Abstract
T-cell/histiocyte-rich diffuse large B-cell lymphoma is characterized by abundant reactive T-cell and histiocyte infiltration within nodal diffuse large B-cell lymphoma, and only limited cases of primary cutaneous T-cell-rich B-cell lymphoma have been documented. These reactive T-cells usually show a T-helper phenotype. Gamma/delta T-cell is a functionally distinct T-cell lineage, which constitutes on average 5% of all T-cells in the peripheral blood. Herein, we report the first documented case of primary cutaneous malignant B-cell lymphoma with abundant reactive gamma/delta+ T-cells within the skin lesion and peripheral blood. An 80-year-old Japanese male presented with a gradually enlarged knee nodule. Histopathological study revealed diffuse infiltration of lymphoid cells in the dermis and subcutis. Proliferation of large-sized atypical lymphoid cells was observed among medium-sized lymphocytes with convoluted nuclei. Immunohistochemically, these large-sized atypical lymphocytes were CD20+, and relatively many gamma/delta+ cell infiltration was also noted. Flowcytometric analysis revealed deviation of lambda+ cells (lambda/kappa 58) and increase of CD3+ gamma/delta+ cells (6%). Peripheral blood had CD3+ gamma/delta+ cells (28.8%). Rearrangement of immunoglobulin heavy chain, but not of T-cell receptor beta and gamma chains, was observed. Accordingly, an ultimate diagnosis of cutaneous B-cell lymphoma with abundant reactive gamma/delta+ cells was made. Recent studies have shown reactive gamma/delta+ T-cell infiltration and/or elevation in the peripheral blood in patients with various types of carcinoma, and that they play a role in the pathogenesis of some carcinomas. Therefore, additional analysis is needed to clarify the role of reactive gamma/delta+ T-cells in malignant lymphoma.
Keywords: Cutaneous B-cell lymphoma, T-cell-rich B-cell lymphoma, gamma/delta T-cell
Introduction
T cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a distinct variant of diffuse large B-cell lymphoma, characterized by a limited number of scattered, large, atypical neoplastic B-cells embedded in a background of abundant T-cells and frequent histiocytes [1]. This type of disease mainly affects middle-aged men and accounts for less than 10% of all diffuse large B-cell lymphomas [1]. The most frequent site of involvement is lymph node, and bone marrow, liver, and spleen are frequently involved at the time of diagnosis [1].
Only limited cases of primary cutaneous B-cell lymphoma with abundant reactive T-cell infiltration have been documented, and most of the previously reported cases were described as “T-cell-rich B-cell lymphoma” [2-16]. Cerroni et al. stated that for diagnosis of cutaneous T-cell/histiocyte-rich B-cell lymphoma, neoplastic large B-cells should not exceed 10% of all infiltrate, and this type of lesion represents a variation of the follicular center lymphoma, diffuse type [17].
Gamma/delta T-cell is a functionally distinct T-cell lineage, which constitutes on average 5% of all T-cells in the peripheral blood, and most gamma/delta T-cells lack CD4 or CD8 antigens [18]. Activated gamma/delta T-cells produce proinflammatory cytokines and chemokines; and are thought to kill infected cells and tumor cells [19,20]. Gamma/delta T-cells have been shown to be associated with some types of malignant tumors, and recent studies have demonstrated a high frequency of gamma/delta T-cells among tumor-infiltrating lymphocytes or in circulation in the peripheral blood in patients with breast or gastric cancers, renal cell carcinoma, squamous cell carcinoma of the head and neck, and acute leukemia [21-25].
It has been well-recognized that reactive T-cells within THRLBCL nodal lesions commonly express a T-helper phenotype (CD3+, CD4+, CD8-) [17,26]. However, malignant B-cell lymphoma with abundant gamma/delta T-cells has not been reported. Herein, we report the first documented case of primary cutaneous B-cell lymphoma with abundant reactive gamma/delta T-cells within the skin lesion and peripheral blood.
