Abstract
The miR-98 is thought to be associated with various cancers. This study was to evaluate the potential predictive value of miR-98 expression in formalin-fixed paraffin-embedded tissue of breast cancer patients. The expression levels of miR-98 were examined in 98 breast cancer patients and 40 cancer-free controls using real-time quantitative PCR. The comparison of miR-98 expression levels between patient and control was performed using the Mann-Whitney test. The miR-98 showed higher expression levels in breast cancer patients compared with cancer free controls (p<0.01). The expression levels of miR-98 were highly correlated with miR24/93/378 in breast cancer patients. The miR-98 exhibited great capability of discriminating between cancer patients and controls by the Receiver-operator characteristic (ROC) curve analysis. The miR-98 was found highly correlated with breast cancer by Univariable logistic regression analysis. These results suggest that over-expression of miR-98 in formalin-fixed paraffin-embedded tissues might serve as a valuable source for biomarker discovery in breast cancer patients.
Keywords: Biomarkers, miR-98, breast cancer, miRNA, cancer
Introduction
MiRNAs are short non-coding RNAs that are single-stranded RNAs of 18-25 nucleotides long [1]. MiRNAs play important roles in diverse regulatory pathways, including cell proliferation [2], cell differentiation [3], cell survival [4], cellular self-renewal [5], apoptosis [6,7], tumor growth [4], cancer development [8,9], tumor invasion and metastasis [10], control of development [11], protein secretion [12] and viral infection [13].
The miR-98 is thought to be associated with various cancers [14-17]. The miR-98 was also found to be dysregulated in breast cancer [18,19]. Moreover, it has been observed as a frequent event aberrantly expressed in patients with breast cancer.
Fresh and frozen tissue samples are considered to be a proper source of DNA, RNA and protein for aim of clinic and research. Due to the limitation and inefficiency of prospectively collecting fresh and frozen samples as well as current insufficiency of bio-banking of patient samples, scientists are looking for alternative stocks of archived samples with potential utility for disease analysis such as formalin-fixed paraffin-embedded (FFPE) tissues [20]. RNA was found to be degraded in FFPE tissue [21]. Interestingly, miRNAs have been proved to be stable in FFPE tissues because of their shorter length. The expression of miRNAs in various cancers has been revealed using FFPE tissue [22]. A high correlation between miRNA expression levels derived from fresh-frozen tissues and matched FFPE tissues has been reported [20]. FFPE tissues are thought to be ideal for miRMA analysis and Immunohistochemistry (IHC) which carries abundant pathological data, thus FFPE tissues might be a valuable source for miRNA expression study, biomarker discovery and validation [23].
Emerging evidence has demonstrated that miR-98 is aberrantly expressed in various cancer samples derived from fresh-frozen tissues and might serve as a potential biomarker for cancer detection. However, little is known about the expression level of miR-98 in FFPE tissue of breast cancer patients and the correlation between miR-98 and breast cancer. In this study, we investigated expression of miR-98 in FFPE tissue of breast cancer patients as well as the correlation between miR-98 and breast cancer. Moreover, we further explored potential predictive value of miR-98 as a novel potential biomarker for breast cancer patients.
Materials and methods
Patients and samples
FFPE tissue samples from 98 breast cancer patients in the Affiliated People’s Hospital of Jiangsu University were collected in this study. Additionally, FFPE tissue samples collected from 40 cancer-free controls. All samples were obtained according to the guidelines of The Affiliated People’s Hospital’s protocol including patient consent and specimen collection. The diagnosis and classification of breast cancer patients were based on the Tumor-Node-Metastasis (TNM) system of American Joint Committee on Cancer (AJCC) [24]. All cases were diagnosed with histologically and clinically confirmed stage I, II and III breast cancer. The clinical characteristics of patients were listed in Table 1.
Table 1.
