Abstract
The patient was a 19-year-old female who presented with a chief complaint of progressive pelvic pain. Preoperative ultrasound of the right ovary revealed an ovarian torsion as the cause of the patient’s progressive pain. Laparoscopy confirmed the torsion and revealed a right ovary measuring 10 cm in greatest diameter. Intraoperative incision into the ovary revealed a simple ovarian cystic mass measuring 3.0 x 1.5 x 0.8 cm. A solid component within the cyst was identified. Histological sections of the cystic mass demonstrated mononuclear and hyperchromatic Sertoli cells with a trabecular growth pattern. Clusters of medium-sized epithelioid cells with abundant eosinophilic cytoplasm consistent with Leydig cells were also identified between the trabeculae of Sertoli cells. In addition, focal areas of intestinal type mucinous epithelium were identified embedded within the trabeculae of Sertoli cells. Immunohistochemical studies revealed that the Sertoli cells were positive for calretinin (bright) while the Leydig cells were positive for calretinin (dim), inhibin, CAM5.2 and AE1&3. CEA showed positivity mainly of the intraluminal contents of the mucinous type intestinal epithelium. The patient had an uneventful post-operative course and was disease-free for 3 years.
Keywords: Sertoli-Leydig cell tumor, heterologous elements, mucinous epithelium
Introduction
Sertoli-Leydig cell tumors (SLCTs) are rare ovarian sex-cord stromal tumors. They account for less than 0.5% of all ovarian tumors [1]. They typically occur in young women and the patients usually present with abdominal swelling or pain. SLCTs are divided into well-differentiated, intermediate differentiation, poorly differentiated, retiform, and mixed [2-7]. Heterologous components may be present, most commonly in the intermediate differentiation and poorly differentiated groups [2-5]. SLCTs are often diagnostically challenging due to significant morphologic overlap with other ovarian tumors [8]. In approximately half of the SLCT patients, endocrine manifestations, such as virilization, have been observed. However, it is less common in SLCTs of retiform type and those with heterologous elements [4]. The prognosis of SLCTs depends on the type of SLCTs. In general, well-differentiated tumors behave benign, while intermediate differentiation, poorly differentiated, retiform type, and SLCTs with heterologous elements have malignant potential [9]. Below, we report a case of ovarian SLCT with heterologous element in a 19-year-old female and a review of the literature.
Case report
The patient was a 19-year-old who came to our hospital with a chief complaint of progressive abdominal pain. Preoperative ultrasound revealed a right ovarian torsion as the cause of the patient’s progressive pain. In addition, the right ovary was enlarged. The patient was sent for emergency laparotomy and exploration. Laparoscopy confirmed the torsion and revealed a right ovary measuring 10 cm in greatest diameter. The left ovary and fallopian tube were unremarkable. The right ovary was removed.
Grossly, the external surface of the right ovary was smooth and glistening with an ovarian wall measuring 0.2 cm in thickness. Section of the ovary revealed an ovarian cystic mass measuring 3.0 cm in maximum diameter, which contained approximately 250 cc of serous fluid. The internal surface was smooth and glistening. A solid component measuring 2 x 1.5 x 0.8 cm was identified within the cyst.
Microscopically, the cyst wall was mostly fibrous tissue. Histologic sections of the solid component showed alternating cellular lobules with areas of less cellular stroma (Figure 1). The cellular lobules were composed of mononuclear, hyperchromatic Sertoli cells with a trabecular growth pattern (Figure 2). Clusters of medium sized cells with abundant eosinophilic cytoplasm consistent with Leydig cells were identified between the trabeculae of Sertoli cells (Figure 2). The stroma separating the Sertoli and Leydig cells ranged from fibromatous to edematous. Interestingly, focal areas of mucinous epithelium were identified amongst the trabeculae of Sertoli cells. This single layer, benign appearing epithelium contained numerous goblet cells and demonstrated abundant intraluminal and intracytoplasmic mucin (Figure 3).
Figure 1.
