Abstract
Transmission electron microscopy (TEM) of sputum deposition (SD) is an important method to assist in the diagnosis of pulmonary alveolar proteinosis (PAP). However, the low positive rate and poor quality of slices restrict the application of sputum samples in the diagnosis of PAP. Furthermore, it can be more difficult to obtain a sufficient amount of sample for TEM if the patients have little or no sputum. In this paper, we successfully diagnosed a patient with PAP using induced sputum deposition (ISD) with TEM, which is a novel and noninvasive method for PAP diagnosis. Therefore, ISD combined with TEM can be an effective method for PAP diagnosis, especially when a lung biopsy and bronchoalveolar lavage (BAL) cannot be performed, or little or no sputum can be obtained.
Keywords: Pulmonary alveolar proteinosis, induced sputum, electron microscopy, ultrastructure, diagnosis
Introduction
Pulmonary alveolar proteinosis (PAP), first described in 1958, is a rare diffuse lung disease characterized by the accumulation of periodic acid Schiff (PAS)-positive surfactant-associated lipoproteinaceous material in the alveoli resulting in gas exchange impairment [1]. Due to its nonspecific clinical symptoms and radiographic findings, the diagnosis of PAP represents a challenge for clinicians and pathologists. Many studies had supported the idea that an open lung biopsy was the “gold standard” for PAP diagnosis [2]. However this conventional method represents substantial trauma and distress and therefore has been replaced by transbronchial biopsy and bronchoalveolar lavage (BAL) with computer tomography (CT) scanning [3,4]. However, some patients have contraindication against lung biopsy and BAL, and some scholars have proposed that sputum deposition (SD) samples are appropriate for PAP diagnosis in these patients [3-6]. For patients with little or no sputum, however, it can be difficult to obtain sufficient samples for transmission electron microscopy (TEM). In addition, the cellular components of sputum are few, with some degeneration and indistinct ultrastructures that affect electron microscopy observations. Overall, these drawbacks limit the clinical use of SD and contribute to its low diagnostic rate. To improve the diagnostic rate, we developed a method to overcome the weaknesses of SD. In this paper, we present a novel, effective and noninvasive method to diagnose PAP: induced sputum deposition (ISD) in conjunction with TEM.
Case report
Medical history and symptoms
A 38-year-old male patient was referred to the Respiratory Department of Tongji Hospital, affiliated with the Tongji University School of Medicine, because of an aggravating cough and exercise-associated anhelation for half a year. The patient complained he had suffered a dry cough for 2 years; because the cough often relieved itself initially, the patient did not pay more attention to it. In the past 6 months, the symptoms of the cough worsened, and shortness of breath after activities developed. Sometimes, the severe cough affected the patient’s sleep. He had received supportive therapy to suppress the cough and relieve inflammation on 4 occasions at other hospitals before he was admitted to our hospital. However, his symptoms did not decrease obviously. Previously, the patient had smoked 20 cigarettes per day for 18 years. The patient had no fever, night sweats, hemoptysis or other symptoms other than the medical history described above.
Physical and laboratory examinations
The physical examination revealed coarse breath sounds in both lungs, but no dry or wet rales. Enlarged superficial lymph nodes were not observed. The results of the arterial blood gas analysis were pH 7.34, PaO2 86mmHg and PaCO2 41mmHg. Pulmonary function tests indicated FVC 3.69L, FEV1 2.96L and FEV1/FVC 80.02%. The CT-scan revealed the bilateral lung concentric ground-glass opacification mainly around the bilateral hilus pulmonis and inferior lobes (Figure 1A, 1B). PAP was highly suspected and had to be distinguished from pulmonary hemosiderosis syndrome, pneumocystis jiroveci, pneumonia and other diseases. A lung biopsy was highly recommended to confirm the diagnosis. However, the patient requested a noninvasive technique and he failed to produce sputum.
Figure 1.
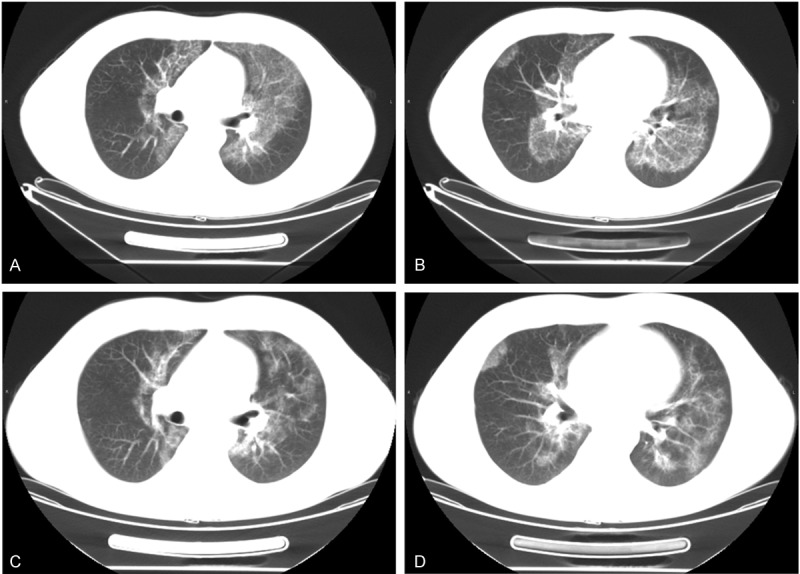
Chest CT-scan of this patient before and after therapy. Before therapy, CT-scan of the PAP patient presented bilateral, diffuse ground-glass opacities mainly distributed in bilateral superior lobes (A) and inferior lobes of lung (B). (C, D) At the patient’s one-year follow-up, CT showed the same scanning position, the ground-glass opacity palliated and the extent of lung opacity shrink, especially in superior lobes (C).
