Abstract
Malignant mesonephric mixed tumor (MMMT), or mesonephric carcinosarcoma, is a rare tumor with malignant epithelial and mesenchymal components, and is found mostly in the uterine cervix. While diagnosed at the early stage in most cases, MMMT can have an aggressive course. The clinical significance of the presence of sarcomatous components remains unsettled. We report a case of MMMT of the uterine cervix in a patient who presented with tumor rupture, instead of the common presentation, vaginal bleeding. This unusual presentation has not been reported in the literature. It implies that MMMT may progress rapidly without any prodrome and pose a surgical emergency. Unlike most cervical adenocarcinomas, both mesonephric adenocarcinoma and MMMT are not related to human papilloma virus (HPV) infection. Because mesonephric neoplasms have a different etiology, their prevention, screening, and treatment should be further investigated. Thirteen cases of MMMT reported in the literature are also reviewed.
Keywords: Mesonephric carcinosarcoma, malignant mesonephric mixed tumor, rupture, uterine cervix
Introduction
Cervical carcinosarcoma, which has both malignant epithelial and mesenchymal components, is far less common than its counterpart in the uterine corpus [1]. Mesonephric carcinosarcoma, or malignant mesonephric mixed tumor (MMMT), is an extremely rare but well documented entity. Mesonephric adenocarcinoma and MMMT both arise from mesonephric remnants. Mesonephric remnants are not uncommon findings in the lateral wall of the vagina and cervix, and in the broad ligament, mesosalpinx, or ovarian hilus [2]. Despite the prevalence of mesonephric remnants, mesonephric neoplasm is one of the rarest tumors of the uterine cervix. To our best knowledge, only thirteen MMMTs, including the present case, have been reported in the literature (Table 1). We herein present a case of MMMT with atypical clinical manifestations and review the literature.
Table 1.
Malignant mesonephric mixed tumors in the literature and the present case
| Cases | Age | Symptom | Site | Size | Sarcomatous component | Mesonephric remnants/hyperplasia | Figo stage | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 [6] | 65 | PMB | Cervix | 3 cm | Spindle cells, osteosarcoma | Yes | Ib | RAH+BSO+RT | 23 mo, NED |
| 2 [4] | 37 | Postcoital bleeding | Cervix | NA | Spindle cells | Yes | Ib | Hyst+BSO+LD+ChT | 11 yr, AWD |
| 3 [4] | 40 | Menorrhagia+ cervical polyp | Cervix | NA | Spindle cells | Yes | Ib | Hyst+BSO+RT | 2.3 yr, NED |
| 4 [4] | 73 | Vaginal bleeding | Cervix | NA | Spindle cells | Yes | Ib | Hyst+BSO+RT | 3 yr, NED |
| 5 [4,7] | 39 | Menorrhagia | Cervix | NA | Spindle cells, osteosarcoma | Yes | Ib | Hyst+BSO | 6.2 yr, DOD |
| 6 [8] | 37 | Intermenstrual bleeding, Endometrial polyp | Uterus (posterior wall) | 3.5 cm | Spindle cells | No | Ic | Hyst+BSO | 45 mo, NED |
| 7 [8] | 38 | Coitalgia | Vagina | NA | Spindle cells | NA | NA | tumorectomy | NA |
| 8 [8] | 54 | Vaginal bleeding | Cervix | 6 cm | Spindle cells | Yes | IIa | Hyst+BSO+LD+ omentectomy | 7 mo, DOD |
| 9 [8] | 54 | NA | Cervix | 3.5 cm | Spindle cells, chondrosarcoma | Yes | Ib | Hyst+BSO+LD+ omentectomy | 13 mo, NED |
| 10 [8] | 62 | NA | Cervix | 8 cm | Spindle cells, rhabdomyosarcoma | No | IVb | Hyst+BSO+ChT+RT | 36 mo, AWD |
| 11 [5] | NA | NA | NA | NA | Spindle cells | NA | NA | NA | NA |
| 12 [11] | 63 | PMB | Cervix | 1.8 cm | Spindle cells | Yes | IIa | RAH+BSO+LD | 10 mo, NED |
| 13 (Our case) | 59 | Tumor rupture | Cervix | 6.3 cm | Spindle cells | Yes | IIIb | Hyst+BSO+LD+ ChT+RT | 4 mo, NED |
AWD: alive with disease; BSO: bilateral salpingo-oophorectomy; ChT: chemotherapy; DOD: died of disease; Hyst: hysterectomy; LD: lymphadenectomy; mo: months; NA: not available; NED: no evidence of disease; PMB: postmenopausal bleeding; RAH: radical hysterectomy; RT: radiotherapy; yr: year.