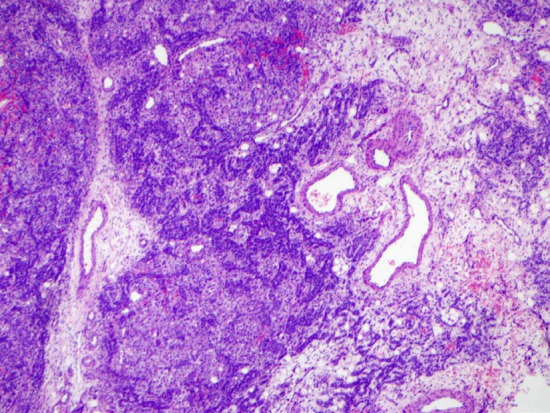
Low-power H&E of the ovarian tumor shows the alternating cellular nodules and hypocellular stroma (4x).
Figure 2.
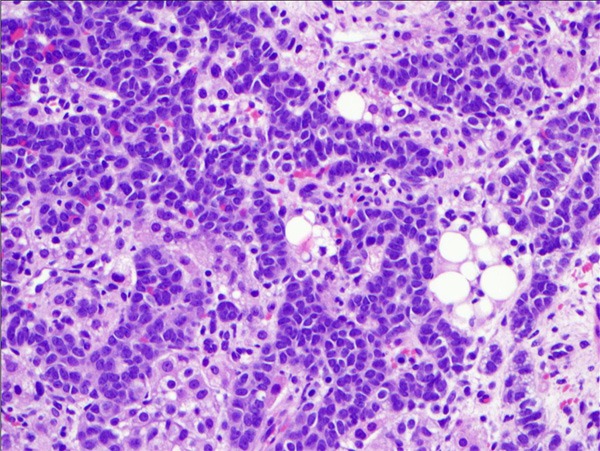
High-power H&E of the ovarian tumor shows the cords and ribbons of Sertoli cells mixed with round Leydig cells with eosinophilic cytoplasm (40x).
Figure 3.
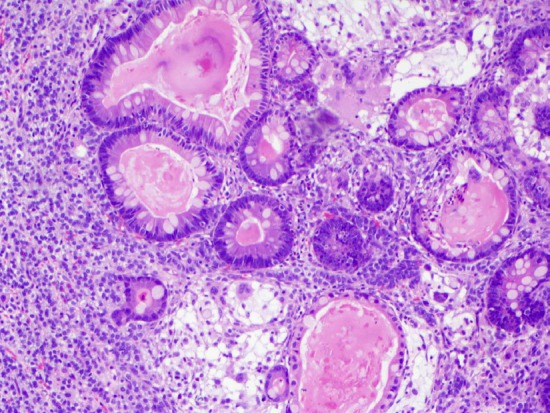
High-power H&E of the ovarian tumor shows the presence of intestinal-type mucinous epithelium surrounded by Sertoli and Leydig cells (40x).
Immunohistochemical studies revealed that the Sertoli cells were positive for calretinin (bright) while the Leydig cells were positive for calretinin and inhibin (Figures 4, 5). The Sertoli cells are also positive for CAM5.2 and AE1&AE3 (Figure 6).
Figure 4.
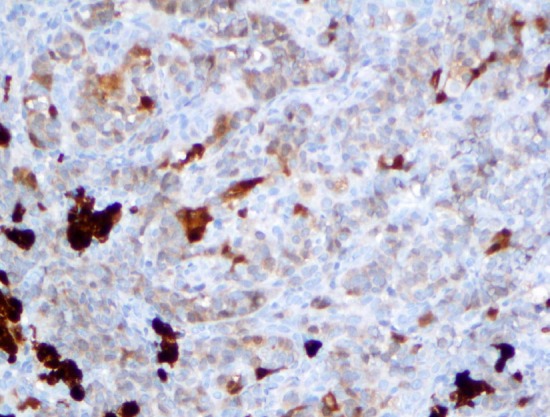
Calretinin immunostain highlights the Sertoli and Leydig cells (40x). Noted the nuclear and the cytoplasmic staining pattern.