Case report
An 80-year-old Japanese male with past history of hypertension, diabetes mellitus, and Alzheimer-type dementia presented with a gradually enlarged nodular lesion in his left knee, which had been first noticed approximately 1 year earlier. Physical examination revealed an indurated erythema, measuring 6 x 7 cm in diameter, in his left knee. A nodule, measuring 2 cm in diameter, was present in the central portion of the erythema. Laboratory tests demonstrated elevated leukocytes (red blood cells 5.24 x 1012/L (range 4.1-5.3), white blood cells 16.6 x 109/L (3.0-8.0), platelets 241 x 109/L (150-400)), lactate dehydrogenase 224 U/L (119-229), and soluble interleukin-2 receptor 709 IU/mL (135-483). Flowcytometric analysis of the peripheral blood revealed the presence of CD3+ CD4- CD8- gamma/delta+ cells (28.8%) (Figure 1). Positron emission tomography demonstrated an accumulation in the left knee and the left external iliac and thigh lymph nodes. Biopsy from the nodule of the left knee was performed, and flowcytometric and gene analyses of the skin lesion were also performed.
Figure 1.
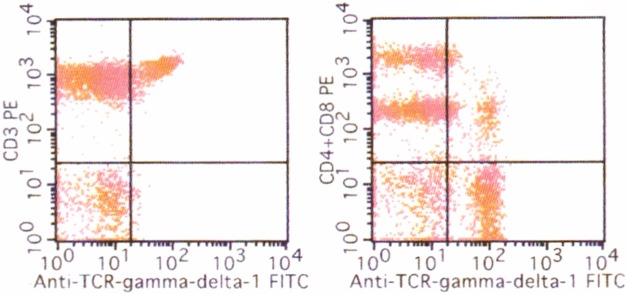
Flowcytometric analysis of the peripheral blood. Presence of CD3+ CD4- CD8- gamma/delta+ cells in the peripheral blood (28.8%).
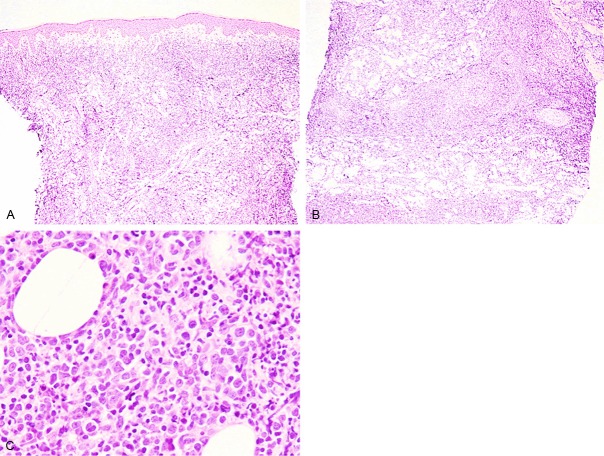
Histopathological examination of the biopsy from the knee nodule revealed diffuse infiltration of lymphoid cells invading into the entire dermis and subcutis (Figure 2A, 2B). Proliferation of large-sized atypical lymphoid cells containing large cleaved nuclei with conspicuous nucleoli was observed (Figure 2C). Medium-sized lymphocytes possessing convoluted nuclei with small nucleoli were scattered among these large-sized atypical lymphoid cells (Figure 2C). No epidermal involvement of lymphoid cells was noted (Figure 2A). Neither necrosis nor vascular destructive growth was observed.
Figure 2.
Histopathological features of the knee nodule. A, B: Diffuse proliferation of lymphoid cells invading into the entire dermis and subcutis. No epidermal involvement is noted. HE, x 40. C: Large-sized atypical lymphoid cells with cleaved nuclei containing conspicuous nucleoli are present among medium-sized lymphoid cells with convoluted nuclei. HE, x 400.