Clinical history
A 59-year-old G6P3A3 postmenopausal female patient visited our gynecological department for lower abdominal pain. The pain had lasted for two weeks and she had dysuria as well. Tracing her history, she had diabetes mellitus and hypertension (both under medication control for years). Neither vaginal bleeding nor other discomfort was noted before this event. Because of lower abdominal pain, abdominal sonography and computed tomography were performed and both revealed a large pelvic mass (Figure 1A and 1B). The tumor markers of CA199 and CA125 are 372.9 U/ml (0-37 U/ml) and 253.9 U/ml (0-35 U/ml), respectively. An operation was performed immediately. During the operation, a large mass arising from posterior uterine cervix was found. The tumor had perforated into the retroperitoneum and adhered to the ureter, bladder base, and rectum. Intraoperative frozen section of the pelvis mass was sent to surgical pathology and revealed malignancy. Then, total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and bilateral pelvic lymphadenectomy were done. No invasion was found, so the ureter, urinary bladder, and rectum were preserved after removal of the tumor adhesion. The post-operative clinical course was uneventful and the patient received concomitant chemoradiation (epirubicin- and cisplatin-based chemotherapy and radiotherapy [total dose 5040 cGy/28 fractions]). At 4 months follow up, the patient remains alive and free of recurrence or metastasis.
Figure 1.
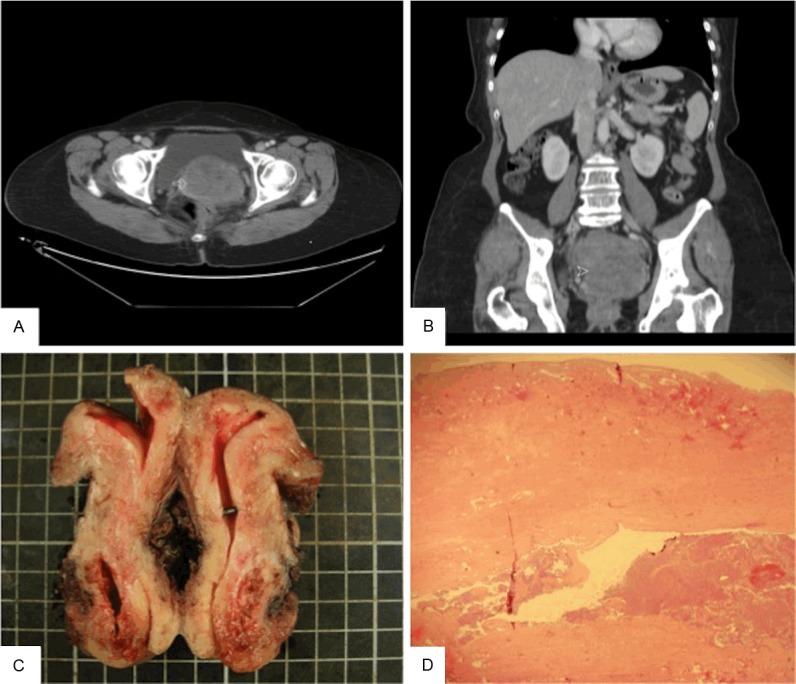
A: The axial image post enhancement shows confluent uterine masses (arrow) with heterogeneous enhancement. The largest one measures 6.8 cm × 7.8 cm × 8.8 cm (depth × width × length in size). B: Coronal reformation of the above uterine masses (arrowhead). C: Grossly, the tumor is located on the posterior aspect of the uterine cervix while the endometrium and cervical canal are spared. D: The cervical mucosa is spared while the tumor rests at the underlying stroma.