Figure 5.
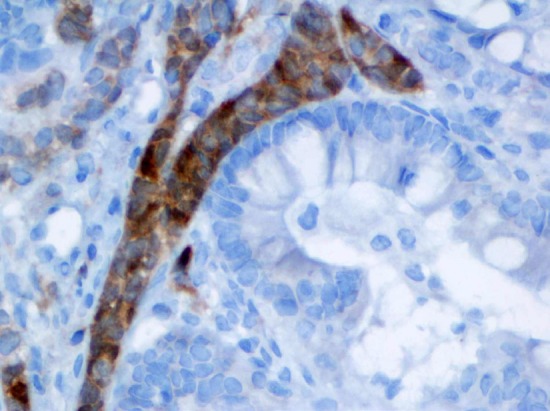
Inhibin immunostain highlights the cord of Sertoli cells around the mucinous epithelium (60x).
Figure 6.
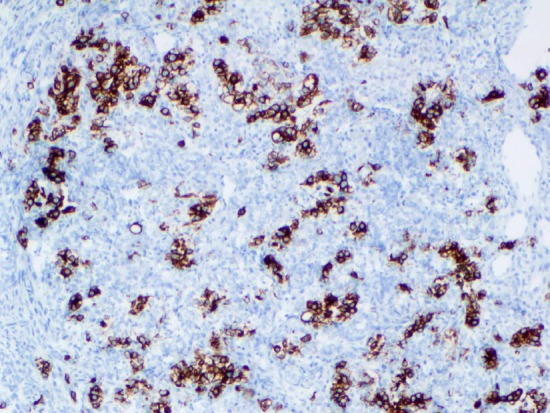
Cytokeratin (CAM5.2) immunostain shows the Sertoli cells are positive (20x).
The diagnosis of SLCT, intermediate differentiation with heterologous element (mucinous epithelium), was rendered. The patient had an uneventful post-operative course and was disease-free with a follow-up of 3 years.
Discussion
Sertoli-Leydig cell tumors (SLCTs), also known as androblastomas, are defined as tumors composed of variable proportions of Sertoli cells, Leydig cells, and/or fibroblastic cells in variable proportions and levels of differentiation [1]. They are extremely rare and account for less than 0.5% of all ovarian tumors [1,9]. There are generally divided into the following subtypes: well differentiated, of intermediate differentiation, poorly differentiated, with heterologous elements, retiform and mixed. The most common subtypes are intermediate and poorly differentiated [1]. Heterologous elements occur in approximately 20% of SLCTs and are seen in all subtypes with the exception of the well differentiated ones [2,3,5]. In the largest series of SLCTs with greater than 200 tumors, 18% contains glands and cysts lined by well-differentiated intestinal type or gastric-type epithelium, 16% has microscopic foci of carcinoid tumor and 5% has stromal heterologous elements, including islands of cartilage and/or areas of embryonal rhabdomyosarcoma [1]. Other heterologous elements that have been associated with SLCTs include hepatocyte-like cells, retinal tissue, neuroblastoma, and mucinous adenocarcinoma [3,5].
SLCTs have been reported in females between 2 and 75 years of age. The majority of cases of SLCTs with heterologous elements present in the second and third decades of life with an average age at diagnosis of twenty-five years [1,9]. Approximately 30% of patients with SLCTs present with virilization but the majority of patients present without endocrine manifestations and instead, present with nonspecific abdominal symptoms secondary to the physical presence of an ovarian mass [4]. This is especially common for SLCTs with heterologous elements [2,3,5,10]. Our patient did not experience any symptoms of virilization and had no increase in her serum testosterone levels but instead presented with progressive abdominal pain secondary to ovarian torsion induced by the presence of an enlarged ovary. Androgenic manifestations are very uncommon in tumors with heterologous elements [2,3]. Almost all SLCTs are unilateral and 95% are confined to the ovaries. Tumor rupture is present in 10% of cases and 4% of patients develop ascites [1,9]. Our patient’s SLCT was unilateral and there was no evidence of either preoperative rupture or ascites. Approximately 2.5% of tumors spread beyond the ovary [1,6,9]. There was no evidence of extraovarian spread in our patient.