Materials and methods
SD/ISD in conjunction with transmission electron microscopy
The sputum sample was expectorated naturally from the patient after he gargled and brushed his teeth. To obtain an induced sputum sample according to the method of Iredale et al [7], the patient inhaled salbutamol aerosol, rinsed with warm water to clean the mouth, and then inhaled 4% hypertonic saline by ultrasonic nebulization for 20-30 minutes. The patient cleaned his nose and mouth and coughed forcefully to discharge deep sputum every 5 minutes. The first sputum sample with more saliva was discarded, and subsequent sputum specimens (2-3 ml) were collected and placed in a clean and sterile container. The sputum sample or induced sputum sample was centrifuged at 2000r/min for 20 minutes, and the supernatant was discarded. The sediment was retained, fixed with 2.5% glutaraldehyde for 2h and 1% osmium tetroxide for 3h in sequence. The sample was dehydrated with a series of ethanol and acetone for 15 minutes, embedded in epoxy resin 618, cut into ultrathin sections and observed under a transmission electron microscope (PHILIPS CM-120, Netherland).
Results
Under TEM, numerous necrotic granules in the matrix and background mucus were observed, and only a small number of concentric myelin-like lamellar bodies could be observed in the SD section (Figure 2A). However, in the ISD sample, there were numerous concentric lamellated myelin-like bodies indicative of surfactant found in the extracellular spaces (Figure 2B). In addition, a few myelin-like bodies in the alveolar epithelial cells and within the phagosomes of macrophages were observed, consistent with PAP. Cells and lamellated bodies exhibited no degeneration and the background was clear. Combining the results of TEM with clinical and imaging manifestations, we diagnosed this patient with PAP.
Figure 2.
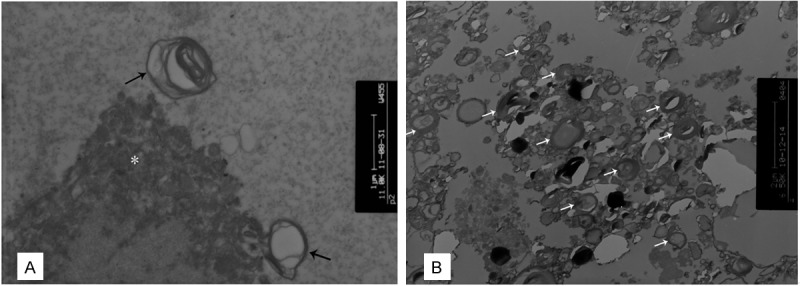
TEM of SD and ISD from this patient. TEM of SD (A) revealed a few of cellular components (*) of sample with serious degeneration. Numerous necrotic granular matrixes were in the background, while rare concentric myelin-like lamellar bodies (→) could be seen individually. However the ISD one (B) presented a large number of lamellar bodies (white arrows) and the background was much clearer. (A, B×8000).
The patient was given BAL as a treatment under general anesthesia. The BAL fluid sample presented as milky sediment. The BAL fluid was centrifuged, made into a cell block, fixed, embedded in paraffin and cut into 5μm-thick sections. The sections were stained with hematoxylin-eosin (H&E) and PAS-diastase (D-PAS), and immunohistochemistry (IHC) was performed. The eosinophilic granular, globular materials, which were abundant in the extracellular spaces and in the cytoplasm of macrophages, were positive for the D-PAS-stained and the H&E-stained sections (Figure 3A, 3B). IHC staining revealed that the macrophages were positive for CD68 (polyclonal KP-1) (Figure 3C). The eosinophilic granules in the background of the samples stained with H&E were also positive in the SP-A staining (polyclonal PE-10) for IHC (Figure 3D). Hence, the diagnosis of PAP was validated via BAL sample examinations. The patient was stable and was discharged from hospital. One year later, he came back for a follow-up visit. The results of the pulmonary function tests were FVC 3.98L, FEV1 3.37L and FEV1/FVC 84.57%, which illustrated that his pulmonary function was significantly improved. CT demonstrated, after therapy, a decrease in the presence of ground-glass opacity and a decrease in the extent of pulmonary opacity, especially in the superior lobes (Figure 1C, 1D). These findings suggest that the last treatment was effective and that the disease progressed slowly.
