 2008;17:684–688. doi: 10.1016/s1472-6483(10)60316-1. [DOI] [PubMed] [Google Scholar]
- 22.Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertility and Sterility. 2009;91:1023–1034. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, Zhu G. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–1327. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, Guo L, Chao SB, Chen Z, Liang XW, Hou Y, Schatten H, Sun QY. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. 2012;27:2130–2145. doi: 10.1093/humrep/des137. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74:295–298. doi: 10.1016/s0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Xing Q, Zhang ZG, Wei ZL, Zhou P, Cong L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod Biomed Online. 2009;19:369–373. doi: 10.1016/s1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 28.Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–891. doi: 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- 29.Walls M, Junk S, Ryan JP, Hart R. IVF versus ICSI for the fertilization of in-vitro matured human oocytes. Reprod Biomed Online. 2012;25:603–607. doi: 10.1016/j.rbmo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Van Blerkom J, Davis P, Alexander S. Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod. 2003;18:2429–2440. doi: 10.1093/humrep/deg466. [DOI] [PubMed] [Google Scholar]
- 31.Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- 32.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Li Y, Gao X, Yan JH, Chen ZJ. Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril. 2010;93:1550–1555. doi: 10.1016/j.fertnstert.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 34.Barnes FL, Crombie A, Gardner DK, Kausche A, Lacham-Kaplan O, Suikkari AM, Tiglias J, Wood C, Trounson AO. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995;10:3243–3247. doi: 10.1093/oxfordjournals.humrep.a135896. [DOI] [PubMed] [Google Scholar]
- 35.Chang CC, Lin CJ, Sung LY, Kort HI, Tian XC, Nagy ZP. Impact of phase transition on the mouse oocyte spindle during vitrification. Reprod Biomed Online. 2011;22:184–191. doi: 10.1016/j.rbmo.2010.10.009. [DOI] [PubMed] [Google Scholar]