Macroscopically, the majority of SLCTs range in size between 5 and 15 cm in diameter. In addition, SLCTs can be solid, solid and cystic or cystic [1-3]. Interestingly, tumors that contain heterologous or the retiform type are more frequently cystic, as in our case. Microscopically, intermediate differentiation SLCTs exhibit cellular lobules separated by loose or edematous fibrous stroma. Immature Sertoli-type cells can be arranged in short, thin cords, true hollow tubules, or poorly circumscribed nests and sheets. These Sertoli-type cells have small oval or angular nuclei and scanty cytoplasm. Mitoses are infrequent. The stroma consists of closely packed spindle-shaped cells. Mature Leydig cells are typically apparent in the stroma occurring in sheets, clusters or single cells. They are typically located around the perimeter of the tumor or at the margins of the lobules [1-7]. In our case, the Sertoli cells were mainly arranged in cords and the Leydig cells were found filling the space in between the cords of Sertoli cells. Mitoses were extremely infrequent. The grading of SLCTs is based on the degree of tubular differentiation of the Sertoli cell component and the quantity of the primitive gonadal stroma. A higher grade is associated with mainly sheets of Sertoli cells with very few Leydig cells and frequent mitoses [9].
SLCTs have many patterns and thus the differential diagnosis is quite wide [8]. The general differential diagnosis for all SLCTs should include granulosa cell tumor, moderately to poorly differentiated endometrioid carcinoma and female adnexal tumor of probable wolffian origin (FATWAO). In the cases of SLCTs of intermediate differentiation with heterologous element of mucinous type intestinal epithelium, however, the differential diagnosis should be expanded to include mature cystic teratoma and mucinous cystadenoma.
Mature cystic teratomas are typically less than 15 cm in diameter with a smooth, gray-white glistening surface. The cyst cavity is typically unilocular and filled with fatty material and hair surrounded by a firm capsule of varying thickness. A protuberance can be found arising from the cyst wall, which is composed of a variety of different tissues. These tissues arise from all three germ layers and are arranged in an orderly manner [11]. Rare case of malignant transformation to mucinous adenocarcinoma has been reported [11]. In contrast, SLCTs may have heterogenous elements similar to those seen in a teratoma but typically to a much lesser degree. In our case, there was floating tissue that may have arisen from the cyst wall but the cyst contents were mainly serosanguineous and extensive sampling of the floating tissue revealed only a focal area of mucinous type intestinal epithelium without other mature heterologous tissues. The clusters of Leydig cells may be confused with heterologous hepatocytes but immunohistochemical analysis with hepatocyte specific marker, Hep-Par1, and inhibin would demonstrate that these are not hepatocytes.
The majority of cases of SLCTs of intermediate differentiation with heterologous elements of mucinous type intestinal epithelium have only focal areas of this intestinal epithelium. However, rare cases can have extensive amounts of intestinal type epithelium which hide the Sertoli-Leydig cell component, leading to a misdiagnosis of mucinous cystadenoma of the ovary [2,8]. In contrast to SLCTs, mucinous cystadenomas are typically large multiloculated cystic tumors and simple cysts, as in our case, are extremely rare. In addition, the cysts contain thick gelatinous material that can range in color from pale yellow to brown. Histologically, the stroma of mucinous cystadenomas is fibrocollagenous with variable cellularity whereas the stroma of SLCTs is often cellular with a minimal fibromatous component. The mucinous type intestinal epithelium in our case is so minimal in comparison to Sertoli and Leydig cells that it would be difficult to misdiagnosis this case as a mucinous cystadenoma. Staats et al. reported a case of micinous cystic tumor with prominent theca cell proliferation and focal granulosa cells in the stroma. The authors pointed out that the abundant mucinous epithelium and the focal theca/granulosa cell proliferation are the key features to distinguish from Sertoli-Leydig cell tumor with heterologous element [12]. Virk et al. recently reported a case of mucinous adenocarcinoma as heterologous element in an ovarian Sertoli-Leydig cell tumor [13]. In our case, the mucinous epithelium is completely benign.