Material and methods
Paraffin-embedded blocks were sectioned for hematoxylin and eosin and immunohistochemical staining. Immunostaining was performed using an automated staining machine (Bondmax, Leica, Newcastle, UK). Antibodies to the following proteins were utilized, paired with controls: estrogen receptor (ER) (Leica, 1:50), progesterone receptor (PR) (Leica, 1:100), cytokeratin (CK) (Genemed Synthesis Inc., San Francisco, CA, 1:200), vimentin (Dako Corp., Carpinteria, CA, 1:200), calretinin (Leica, 1:100), anti-CD10 (Leica, 1:100), actin-M851 (Leica, 1:100), S100 protein (Dako, 1:400), and WT-1 (Leica, 1:100).
Results
Grossly, both the endometrium and uterine cervical canal were intact (Figure 1C), but a mass measuring 6.3 × 4.5 × 2.5 cm on the posterior cervical wall and a hole measuring 6.5 × 6.0 cm on the pelvic side of the cervix were noted. Microscopically, the tumor was located on the outer half of the cervix, and the cervical mucosa and endometrium were spared (Figure 1D). The tumor was composed primarily of spindle cells with occasional malignant glandular components. The mass was mainly solid but contained tubular and ductal but not retiform or sex cord-like elements. Some mesonephric remnants with eosinophilic secretion were found to be focally intermingled with the tumor centrally and peripherally (Figure 2). The tumor cells demonstrated marked atypia and brisk mitotic figures (30 over 10 high power fields). Immunohistochemically, the tumor cells were diffusely positive for vimentin, cytokeratin, and S-100 protein; focally positive for CD10, ER, and calretinin, and negative for WT-1, PR, and actin-M851 (Figure 3). The final pathological diagnosis was MMMT (mesonephric carcinosarcoma) of the uterine cervix with tumor rupture. A single regional lymph node metastasis was documented and the pathological staging was pT3bN1, stage IIIB (AJCC Cancer Staging Manual, 7th edition).
Figure 2.
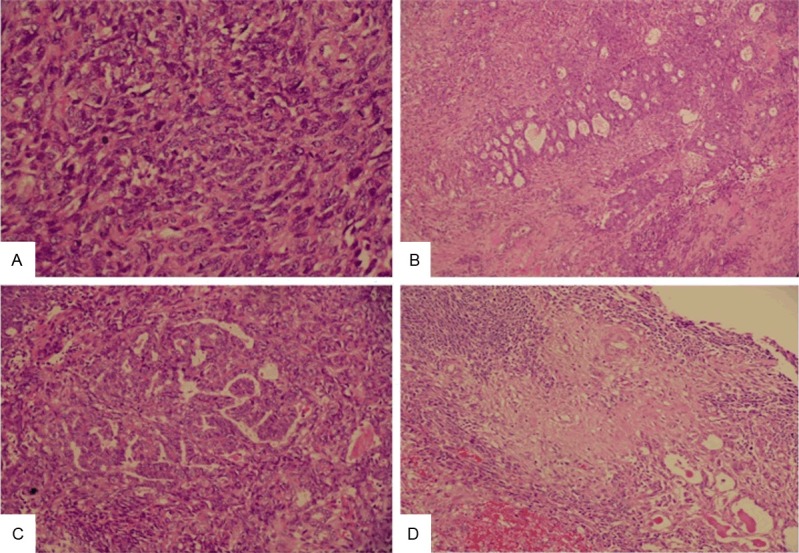
The tumor can be solid (A) (Original magnification × 400), tubular (B) (Original magnification × 100), and ductal (C) (Original magnification × 200). Transition between mesonephric remnants in the right lower quarter and the spindle cell component (D) (Original magnification × 200).
Figure 3.