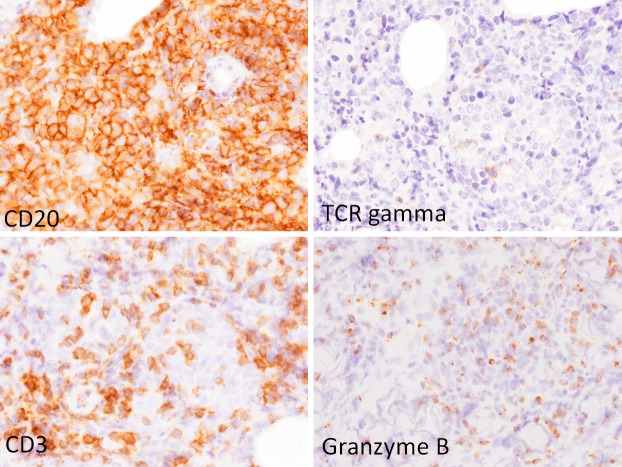
Immunohistochemical and in situ hybridization analyses were performed using an autostainer (Ventana) as the same method previously reported [27-31]. CD20 was expressed in the large-sized atypical lymphoid cells (Figure 3). These cells were also positive for CD10, bcl-2, and bcl-6, but negative for CD5, CD138, and cyclin D1. Approximately half of these lymphoid cells were also positive for MUM-1. Moreover, many CD3-positive T-cells had also infiltrated (Figure 3), and the CD4- and CD8-positive cells were almost evenly distributed. Alpha/beta (clone beta F1)-positive cells were dominant, however, relatively many gamma/delta (clone gamma3.20)-positive cells were also found (Figure 3). Many granzyme B and/or TIA-1-positive cells were observed (Figure 3). Neither CD56- nor EBER-positive cells were noted.
Figure 3.
Immunohistochemical features of the knee nodule. CD20 is expressed in the large-sized atypical lymphoid cells. CD3, and gamma/delta-positive cells are scattered among the large-sized lymphoid cells. Granzyme B-positive cells are scattered. x 400.
Flowcytometric analysis of the knee nodule demonstrated deviation of lambda-positive B-cells and increase of CD3+ gamma/delta+ cells (CD3 (40%), CD19 (50%), CD20 (67%), CD3+ alpha/beta+ (31%), CD3+ gamma/delta+ (6%), kappa+ (1%), and lambda+ (58%)) (Figure 4).
Figure 4.
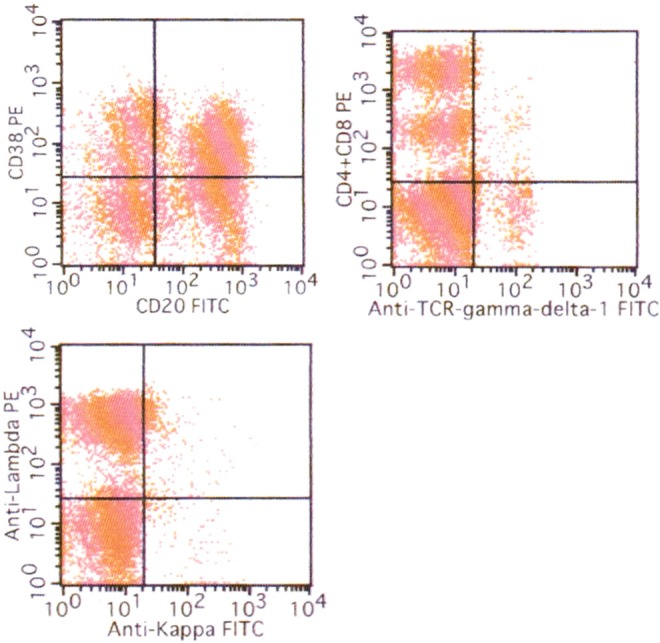
Flowcytometric analysis of the knee nodule. Lambda-positive cells are predominant (lambda/kappa 58), and CD3+ gamma/delta+ cells are also present (6%).
Moreover, rearrangement of immunoglobulin heavy chain (JH) was observed by the polymerase chain reaction method. However, rearrangement of T-cell receptor beta and gamma chains (C beta and J gamma) was not detected from the skin specimen and peripheral blood.
According to these results, an ultimate diagnosis of primary cutaneous B-cell lymphoma with abundant reactive gamma/delta T-cells within the skin lesion and peripheral blood was made with consideration of the results of the flowcytometric and gene analyses.