The differentiation of granulosa cell tumors from SLCTs without heterologous elements is based on the presence of more mature cells with pale, grooved nuclei, a prominent fibromatous or thecomatous component, absence of steroid-type cells, and absence of both retiform and heterologous elements. The majority of the cells in our case had prominent, hyperchromatic nuclei with a cellular stroma and an abundance of Leydig cells interspersed amongst the trabeculae of Sertoli cell. In addition, SLCTs lack Call-Exner bodies [14,15].
The differentiation of endometrioid carcinoma from SLCT is supported by the typical older age of the patient, the absence of virilization, glands that are larger than the tubules of SLCT, and an epithelium that is less well differentiated than that of SLCT. In addition, endometrioid carcinomas typically exhibit mucin secretion as well as areas of squamous differentiation. For difficult cases, immunohistochemical studies with epithelial membrane antigen (EMA), CK7, inhibin, and calretinin may be helpful. EMA and CK7 are usually diffusely positive in endometrioid carcinoma and the neoplastic glands are negative for inhibin and calretinin [16,17]. McCluggage studied cases of ovarian Sertoli-Leydig cell tumor with pseudoendometrioid tubules and again found that EMA, CK7, inhibin, and calretinin are helpful immunohistochemical markers to differentiate it from endometrioid carcinoma [10].
The differentiation of a FATWAO from SCLTs without heterologous elements can be difficult because the tubules can be morphologically similar in the two tumors. In addition, immunohistochemistry is generally not useful since both FATWAO and SLCTs express alpha-inhibin. However, the typical morphology of a FATWAO consists of a mixture of patterns which is diagnostic and helpful in distinguishing them from SLCTs. These patterns include a tubular pattern with closely packed tubules or solid cords, a sieve-like growth pattern produced by cysts of varying sizes, and a diffuse growth pattern composed of spindle or polygonal cells [18].
The incidence of clinical malignancy in SLCTs is 10-30%. While the degree of differentiation and the presence of heterologous elements correlate to some extent with clinical outcome, the most reliable indication of prognosis is the presence of extraovarian spread or metastases. SLCTs with extraovarian spread and/or metastases typically pursue an aggressive course. Without these factors, the presence of a unilateral well-differentiated Stage I tumor in a young patient carries a good prognosis and unilateral salpingo-oophorectomy with preservation of fertility is ideal [9]. For the patient in our case, she is being treated with combination chemotherapy consisting of cisplatin, etoposide and bleomycin with particular attention to serum levels and radiograph analyses.
Disclosure of conflict of interest
None.