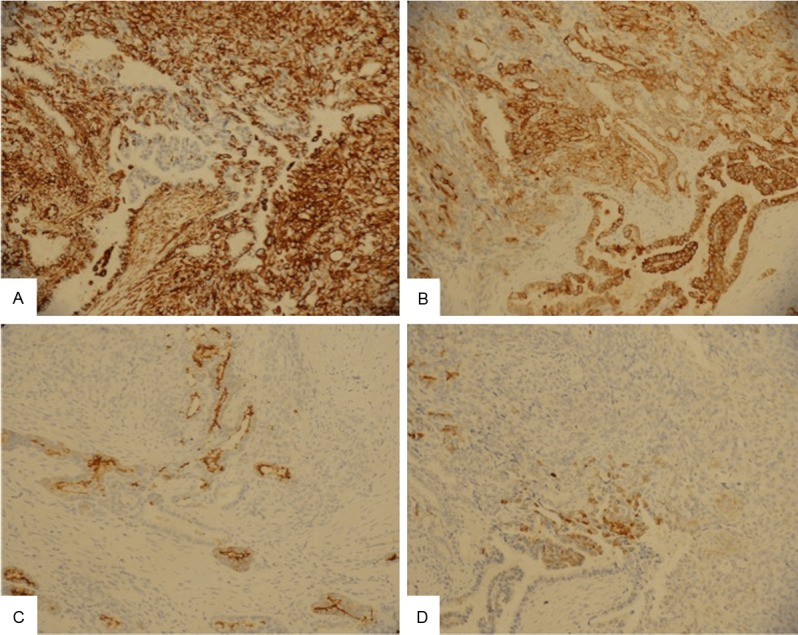
The tumor cells are diffusely positive for vimentin (A) and cytokeratin (B), while focally positive for CD10 (C) and calretinin (D) (Original magnification × 200).
Discussion
Nogales divided the Wolffian (mesonephric) duct into two zones: the upper zone (including rete ovarii) and lower zone (including cervical or vaginal mesonephric remnants) [3]. The former gives rise to Wolffian adnexal tumors and the latter gives rise to mesonephric adenocarcinomas and MMMTs. As indicated by the distribution of mesonephric remnants, mesonephric adenocarcinomas and MMMTs arise most often from the uterine cervix and less often from the vagina and uterine corpus. MMMTs are associated with mesonephric remnants or hyperplasia in 37.5% (3/8) to 100% (8/8) of cases in different series [4,5] and are associated with mesonephric remnants or hyperplasia in 83% of cases reviewed herein (Table 1). In the present case, both remnants and hyperplasia of the mesonephric ducts were within the focally central and peripheral parts of the malignant component. A transitional zone between normal mesonephric ducts, hyperplastic ducts, and the malignant component confirmed that this tumor originated from a mesonephric duct.
Only 13 cases of MMMT, including the current one, have been reported in the literature (Table 1). The median age at onset of MMMT is 54 years (range, 37-73). The common presentations of MMMT include vaginal bleeding, menorrhagia, postmenopausal bleeding, and postcoital bleeding. Spindle cells were present in all cases (13/13, 100%) and other sarcomatous components such as osteosarcoma (n=2), rhabdomyosarcoma (n=2), and chondrosarcoma (n=2) occurred infrequently. In 10 of 12 cases (83%), mesonephric remnant and/or hyperplasia was concomitantly present. The mean tumor size was 3.5 cm (range, 1.8-8.0). Seven of eleven (64%) patients presented with FIGO stage I and the most advanced stage was IVb. Hysterectomy with bilateral salpingo-oophorectomy was performed in 11 of 12 MMMT cases (92%). Post-operative treatments included radiotherapy (n=3), concomitant chemoradiative therapy (n=2), and chemotherapy (n=1). The mean follow-up period was 36 months (range, 3-132) with 7, 2, and 2 patients, respectively, having no tumor recurrence, dying of MMMT, and remaining alive with disease.
The most common presentation of either mesonephric adenocarcinoma or MMMT is vaginal bleeding. Other presentations include pelvic pain, coitalgia, incidental findings, uterine prolapse, and abnormal Pap smear [4,7]. Tumor rupture as an initial presentation has not been previously reported in the literature. Most mesonephric adenocarcinomas or MMMTs show mucosal and even full-thickness involvement [4,7-9]. We postulated that vaginal bleeding (the most common presentation) is related to mucosal involvement. While mucosal involvement is a frequent finding, few mesonephric adenocarcinomas are detected by Pap smear. In the present case, there was no clinical discomfort before this event. We believe that the absence of uterine cervical mucosal and endometrial involvement and consequent bleeding allowed the tumor to progress in a silent fashion and led to tumor rupture.