After the diagnosis, the patient underwent 10 cycles of rituximab therapy. The knee lesion and lymph nodes decreased slightly in size. Flowcytometric analysis of the peripheral blood after rituximab therapy revealed a slight decrease of gamma/delta+ T-cells and disappearance of B-cells (CD3+ (91.7%), CD19+ (0%), alpha/beta+ (71%), gamma/delta+ (23.4%), and CD3+ gamma/delta+ (17.9%)).
However, the skin lesion enlarged again, and radiation therapy was performed, which led to slight decrease in size of the skin lesion. The patient is alive with disease 6 months after the first visit to our hospital.
Discussion
In this report, we describe the first documented case of primary cutaneous B-cell lymphoma with abundant reactive gamma/delta T-cells within the skin lesion and peripheral blood. It has been well-recognized that reactive T-cells within THRLBCL nodal lesion commonly show a T-helper phenotype (CD3+, CD4+, CD8-) [26]. Moreover, reactive T-cells within the lesion of cutaneous T-cell rich B-cell lymphoma also usually show the same phenotype [17]. Primary cutaneous T-cell-rich B-cell lymphoma is extremely rare, and only 20 cases have been reported in the English-language literature [2-16]. Table 1 summarizes the clinicopathological features of the previously reported cases of cutaneous T-cell-rich B-cell lymphoma, which are as follows: i) this type of tumor mainly affects males (male: female 17: 3) with a large age range (20 to 86 years); ii) clinically erythematous papules, plaques or nodules are present on the scalp, face, trunk, and extremities; iii) histopathologically, large neoplastic B-cells admix with abundant small-sized lymphocytes; and iv) immunohistochemically, these neoplastic large-sized B-cells are CD20+ and bcl-6+, and reactive small-sized lymphocytes are usually CD3+, CD4+, CD8- [2-16]. However, predominantly CD8+ reactive T-cell infiltration among the neoplastic B-cells has also been reported in a few cases [15,16]. Only a few cases of Epstein-Barr virus-associated cases have been documented [7,13]. The prognosis of this type of disease is usually favorable as compared to nodal THRLBCL.
Table 1.
Clinicopathological features of primary cutaneous T-cell-rich B-cell lymphoma
| Case No. | Age | Gender | Location | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|
| 1 | 50 | Male | Not available | Chemotherapy | Resolved at 3 months | [2] |
| 2 | 61 | Male | Axilla | Excision | Remission for 21 months | [3] |
| 3 | 30 | Male | Face | Radiation | Died of AIDS after 8 months | [4] |
| 4 | 74 | Male | Back | Chemotherapy | Remission for 26 months | [4] |
| 5 | 59 | Male | Preauricular | Excision and radiation | Remission for 10 years | [5] |
| 6 | 41 | Female | Ear | Excision | Remission for 15 months | [6] |
| 7 | 58 | Male | Back | Excision | Remission for 20 months | [6] |
| 8 | 74 | Male | Neck | Chemotherapy | Died of pneumonia after 1 year | [7] |
| 9 | 45 | Male | Leg | Interferon | Remission for 12 months | [8] |
| 10 | 72 | Female | Scalp | Radiation | Remission for 12 months | [9] |
| 11 | 33 | Male | Scalp | Chemotherapy and radiation | Remission for 7 months | [9] |
| 12 | 71 | Male | Shoulder | Excision | Remission for 10 months | [9] |
| 13 | 50 | Male | Arm | Excision | Hodgkin lymphoma occurred, remission for 74 months | [10] |
| 14 | 52 | Female | Arm | Chemotherapy | Relapsed at 2 months | [11] |
| 15 | 51 | Male | Lip | Chemotherapy and radiation | Remission for 4 months | [12] |
| 16 | 62 | Male | Scalp | Chemotherapy and radiation | Remission for 66 months | [12] |
| 17 | 86 | Male | Abdomen | None | No remarkable change | [13] |
| 18 | 46 | Male | Scalp | Excision and chemotherapy | Remission for 2 years | [14] |
| 19 | 20 | Male | Submandibular | Not available | Remission for 24 months | [15] |