Clinicopathological characteristics and expression of miR-98 in breast cancer
| characteristics | cases | miR98 expression level | ||
|---|---|---|---|---|
|
|
||||
| Low | high | p | ||
| Age (years) | ||||
| ≤40 | 18 | 12 | 6 | 0.324 |
| 40-55 | 33 | 15 | 18 | |
| >55 | 47 | 23 | 24 | |
| Histological grade | 0.599 | |||
| I | 7 | 3 | 4 | |
| II | 39 | 18 | 21 | |
| III | 52 | 29 | 23 | |
| Tumor stage | 0.548 | |||
| I | 18 | 6 | 12 | |
| II | 11 | 4 | 7 | |
| III | 5 | 3 | 2 | |
| N stage | 0.628 | |||
| 0 | 58 | 28 | 30 | |
| 1-3 | 22 | 12 | 10 | |
| 4-9 | 9 | 6 | 3 | |
| >10 | 8 | 3 | 5 | |
| ER status | 0.513 | |||
| negative | 40 | 22 | 18 | |
| positive | 58 | 28 | 30 | |
| PR status | 0.529 | |||
| negative | 44 | 24 | 20 | |
| positive | 54 | 26 | 28 | |
RNA extraction and reverse transcription
Total RNA was extracted from 98 FFPE tissue of breast cancer patients and 40 controls using the RecoverAll™ Total Nucleic Acid Isolation Kit (Ambion, catalog no: AM 1975) and reverse transcribed to cDNA using miScript Reverse Transcription Kit (Qiagen, catalog no. 218061).
Real-time quantitative PCR
Real-time quantitative PCR (RQ-PCR) was performed according to the manufacturer’s instructions using miScript SYBR green PCR kit (Qiagen, catalog no. 218073) with the manufacturer-provided miScript Universal primer and miRNA-specific forward primer: TGA GGTAGTAAGTTGTATTGT (miR-98). RQ-PCR amplification was described in our previous study [25].
Statistical analyses
All statistical analyses were performed using spss16.0. The comparison of miRNA expression level between patient and control was performed using Mann-Whitney test. Spearman correlation coefficient was used to analyze the correlation between miR-98 and miR-24, miR-93 or miR-378 expression, respectively. The χ2 test was used to analyze the association of miR-98 expression level with clinicopathological characteristics. Receiver-operator characteristic (ROC) curve analysis was applied to assess the diagnostic significance of miR-98. The correlation between miR-98 and breast cancer was further determined using Univariable logistic regression analysis. All values showed are two-sided, and a p-value<0.05 was considered statistically significant.
Results
Expression of miR-98 with clinicopathological characteristics of breast cancer
To evaluate whether high-expression of miR-98 was related to the clinic progression of breast cancer, we analyzed the association of miR-98 expression level with the clinicopathological status of patients with breast cancer. Expression of miR-98 had no significant difference in Age, Histological grade, Tumor stage, N (lymph nodes) stage, ER (estrogen receptor) status, and PR (progesterone receptor) status of patients with breast cancer (Table 1).
Expression of miR-98 and correlation between miR-98 and miR-24/93/378 in FFPE tissues of breast cancer patients
The miR-98 exhibited higher expression levels in breast cancer patients compared with controls (p<0.01) (Figure 1A, Table 2). Furthermore, the spearman correlation scatter plot indicated high association between miR-98 and miR-24/93/378, with r=0.773 between miR-98 and miR-24 (Figure 1B), r=0.657 between miR-98 and miR-93 (Figure 1C), r=0.497 between miR-98 and miR-378 (Figure 1D).
Figure 1.
The miR-98 exhibited higher expression levels in breast cancer patients compared with controls (A). Correlation between miR-98 and miR-24/93/378, the spearman correlation scatter plot indicated association between miR-98 and miR-24/93/378, with r=0.773 between miR-98 and miR-24 (B), r=0.657 between miR-98 and miR-93 (C), r=0.497 between miR-98 and miR-378 (D). miRNA expression levels were presented after log10 transformation.
Table 2.
Expression level of miR-98 and its diagnostic significance in breast cancer patients
| Median in cancer patients | Median in controls | P value | AUC | Cutoffs | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| miR-98 | 0.763 | 0.097 | <0.001 | 0.76 | 0.084 | 82.7% | 53.8% |
Highly specificity and sensitivity of miR-98 for determination of breast cancer
ROC analysis then performed to evaluate the capability of miR-98 to discriminate between breast cancer patients and controls. When optimum cutoff value was determined, miR-98 showed 0.76 AUC value and yielded 82.7% sensitivity and 53.8% specificity (Figure 2). The miR-98 expression level was also found highly correlated with breast cancer by univariate logistic regression analysis (p<0.01, Table 3).