Histologically, distinguishing between mesonephric hyperplasia, mesonephric adenocarcinoma, and MMMT is a challenge. The histological findings of mesonephric adenocarcinoma vary from case to case. Clement et al. classified the morphologies into five patterns: ductal, tubular, solid, retiform, and sex cord-like [4]. The differential diagnosis includes endometrioid adenocarcinoma, clear cell carcinoma, serous adenocarcinoma, adenoma malignum, and mesonephric hyperplasia. The immunoprofile of mesonephric neoplasm is mostly but not consistently positive for vimentin, calretinin, CD10, and CK7 while negative for ER and PR [7]. Histology (including the presence of adjacent mesonephric remnants) combined with tumor immunoprofile is the best way to diagnose MMMT. The immunoprofiles of mesonephric hyperplasia and mesonephric adenocarcinoma are similar and thus the former can mimic the latter. Cytological atypia, infiltrative border, and Ki-67 can assist in differential diagnosis [7]. The presence of sarcomatous components, either spindle cells or heterologous elements, is the key to MMMT (or mesonephric carcinosarcoma) diagnosis. Although the impact of more metastasis events on survival in MMMTs is clear, the impact of sarcomatous components on overall survival is controversial [10]. Meguro et al. observed that sarcomatous components occur more frequently in cervical mesonephric tumors than in Müllerian tumors; albeit further proof is needed, mesonephric carcinosarcoma should be added to the differential diagnosis of tumors with malignant epithelial and mesenchymal components [11].
The incidence of adenocarcinoma, which accounts for 10-15% of cervical cancers, is increasing despite cytological screening [12,13]. The most and least common subtypes are the endocervical and mesonephric subtypes, respectively [14]. HPV is not part of the etiology of mesonephric adenocarcinoma, unlike the usual types of uterine cervical adenocarcinoma [5]. Thus, HPV may be of less value for preventing mesonephric adenocarcinomas and MMMTs [15]. Our present case expanded the clinical spectrum of uterine adenocarcinomas and highlighted the atypical presentation of MMMT, which may be complicated by tumor rupture and pose a surgical emergency. The preventive measures, screening, and treatment measures that apply to the usual type cervical adenocarcinoma may not be suitable for mesonephric tumors. More effort is needed to clarify the etiology and nature of mesonephric adenocarcinomas and MMMTs.
In conclusion, we report the first case of MMMT of the uterine cervix with an unusual clinical presentation of tumor rupture. It reminds clinicians that MMMT may progress rapidly without any prodrome to tumor rupture and pose a surgical emergency. Owing to differences in etiology between MMMTs and other uterine tumors, their prevention, screening, and treatment should be further investigated.
Disclosure of conflict of interest
None.
References
- 1.McCluggage WG, Kubik-Huch RA. World Health Organization classification of tumours. In: Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 284–286. [Google Scholar]
- 2.Huffman JW. Mesonephric remnants in the cervix. Am J Obstet Gynecol. 1948;56:23–40. doi: 10.1016/0002-9378(48)90188-4. [DOI] [PubMed] [Google Scholar]
- 3.Nogales FF. Mesonephric (Wolffian) tumours of the female genital tract: is mesonephric histogenesis a mirage and trap? Curr Diag Pathol. 1995;2:94–100. [Google Scholar]
- 4.Clement PB, Young RH, Keh P, Ostor AG, Scully RE. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol. 1995;19:1158–1171. doi: 10.1097/00000478-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- 6.Bloch T, Roth LM, Stehman FB, Hull MT, Schwenk GR Jr. Osteosarcoma of the uterine cervix associated with hyperplastic and atypical mesonephric rests. Cancer. 1988;62:1594–1600. doi: 10.1002/1097-0142(19881015)62:8<1594::aid-cncr2820620823>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, Tavassoli FA. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. 2001;25:379–387. doi: 10.1097/00000478-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Bague S, Rodriguez IM, Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2004;28:601–607. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol. 1990;14:1100–1111. doi: 10.1097/00000478-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Yap OW, Hendrickson MR, Teng NN, Kapp DS. Mesonephric adenocarcinoma of the cervix: a case report and review of the literature. Gynecol Oncol. 2006;103:1155–1158. doi: 10.1016/j.ygyno.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Meguro S, Yasuda M, Shimizu M, Kurosaki A, Fujiwara K. Mesonephric adenocarcinoma with a sarcomatous component, a notable subtype of cervical carcinosarcoma: a case report and review of the literature. Diagn Pathol. 2013;8:74. doi: 10.1186/1746-1596-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 13.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75:536–545. doi: 10.1002/(sici)1097-0215(19980209)75:4<536::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000;157:1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]