| 20 | 40 | Male | Leg | Excision and radiation | Remission for 1 year | [16] |
In the normal skin, most of the lymphocytes are alpha/beta+, and gamma/delta+ T-cells, which account for ~1% and 15% of CD3+ lymphocytes, are intraepidermally scattered among basal keratinocytes as well as within the dermis [32]. Although it has been reported that accumulation of gamma/delta+ T-cells is observed in some kinds of skin inflammatory conditions, such as granulomatous lesions of leprosy and tuberculosis [33], abundant infiltration of gamma/delta+ T-cells in the skin is extremely rare. Therefore, the diagnosis of the present case was challenging because of the scattered infiltration of gamma/delta+ T-cells within the lesion. Initially, without consideration of the results of the flowcytometric and gene analyses, this lesion was pathologically diagnosed as gamma/delta T-cell lymphoma. The presence of many granzyme B and/or TIA-1-positive lymphocytes led to a suspicion of malignant T-cell lymphoma with cytotoxic activity, thus, we added the immunostaining for alpha/beta and gamma/delta chains. Relatively many gamma/delta+ T-cells misled to an initial diagnosis of gamma/delta T-cell lymphoma. According to the review of the histopathological and immunohistochemical features of the present case, the large-sized atypical lymphoid cells were CD20-positive, and gamma/delta+ cells were medium-sized. With consideration of the deviation of lambda+ B-cells, presence of rearrangement of immunoglobulin heavy chain, and lack of rearrangement of T cell receptor, an ultimate diagnosis of cutaneous B-cell lymphoma with reactive gamma/delta+ cells was made. Moreover, although the present lesion had many gamma/delta+ T-cells, the percentage of neoplastic B-cells present was more than 10%, which did not met the diagnostic criteria of T-cell-rich B-cell lymphoma proposed by Cerroni et al. [17].
Primary cutaneous gamma/delta T-cell lymphoma is a rare and aggressive subtype of T-cell lymphoma and the characteristic histopathological features are as follows: i) the neoplastic cells usually infiltrate into the dermis and subcutis, and show epidermotropism; ii) the neoplastic cell are small to medium or large in size and have pleomorphic nuclei, and angiodestructive growth is commonly seen; and iii) the characteristic immunohistochemical profile of neoplastic lymphocytes is alpha/beta-, CD3+, CD56+, TIA-1+ [34]. This type of disease must be included in the differential diagnosis of the present tumor, however, the results of immunohistochemical, flowcytometric, and gene analyses can differentiate from cutaneous gamma/delta T-cell lymphoma.
Recent studies have shown reactive gamma/delta+ T-cell infiltration and/or elevation in the peripheral blood in patients with various types of carcinomas [21-25]. Lee et al. demonstrated that the proportion of gamma/delta+ T-cells in the peripheral blood of gastric cancer patients was significantly higher in comparison to that in healthy controls [24]. Moreover, Ma et al. clearly demonstrated that gamma/delta+ T-cell infiltration and accumulation in breast cancer sites were general features, and intratumoral gamma/delta+ T-cell numbers were positively correlated with advanced tumor stage and represented the most significant independent prognostic factor [21]. According to these results, gamma/delta+ T-cells appear to play a role in the pathogenesis of some types of carcinomas. Immunohistochemical analysis of gamma/delta+ chain in the paraffin sections has not been possible; however, an anti-gamma/delta antibody for paraffin sections (clone gamma3.20) as used in this study has recently been available [35]. Therefore, additional analyses are needed to clarify the role of reactive gamma/delta+ T-cells in malignant lymphoma.
Disclosure of conflict of interest
None.