Figure 2.
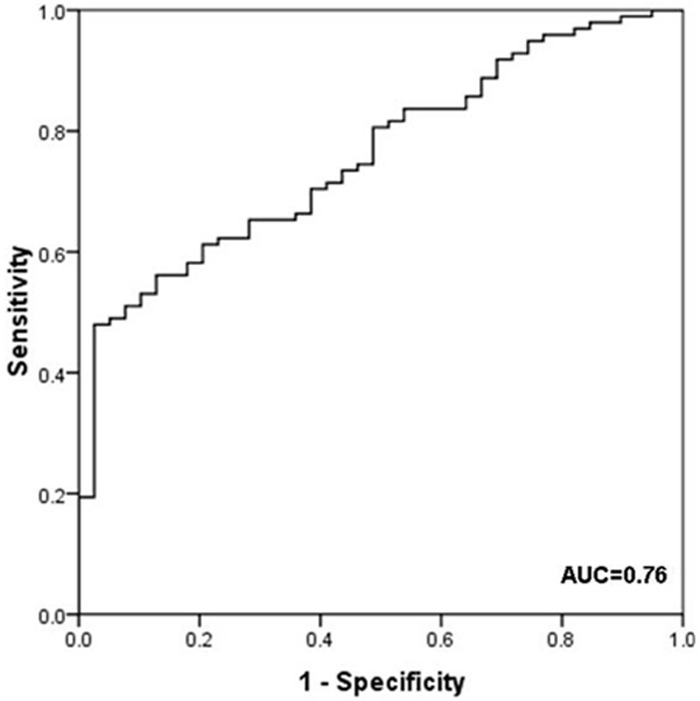
ROC curve analysis of miR-98 expression level in 98 patients diagnosed with breast cancer and 40 controls. The area under the ROC curve (AUC) indicates the accuracy for differentiating breast cancer from patients and controls in terms of sensitivity and specificity. ROC curve of miR-98 showed 0.76 AUC with 82.7% sensitivity and 53.8% specificity.
Table 3.
Univariable Logistic regression model of miRNAs
| Regression coefficient (β) | SE | Wald. χ2 | P value | Exp (β) | |
|---|---|---|---|---|---|
| miR-98 | 1.661 | 0.433 | 14.715 | <0.01 | 5.263 |
Discussion
The correlation between miR-98 expression level and clinicopathological characteristics of breast cancer was also analyzed. We found that expression of miR-98 did not vary with Age, Histological grade, Tumor stage, N stage, ER status, and PR status of patients with breast cancer. It suggested that miR-98 might be a new predictive factor for breast cancer which is independent from other known clinicopathological characteristics.