Figure 3.
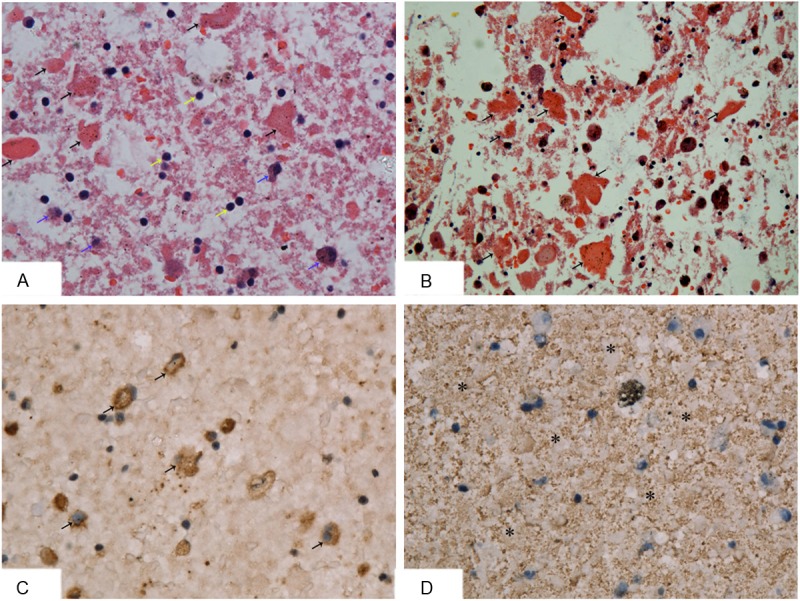
BAL fluid deposition of this patient stained with H&E, D-PAS and IHC. BAL sample was centrifuged, fixed, embedded in paraffin, cut at five microns, and stained with H&E, D-PAS and IHC respectively. (A) HE stained showed a lot of eosinophilic granular, globular materials (black arrows) in extracellular spaces, some macrophages (blue arrows) and lymphocytes (yellow arrows) (HE×200); (B) The eosinophilic granules were positive for D-PAS stain (D-PAS×200). The IHC stain showed macrophages (→) were positive for KP-1 (C) (Envision×400) and the eosinophilic granules (*) in the background of H&E stain were positive for PE-10 (D) (Envision×400).
Discussion
The pathological characteristics of PAP are the accumulation of surfactant and degenerative type II alveolar epithelial cells in the alveoli. Under TEM, osmiophilic lamellar bodies with diagnostic value can be found in the BAL fluid samples of PAP patients [5,8] and provides a basis for PAP diagnosis by TEM of SD, to some extent. Previously, we found that although TEM of SD could make a definitive diagnosis for some PAP patients when combined with clinical and radiographic findings, the technique had some disadvantages, such as poor-quality slices for TEM observation and a low diagnostic rate [9,10]. In the case presented here, we would have been unable to make a diagnosis or differential diagnosis of PAP by TEM using the SD because the patient had little sputum and the section did not meet the requirements for electron microscopy detection. Based on the above experiences, we used a novel method of TEM of ISD to further confirm the diagnosis. The results demonstrated that many myelin-like lamellar bodies existed in the extracellular spaces and inside the phagosomes of macrophages, which are the typical ultrastructural features of PAP. The diagnosis was confirmed via subsequent BAL cytopathological examination, and the patient responded well to the corresponding treatment.
Compared with SD, ISD can collect sufficient samples for electron microscope detection. Moreover, ISD samples are typically obtained from the terminal bronchioles and alveoli, which decrease the electron microscope interference by bacteria or the secretions of the upper respiratory tract and oral cavity. Furthermore, better quality slices make observation much easier and reduces the possibility of false-negatives because the ultrastructure is well-preserved and the background is clearer. Additionally, we found that the ultrastructures of ISD samples were in accordance with BAL because both originated from the terminal bronchiole secretions. ISD samples are superior to BAL because ISD has more indications and is independent of complex instruments. Moreover, reports of patients’ discomfort are rare. For instance, sometimes patients with poor pulmonary function cannot bear BAL, whereas the ISD test is a noninvasive method that is suited for most of PAP patients and can be used to monitor therapeutic effects.
