Abstract
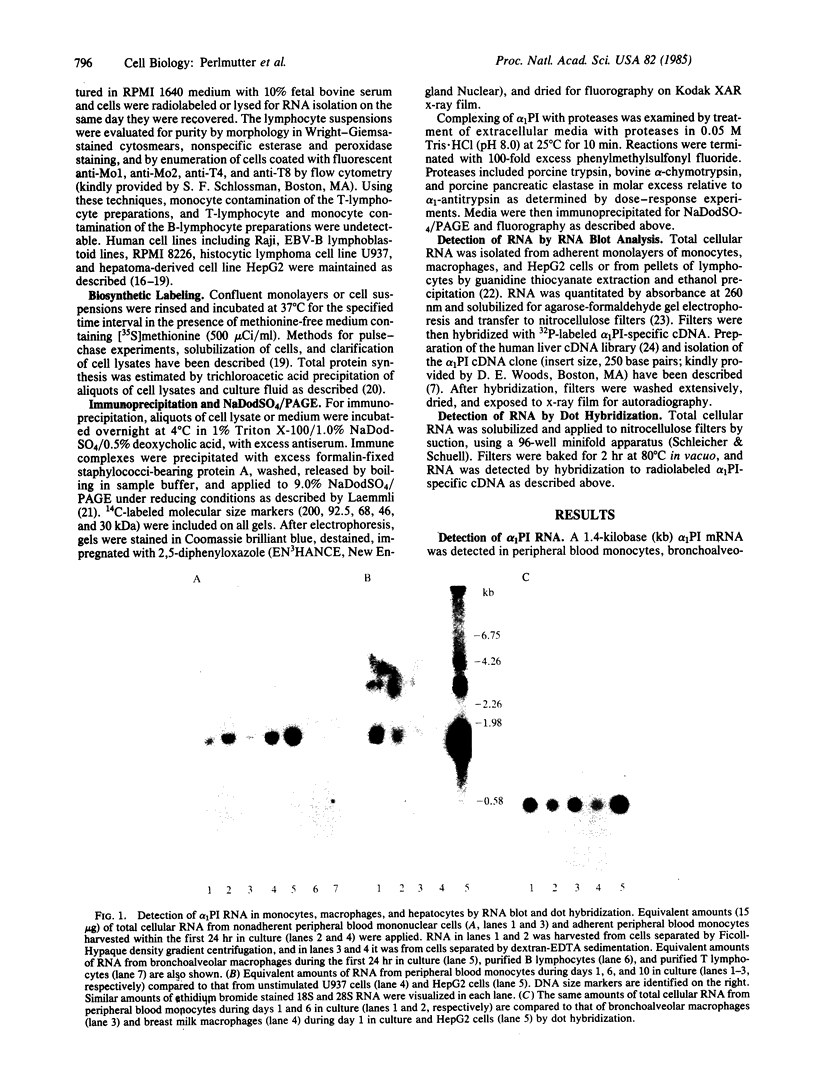
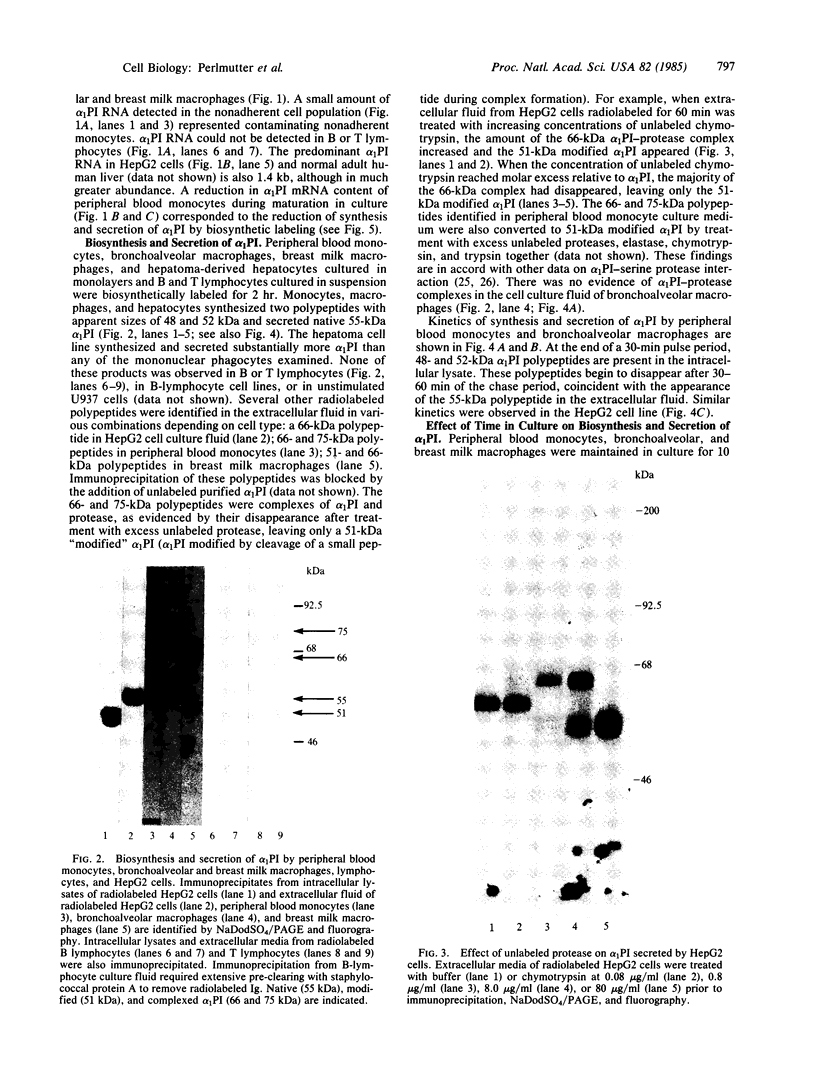
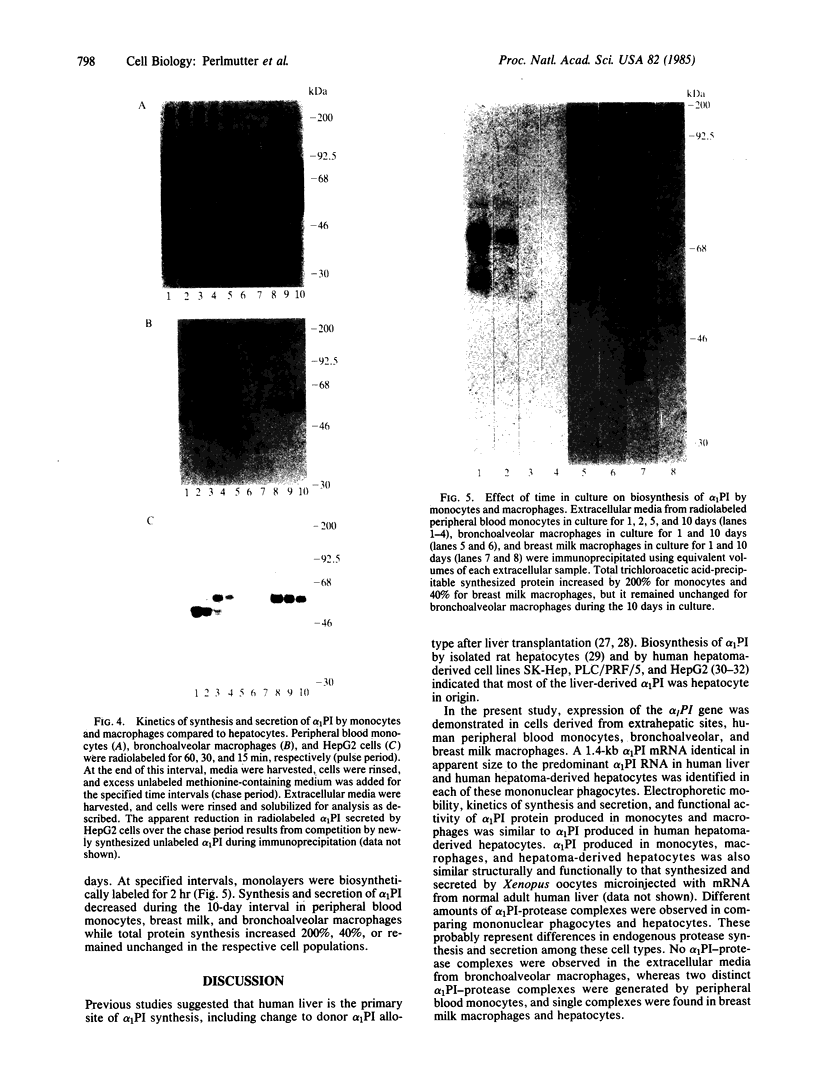
Expression of the alpha 1-proteinase inhibitor (alpha 1PI) gene was studied in human mononuclear cells. Using RNA blot and dot hybridization, alpha 1PI mRNA was detected in human peripheral blood monocytes, bronchoalveolar and breast milk macrophages, but not in B or T lymphocytes. Using incorporation of a radiolabeled amino acid precursor, synthesis and secretion of alpha 1PI were demonstrated in human monocytes and macrophages, but not in lymphocytes. In addition, alpha 1PI was secreted in functionally active form as shown by complexing with serine proteases. Biosynthesis of alpha 1PI by mononuclear phagocytes was greatest during the first 24 hr in culture and progressively decreased over the next 10 days. The reduction in alpha 1PI biosynthesis in vitro involved a mechanism acting at the pretranslational level as alpha 1PI mRNA content also progressively declined over 10 days in culture. The ease of sampling human monocytes and macrophages now permits examination of the biochemical defect in homozygous PiZ and PiS alpha 1PI deficiencies and study of the functional significance of locally produced alpha 1PI in normal tissues and sites of injury or inflammation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Raum D., Awdeh Z. L., Petersen B. H., Taylor P. D., Starzl T. E. Studies of hepatic synthesis in vivo of plasma proteins, including orosomucoid, transferrin, alpha 1-antitrypsin, C8, and factor B. Clin Immunol Immunopathol. 1980 May;16(1):84–89. doi: 10.1016/0090-1229(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Anderson K. C., Bates M. P., Slaughenhoupt B., Schlossman S. F., Nadler L. M. A monoclonal antibody with reactivity restricted to normal and neoplastic plasma cells. J Immunol. 1984 Jun;132(6):3172–3179. [PubMed] [Google Scholar]