Aberrant expression patterns of miRNAs were revealed in various cancers and dysregulation of miRNAs were found highly associated with the progression of different cancers [18,19,26]. Breast cancer is considered to be a heterogeneous neoplasm, which involves aberrant expression patterns of miRNA and mRNA [27]. Abundant results about the expression of different miRNAs and their function in breast cancer patients were reported [18,19,27,28]. Up-regulation of miR-21 and down-regulation of miR-125b have been found in breast cancer patients [29]. Sun Y et al reported that serum miR-155 was over-expressed in breast cancer patients [30]. Recent studies demonstrated that miRNAs could be good potential candidates for the development of therapeutic targets and novel biomarkers [27]. The miR-98 is thought to be associated with various cancers [14-17]. The miR-98 was also found to be dysregulated in breast cancer [18,19]. The validation of miR-98 expression in breast cancer patients should be performed with diversity of samples. FFPE tissues could be long-term stored and can permanently preserve the structure of the tissue. MiRNAs were proved to be stable in FFPE tissue because of their shorter length. Recent studies revealed that miRNAs obtained from FFPE tissues showed reliable expression levels as compared with frozen tissues [31-33]. In this study, we analyzed miR-98 expression level in FFPE tissue of breast cancers using RQ-PCR. We have found that miR-98 shows higher expression levels in breast cancer patients compared with controls, which is consistent with Farazi TA’ study [18]. Our results validated the high expression of miR-98 in breast cancer patients using FFPE tissues. In order to explore diagnostic ability of miR-98 in FFPE for breast cancer patients, we performed ROC curve analysis. The miR-98 expression in FFPE tissues of breast cancer produced an AUC of 0.76 with sensitivity of 82.7% and specificity of 53.8% in the identification of breast cancer. These results showed that miR-98 had considerable diagnostic power to discriminate between breast cancer patients and controls. The correlation between miR-98 expression level and breast cancer was further confirmed by univariate logistic regression. The miR-24, miR-93 and miR-378 are thought to be onco-miRNAs for their abilities of enhancing tumor growth. We revealed that miR-24/93/378 were up-regulated in FFPE tissue of breast cancers and the three miRNAs were significantly correlated with each other in breast cancer patients (data not shown). The miR24/93/378 might be good potential candidates for the development of novel biomarkers in breast cancer. In this study, we have found that miR-98 is associated with miR-24/93/378, respectively. The miR-98 might also be considered as a good potential candidate for the development of novel biomarker in breast cancer.
Taken together, we suppose that over-expression of miR-98 in FFPE tissues of breast cancer patients might suggest miR-98 from FFPE tissues can serve as a valuable source for biomarker discovery and validation in breast cancer patients.
Acknowledgements
This study was supported by National Natural Science foundation of China (81172592, 81270630).
Disclosure of conflict of interest
None.
References
- 1.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutary U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 2.Shatseva T, Lee DY, Deng Z, Yang BB. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J Cell Sci. 2011;124:2826–2836. doi: 10.1242/jcs.077529. [DOI] [PubMed] [Google Scholar]
- 3.Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, Bannard O, Bluestone JA, Matloubian M, Ansel KM, Jeker LT. The microRNA cluster miR-17-92 promotes TFH cell differentiation and represses subset-in appropriate gene expression. Nat Immunol. 2013;14:840–8. doi: 10.1038/ni.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–5. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z, Du WW, Fang L, Shan SW, Qian J, Lin J, Qian W, Ma J, Rutnam ZJ, Yang BB. The intermediate filament vimentin mediates microRNA miR-378 function in cellular self-renewal by regulating the expression of the Sox2 transcription factor. J Biol Chem. 2013;288:319–31. doi: 10.1074/jbc.M112.418830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z, Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 7.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 8.Ye G, Fu G, Cui S, Zhao S, Bernaudo S, Bai Y, Ding Y, Zhang Y, Yang BB, Peng C. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: implications for chemoresistance. J Cell Sci. 2011;124:359–368. doi: 10.1242/jcs.072223. [DOI] [PubMed] [Google Scholar]
- 9.Negrini M, Ferracin M, Sabbioni S, Croce CM. MicroRNAs in human cancer: from research to therapy. J Cell Sci. 2007;120:1833–1840. doi: 10.1242/jcs.03450. [DOI] [PubMed] [Google Scholar]