References
- 1.De Wolf-Peeters C, Delabie J, Campo E, Jaffe ES, Delsol G. T cell/histiocyte-rich large B-cell lymhpoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 238–239. [Google Scholar]
- 2.Osborne BM, Butler JJ, Pugh WC. The value of immunophenotyping on paraffin sections in the identification of T-cell rich B-cell large-cell lymphomas: lineage confirmed by JH rearrangement. Am J Surg Pathol. 1990;14:933–938. doi: 10.1097/00000478-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Arai E, Sakurai M, Nakayama H, Morinaga S, Katayama I. Primary cutaneous T-cell-rich B-cell lymphoma. Br J Dermatol. 1993;129:196–200. doi: 10.1111/j.1365-2133.1993.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan J, Wallberg K, Frizzera G. T-cell-rich large B-cell lymphoma: a study of 30 cases, supporting its histologic heterogeneity and lack of clinical distinctiveness. Am J Surg Pathol. 1994;18:455–465. [PubMed] [Google Scholar]
- 5.Dommann SN, Dommann-Scherrer CC, Zimmerman D, Dours-Zimmermann MT, Hassam S, Burg G. Primary cutaneous T-cell-rich B-cell lymphoma. A case report with a 13-year-follow-up. Am J Dermatopathol. 1995;17:618–624. doi: 10.1097/00000372-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Sander CA, Kaudewitz P, Kutzner H, Simon M, Schirren CG, Sioutos N, Cossman J, Plewig G, Kind P, Jaffe ES. T-cell-rich B-cell lymphoma presenting in skin. J Cutan Pathol. 1996;23:101–108. doi: 10.1111/j.1600-0560.1996.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 7.Take H, Kubota K, Fukuda T, Shinonome S, Ishikawa O, Shirakura T. An indolent type of Epstein-Barr virus-associated T-cell-rich B-cell lymphoma of the skin: report of a case. Am J Hematol. 1996;52:221–223. doi: 10.1002/(SICI)1096-8652(199607)52:3<221::AID-AJH16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Wollina U. Complete response of a primary cutaneous T-cell-rich B cell lymphoma treated with interferon alpha2a. J Cancer Res Clin Oncol. 1998;124:127–129. doi: 10.1007/s004320050144. [DOI] [PubMed] [Google Scholar]
- 9.Dunphy CH, Nahass GT. Primary cutaneous T-cell-rich B-cell lymphomas with flow cytometric immunophenotypic findings: Report of 3 cases and review of the literature. Arch Pathol Lab Med. 1999;123:1236–1240. doi: 10.5858/1999-123-1236-PCTCRB. [DOI] [PubMed] [Google Scholar]
- 10.Kamarashev J, Dummer R, Schmidt MH, Kempf W, Kurrer MO, Burg G. Primary cutaneous T-cell-rich B-cell lymphoma and Hodgkin’s disease in a patient with Gardner’s syndrome. Dermatology. 2000;201:362–365. doi: 10.1159/000051557. [DOI] [PubMed] [Google Scholar]
- 11.Gogstetter D, Brown M, Seab J, Scott G. Angiocentric primary cutaneous T-cell-rich B-cell lymphoma: a case report and review of the literature. J Cutan Pathol. 2000;27:516–525. doi: 10.1034/j.1600-0560.2000.027010516.x. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Griffin CA, Mann RB, Borowitz MJ. Primary cutaneous T-cell-rich B-cell lymphoma: clinically distinct from its nodal counterpart? Mod Pathol. 2001;14:10–13. doi: 10.1038/modpathol.3880250. [DOI] [PubMed] [Google Scholar]
- 13.Watabe H, Kawakami T, Soma Y, Baba T, Mizoguchi M. Primary cutaneous T-cell-rich B-cell lymphoma in a zosteriform distribution associated with Epstein-Barr virus infection. J Dermatol. 2002;29:748–753. doi: 10.1111/j.1346-8138.2002.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 14.Venizelos ID, Tatsiou ZA, Mandala E. Primary cutaneous T-cell-rich B-cell lymphoma: a case report and literature review. Acta Dermatovenerol Alp Panonica Adriat. 2008;17:177–181. [PubMed] [Google Scholar]
- 15.Vezzoli P, Fiorani R, Girgenti V, Fanoni D, Tavecchio S, Balice Y, Mozzana R, Crosti C, Berti E. Cutaneous T-cell/histiocyte-rich B-cell lymphoma: A case report and review of the literature. Dermatology. 2011;222:225–230. doi: 10.1159/000327376. [DOI] [PubMed] [Google Scholar]