- Carlson J., Eriksson S., Alm R., Kjellström T. Biosynthesis of abnormally glycosylated alpha 1-antitrypsin by a human hepatoma cell line. Hepatology. 1984 Mar-Apr;4(2):235–241. doi: 10.1002/hep.1840040211. [DOI] [PubMed] [Google Scholar]
- Carlson J., Stenflo J. The biosynthesis of rat alpha 1-antitrypsin. J Biol Chem. 1982 Nov 10;257(21):12987–12994. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cole F. S., Matthews W. J., Jr, Rossing T. H., Gash D. J., Lichtenberg N. A., Pennington J. E. Complement biosynthesis by human bronchoalveolar macrophages. Clin Immunol Immunopathol. 1983 May;27(2):153–159. doi: 10.1016/0090-1229(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Cole F. S., Schneeberger E. E., Lichtenberg N. A., Colten H. R. Complement biosynthesis in human breast-milk macrophages and blood monocytes. Immunology. 1982 Jun;46(2):429–441. [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow J. E., Bagdasarian A., Colman R. W. Functional alpha 1 protease inhibitor produced by a human hepatoma cell line. J Lab Clin Med. 1982 Jan;99(1):108–117. [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J. M., Koep L. J., Peters R. L., Schröter G. P., Weil R., 3rd, Redeker A. G., Starzl T. E. Liver transplantation for advanced liver disease with alpha-1-antitrypsin deficiency. N Engl J Med. 1980 Jan 31;302(5):272–275. doi: 10.1056/NEJM198001313020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T., Okubo H., Kudo J., Ishibashi H., Inoue T. Alpha 1-antitrypsin synthesis by human lymphocytes. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1509–1516. doi: 10.1016/0006-291x(82)91422-x. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Jones D. B., Judd M. A. Alpha 1-antitrypsin in human macrophages. Lancet. 1979 Nov 3;2(8149):964–965. doi: 10.1016/s0140-6736(79)92665-5. [DOI] [PubMed] [Google Scholar]