- 10.Du WW, Fang L, Li M, Yang X, Liang Y, Peng C, Qian W, O’Malley YQ, Askeland RW, Sugg SL, Qian J, Lin J, Jiang Z, Yee AJ, Sefton M, Deng Z, Shan SW, Wang CH, Yang BB. MicroRNA miR-24 enhances tumor invasion and metastasis by targeting PTPN9 and PTPRF to promote EGF signaling. J Cell Sci. 2013;126:1440–53. doi: 10.1242/jcs.118299. [DOI] [PubMed] [Google Scholar]
- 11.Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, Zhang Y, Xuan JW, Yee SP, Siragam V, Yang BB. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol. 2009;11:1031–1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- 12.Mello CC, Czech MP. Micromanaging insulin secretion. Nat Med. 2004;10:1297–1298. doi: 10.1038/nm1204-1297. [DOI] [PubMed] [Google Scholar]
- 13.Chellappan P, Vanitharani R, Fauquet CM. MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci U S A. 2005;102:10381–10386. doi: 10.1073/pnas.0504439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang Q, Tang H, Yu J, Yin J, Yang X, Lei X. MicroRNA-98 sensitizes cisplatin-resistant human lung adenocarcinoma cells by up-regulation of HMGA2. Pharmazie. 2013;68:274–81. [PubMed] [Google Scholar]
- 15.Ting HJ, Messing J, Yasmin-Karim S, Lee YF. Identification of microRNA-98 as a therapeutic target inhibiting prostate cancer growth and a biomarker induced by vitamin D. J Biol Chem. 2013;288:1–9. doi: 10.1074/jbc.M112.395947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer. 2007;6:5–16. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, Yang BB. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;3:1370–85. doi: 10.18632/oncotarget.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, van Kouwenhove M, Kreike B, Sie D, Hovestadt V, Wessels LF, van de Vijver MJ, Tuschl T. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–53. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan LX, Huang XF, Shao Q, Huang MY, Deng L. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami RS, Waldron L, Machado J, Cervigne NK, Xu W, Reis PP, Bailey DJ, Jurisica I, Crump MR, Kamel-Reid S. Optimization and analysis of a quantitative real-time PCR-based technique to determine microRNA expression in formalin-fixed paraffin-embedded samples. BMC Biotechnol. 2010;10:47–59. doi: 10.1186/1472-6750-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macabeo-Ong M, Ginzinger DG, Dekker N, McMillan A, Regezi JA, Wong DT, Jordan RC. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod Pathol. 2002;15:979–987. doi: 10.1097/01.MP.0000026054.62220.FC. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Lu Y, Chen Y, Lu W, Xie X. MicroRNA profile of paclitaxel-resistant serous ovarian carcinoma based on formalin-fixed paraffin-embedded samples. BMC Cancer. 2013;13:216–223. doi: 10.1186/1471-2407-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu SW, Chen L, Man YG. miRNA Biomarkers in Breast Cancer Detection and Management. J Cancer. 2011;2:116–22. doi: 10.7150/jca.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Staging system for breast cancer: revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin North Am. 2013;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 25.Qian J, Lin J, Qian W, Ma JC, Qian SX, Li Y, Yang J, Li JY, Wang CZ, Chai HY, Chen XX, Deng ZQ. Overexpression of miR-378 is frequent and may affect treatment outcomes in patients with acute myeloid leukemia. Leuk Res. 2013;37:765–8. doi: 10.1016/j.leukres.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Colangelo T, Fucci A, Votino C, Sabatino L, Pancione M, Laudanna C, Binaschi M, Bigioni M, Maggi CA, Parente D, Forte N, Colantuoni V. MicroRNA-130b Promotes Tumor Development and Is Associated with Poor Prognosis in Colorectal Cancer. Neoplasia. 2013;15:1086–99. doi: 10.1593/neo.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu SW, Chen L, Man YG. miRNA Biomarkers in Breast Cancer Detection and Management. J Cancer. 2011;2:116–22. doi: 10.7150/jca.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y, Li C, Chong M, Ibrahim T, Mercatali L, Amadori D, Lu X, Xie D, Li QJ, Wang XF. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–294. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mar-Aguilar F, Luna-Aguirre CM, Moreno-Rocha JC, Araiza-Chávez J, Trevino V, Rodríguez-Padilla C, Reséndez-Pérez D. Differential expression of miR-21, miR-125b and miR-191 in breast cancer tissue. Asia Pac J Clin Oncol. 2013;9:53–9. doi: 10.1111/j.1743-7563.2012.01548.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wang M, Lin G, Sun S, Li X, Qi J, Li J. Serum microRNA-155 as a potential biomarker to track disease in breast cancer. PLoS One. 2012;7:e47003. doi: 10.1371/journal.pone.0047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoefig KP, Thorns C, Roehle A, Kaehler C, Wesche KO, Repsilber D, Branke B, Thiere M, Feller AC, Merz H. Unlocking pathology archives for microRNA-profiling. Anticancer Res. 2008;28:119–123. [PubMed] [Google Scholar]
- 33.Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, O’ Leary J. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35–49. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]