There are 3 forms of PAP: congenital, acquired and idiopathic. The latter 2 forms are predominant in the adult population. Previous case studies have determined that the acquired form can be secondary to various autoimmune, infectious, malignant and environmental etiologies. Recent advances in the understanding of the pathophysiology of PAP demonstrate that the idiopathic form is due to anti granulocyte macrophage-colony stimulating factor (anti GM-CSF) antibodies [11]. Because our hospital has not developed a test for anti-GM-CSF neutrality antibodies in the blood (or BAL fluid) and because this report mainly focused on the method for pathological morphology diagnosis, we did not provide data regarding this aspect. Our patient exhibited no familial history and had not suffered primary pulmonary diseases, cancer, infections or intimate contact history with dust. Therefore we suggest that this case might be the idiopathic form rather than congenital and acquired. In the future, we plan to launch the test to differentiate PAP forms.
In brief, PAP can be definitively diagnosed through electron microscopy of diagnostic osmiophilic lamellar bodies in ISD samples when combined with clinical and radiographic findings. Today, TEM is convenient and substantially more cost-effective than in the past. Therefore, for patients with contraindications to lung biopsy and BAL and for those patients with little or no sputum, TEM examination of induced sputum deposition is an effective, non-invasive method to assist the diagnosis of PAP.
Acknowledgements
Sincerely thank Dr. Weizhe Qiu, Long Zhang (in Department of Pathology, Tongji Hospital, Tongji University School of Medicine) and Zhang Yu (in Electron microscope room, Shanghai medical college of Fudan University) for their skillful technical assistance. This study is supported by Shanghai Science and Technology Commission foundation of key medical research (034119868, 09411951600).
Disclosure of conflict of interest
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing the paper.
Abbreviations
- BAL
Bronchoalveolar lavage
- CT
Computed tomography
- D-PAS
PAS-diastase
- ISD
Induced sputum deposition
- IHC
Immunohistochemistry
- PAP
Pulmonary alveolar proteinosis
- PAS
Periodic acid-Schiff
- SD
Sputum deposition
- TEM
Transmission electron microscopy
References
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Prakash UB, Barham SS, Carpenter HA, Dines DE, Marsh HM. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987;62:499–518. doi: 10.1016/s0025-6196(12)65477-9. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Lazar CA, Fishbein MC, Lynch JP 3rd. Pulmonary alveolar proteinosis. Semin Respir Crit Care Med. 2012;33:498–508. doi: 10.1055/s-0032-1325160. [DOI] [PubMed] [Google Scholar]
- 4.Costello JF, Moriarty DC, Branthwaite MA, Turner-Warwick M, Corrin B. Diagnosis and management of alveolar proteinosis: the role of electron microscopy. Thorax. 1975;30:121–132. doi: 10.1136/thx.30.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maygarden SJ, Iacocca MV, Funkhouser WK, Novotny DB. Pulmonary alveolar proteinosis: a spectrum of cytologic, histochemical, and ultrastructural findings in bronchoalveolar lavage fluid. Diagn Cytopathol. 2001;24:389–395. doi: 10.1002/dc.1086. [DOI] [PubMed] [Google Scholar]
- 6.Carlson DJ, Mason EW. Pulmonary alveolar proteinosis. Diagnosis of probable case by examination of sputum. Am J Clin Pathol. 1960;33:48–54. doi: 10.1093/ajcp/33.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Iredale MJ, Wanklyn SA, Phillips IP, Krausz T, Ind PW. Non-invasive assessment of bronchial inflammation in asthma: no correlation between eosinophilia of induced sputum and bronchial responsiveness to inhaled hypertonic saline. Clin Exp Allergy. 1994;24:940–945. doi: 10.1111/j.1365-2222.1994.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Wakefield SJ, Naran S, Lallu S, Fauck R. Cytohistologic and electron microscopic findings in bronchoalveolar lavage fluid in a case of pulmonary alveolar proteinosis. Diagn Cytopathol. 2002;27:63–65. doi: 10.1002/dc.10112. [DOI] [PubMed] [Google Scholar]
- 9.Yi X, Li H, Zeng Y, Fang X, Wang L, Lv H, Luo B, Zhang Z, Chu H, Zhu X, Li X. Transmission electron microscopy of sputum deposition in the diagnosis of pulmonary alveolar proteinosis. Ultrastruct Pathol. 2012;36:153–159. doi: 10.3109/01913123.2011.639134. [DOI] [PubMed] [Google Scholar]
- 10.Luo BF, Li HP, Yi XH, Tao JW, Lu HJ, Fang X, Zhang ZM, Zhang L, Ren SX, Chu HQ, Li X, Zeng Y. The ultrastructural features of sputum deposition and its value in the diagnosis of pulmonary alveolar proteinosis. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:887–891. [PubMed] [Google Scholar]
- 11.Patel SM, Sekiguchi H, Reynolds JP, Krowka MJ. Pulmonary alveolar proteinosis. Can Respir J. 2012;19:243–245. doi: 10.1155/2012/841530. [DOI] [PMC free article] [PubMed] [Google Scholar]