References
- 1.Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985;9:543–569. doi: 10.1097/00000478-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Young RH, Prat J, Scully RE. Ovarian Sertoli-Leydig cell tumor with heterologous elements. Gastrointestinal epithelium and carcinoid: a clinicopathological analysis of thirty-six cases. Cancer. 1982;50:2448–2456. doi: 10.1002/1097-0142(19821201)50:11<2448::aid-cncr2820501133>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Prat J, Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumor with heterologous elements II, cartilage and skeletal muscles: a clinicopathologic analysis of twelve cases. Cancer. 1982;50:2465–2475. doi: 10.1002/1097-0142(19821201)50:11<2465::aid-cncr2820501135>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Young RH, Scully RE. Sex Cord-Stromal, Steroid Cell, and Other Ovarian Tumors with Endocrine, Paraendocrine, and Paraneoplastic Manifestations. In: Kurman R, editor. Blaustein’s Pathology of the Female Genital Tract. 5th edition. New York, New York: Springer-Verlag New York, Inc; 2002. pp. 929–39. [Google Scholar]
- 5.Mooney EE, Nogales FF, Tavassoli FA. Hepatocytic differentiation in retiform Sertoli-Leydig cell tumors: Distinguishing a heterologous element from leydig cells. Hum Pathol. 1999;30:611–617. doi: 10.1016/s0046-8177(99)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Mooney EE, Nogales FF, Bergeron C, Tavassoli FA. Retiform Sertoli-Leydig cell tumors: clinical, morphological and immunohistochemical findings. Histopathology. 2002;41:110–117. doi: 10.1046/j.1365-2559.2002.01426.x. [DOI] [PubMed] [Google Scholar]
- 7.Talerman A. Ovarian Sertoli-Leydig cell tumor with retiform pattern. A clinicopathologic study. Cancer. 1987;60:3056–3064. doi: 10.1002/1097-0142(19871215)60:12<3056::aid-cncr2820601233>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Scully RE. Differential diagnosis of ovarian tumors based primarily on their patterns and cell types. Semin Diagn Pathol. 2001;18:161–235. [PubMed] [Google Scholar]
- 9.Gui T, Cao D, Shen K, Yang J, Zhang Y, Yu Q, Wan X, Xiang Y, Xiao Y, Guo L. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol. 2012;127:384–389. doi: 10.1016/j.ygyno.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 10.McCluggage WG, Young RH. Ovarian Sertoli-Leydig cell tumor with pseudoendometrioid tubules (pseudoendometrioid Sertoli-Leydig tumors) Am J Surg Pathol. 2007;31:592–597. doi: 10.1097/01.pas.0000213365.56498.72. [DOI] [PubMed] [Google Scholar]
- 11.Hinshaw HD, Smith AL, Olawaiye AB. Malignant transformation of a mature cystic ovarian teratoma into thyroid carcinoma, mucinous adenocarcinoma, and strumal carcinoid: a case report and literature review. Case Rep Obstet Gynecol. 2012;2012:269489. doi: 10.1155/2012/269489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staats PN, Coutts MA, Young RH. Primary ovarian mucinous cystic tumor with prominent theca cell proliferation and focal granulosa cell tumor in its stroma: case report, literature review, and comparison with Sertoli-Leydig cell tumor with heterologous elements. Int J Gynecol Pathol. 2010;29:228–233. doi: 10.1097/PGP.0b013e3181c04007. [DOI] [PubMed] [Google Scholar]
- 13.Virk R, Lu D. Mucinous adenocarcinoma as heterologous element in intermediate differentiated Sertoli-Leydig cell tumor of the ovary. Pathol Res Pract. 2010;206:489–492. doi: 10.1016/j.prp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Stenwig JT, Hazekamp JT, Beecham JB. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 1979;7:136–152. doi: 10.1016/0090-8258(79)90090-8. [DOI] [PubMed] [Google Scholar]
- 15.Vang R, Herrmann ME, Tavassoli FA. Comparative immunohistochemical analysis of granulosa and Sertoli components in ovarian sex cord-stromal tumors with mixed differentiation: potential implications for derivation of Sertoli differentiation in ovarian tumors. Int J Gynecol Pathol. 2004;23:151–161. doi: 10.1097/00004347-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Baker PM, Oliva E. Immunohistochemistry as a Tool in the Differential Diagnosis of Ovarian Tumors: An Update. Int J Gynecol Pathol. 2004;24:39–55. [PubMed] [Google Scholar]
- 17.McCluggage WG, Maxwell P. Immunohistochemical staining for calretinin is useful in the diagnosis of ovarian sex cord-stromal tumours. Histopathology. 2001;38:403–408. doi: 10.1046/j.1365-2559.2001.01147.x. [DOI] [PubMed] [Google Scholar]
- 18.Tiltman AJ, Allard U. Female adnexal tumours of probable Wolffian origin: an immunohistochemical study comparing tumours, mesonephric remnants and paramesonephric derivatives. Histopathology. 2001;38:237–42. doi: 10.1046/j.1365-2559.2001.01086.x. [DOI] [PubMed] [Google Scholar]