- Issekutz T., Chu E., Geha R. S. Antigen presentation by human B cells: T cell proliferation induced by Epstein Barr virus B lymphoblastoid cells. J Immunol. 1982 Oct;129(4):1446–1450. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Morii M., Odani S., Ikenaka T. Characterization of a peptide released during the reaction of human alpha 1-antitrypsin and bovine alpha-chymotrypsin. J Biochem. 1979 Oct;86(4):915–921. doi: 10.1093/oxfordjournals.jbchem.a132623. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Todd R. F., 3rd, Distaso J. A., Schlossman S. F. The role of the macrophage in in vitro primary anti-DNP antibody production in man. J Immunol. 1981 Sep;127(3):1137–1141. [PubMed] [Google Scholar]
- Morse J. O. Alpha1-antitrypsin deficiency (second of two parts). N Engl J Med. 1978 Nov 16;299(20):1099–1105. doi: 10.1056/NEJM197811162992003. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Goldberger G., Colten H. R. Distinct primary translation products from human liver mRNA give rise to secreted and cell-associated forms of complement protein C2. J Biol Chem. 1984 Aug 25;259(16):10380–10385. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J., Landis J. A., Cox F. R., Kuhn C., Koren H. S. Elastase of U-937 monocytelike cells. Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982 Feb;69(2):384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Sztein M. B., Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of human peripheral blood monocyte DR antigen expression in vitro by lymphokines and recombinant interferons. J Clin Invest. 1984 Feb;73(2):556–565. doi: 10.1172/JCI111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Banda M. J., Jones P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980 Nov 1;152(5):1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. B., Walker J. H., Jr, Watkins J. H., Jr, Wolgroch D. Determination of subpopulations of leukocytes involved in the synthesis of alpha 1-antitrypsin in vitro. Proc Soc Exp Biol Med. 1980 May;164(1):105–114. doi: 10.3181/00379727-164-40832. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Kramps J. A., Diesselhof-den Dulk M. M. Synthesis of alpha 1-anti-trypsin by human monocytes. Clin Exp Immunol. 1983 Mar;51(3):551–557. [PMC free article] [PubMed] [Google Scholar]