- 16.Kambayashi Y, Fujimura T, Tsukada A, Hashimoto A, Aiba S. Extranodal T-cell/hisitiocyte-rich large B-cell lymphoma presenting primarily on the skin. Acta Derm Venereol. 2012;92:637–639. doi: 10.2340/00015555-1435. [DOI] [PubMed] [Google Scholar]
- 17.Cerroni L, Gatter K, Kerl H. Other cutaneous B-cell lymphoma. In: Cerroni L, Gatter K, Kerl H, editors. Skin lymphoma: the illustrated guide. Chichester: Wiley-Blackwell; 2008. pp. 164–165. [Google Scholar]
- 18.Lauritsen JP, Haks MC, Lefebvre JM, Kappes DJ, Wiest DL. Recent insights into the signals that control alphabeta/gammadelta-lineage fate. Immunol Rev. 2006;209:176–190. doi: 10.1111/j.0105-2896.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler HK. The role of gamma/delta T cells in immunity to infection and regulation of inflammation. Immunol Res. 2004;29:293–302. doi: 10.1385/ir:29:1-3:293. [DOI] [PubMed] [Google Scholar]
- 20.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human gammadelta T cells: a nonredundant system in the immunesurveillance against cancer. Trends Immunol. 2002;23:14–18. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 21.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, Schwartz T, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, Peng G. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalczyk D, Skorupski W, Kwias Z, Nowak J. Flow cytometric analysis of tumor-infiltrating lymphocytes in patients with renal cell carcinoma. Br J Urol. 1997;80:543–547. doi: 10.1046/j.1464-410x.1997.00408.x. [DOI] [PubMed] [Google Scholar]
- 23.Bas M, Bier H, Schirlau K, Friebe-Hoffmann U, Scheckenbach K, Balz V, Whiteside TL, Hoffmann TK. Gamma-delta T-cells in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:691–697. doi: 10.1016/j.oraloncology.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Lee AJ, Kim SG, Chae HD, Lee GH, Shin IH. Gammadelta T cells are increased in the peripheral blood of patients with gastric cancer. Clin Chim Acta. 2012;413:1495–1499. doi: 10.1016/j.cca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Meeh PF, King M, O’Brien RL, Muga S, Buckhalts P, Neuberg R, Lamb LS Jr. Characterization of the gammadelta T cell response to acute leukemia. Cancer Immunol Immunother. 2006;55:1072–1080. doi: 10.1007/s00262-005-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achten R, Verhoef G, Vanuytsel L, De Wolf-Peeters C. Histiocyte-rich, T-cell-rich B-cell lymphoma: a distinct diffuse large B-cell lymphoma subtype showing characteristic morphologic and immunophenotypic features. Histopathology. 2002;40:31–45. doi: 10.1046/j.1365-2559.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 27.Ishida M, Fukami T, Nitta N, Iwai M, Yoshida K, Kagotani A, Nozaki K, Okabe H. Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2242–2246. [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida M, Kodama N, Takemura Y, Iwai M, Yoshida K, Kagotani A, Matsusue Y, Okabe H. Primary bone carcinosarcoma of the fibula with chondrosarcoma and squamous cell carcinoma components. Int J Clin Exp Pathol. 2013;6:2216–2223. [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida M, Igarashi T, Teramoto K, Hanaoka J, Iwai M, Yoshida K, Kagotani A, Tezuka N, Okabe H. Mucinous brobchioloalveolar carcinoma with K-ras mutation arising in type 1 congenital cystic adenomatous malformation: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2597–2602. [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 32.Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antingen-specific gammadelta t-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 34.Cerroni L, Gatter K, Kerl H. Primary cutaneous gamma/delta T-cell lymphoma. In: Cerroni L, Gatter K, Kerl H, editors. Skin lymphoma: the illustrated guide. Chichester: Wiley-Blackwell; 2008. pp. 100–104. [Google Scholar]
- 35.Roullet M, Gheith SM, Mauger J, Junkins-Hopskins JM, Choi JK. Percentage of gammadelta T cells in panniculitis by paraffin immunohistochemical analysis. Am J Clin Pathol. 2009;131:820–826. doi: 10.1309/AJCPMG37MXKYPUBE. [DOI] [PubMed] [Google Scholar]