Abstract
Extensive melanosis of breast tissue due to melanin in the absence of involvement by melanoma either primary or secondary has been rarely encountered. Herein we report a first and unique case of extensive macroscopic and microscopic melanosis of mammary parenchyma between carcinoma cells due to melanin in a patient with a poorly differentiated invasive ductal carcinoma of the breast with no evidence of melanocytic differentiation or melanoma. In contrast to previously reported cases in the literature, there is no breach of dermal-epidermal junction and there is no dermal infiltrate in the skin overlying the carcinoma, or Pagetoid disease in the nipple.
Keywords: Invasive ductal carcinoma, breast, melanin melanosis
Introduction
Melanosis refers to excessive deposits of melanin or other types of pigments in cells and/or tissues. This phenomenon is encountered most commonly in skin and mucocutaneous tissues such as the oral cavity [1], colon [2,3], genitalia, conjunctiva, and the urinary bladder [4,5]. Melanosis of the breast is rare [6-8], but when it occurs, it is usually associated with primary malignant melanoma [9,10] and carcinoma with melanocytic differentiation [11], in which a metaplastic carcinoma of the breast was entertained [11]. However, melanin melanosis associated with carcinoma of the breast in the absence of either primary or metastatic melanoma and without melanocytic differentiation has never reported before. Herein we report a case of a poorly-differentiated invasive ductal carcinoma of the breast in which an extensive melanin melanosis from the dysplastic stroma is present. The carcinoma has no melanocytic features and this patient has no history of melanoma. To the best of our knowledge, this is the first such case described here.
Report of a case
Clinical presentation
A 41-year-old woman was admitted to the clinic because a left breast mass has been noted on routine mammography. By physical examination, although there is no change in color of the overlying skin, and there is no invagination of the nipple, the lesion was felt, nevertheless, to represent an irregular mass, highly suspicious for a malignancy. Ultrasonic examination revealed a mass lesion with 3.4 cm in greatest diameter and it is located in her left upper quadrant of her breast. A fine needle aspiration (FNA) revealed scattered clusters of atypical cohesive epithelioid cells. Permanent H&E examination of frozen specimen together with extensive immunohistochemistry confirmed a diagnosis of invasive ductal carcinoma (see below) with no melanocytic differential. The patient underwent a modified radical mastectomy. Magnetic resonance imaging (MRI) and CT showed no lesions in the chest, abdomen, or extremities. Left axillar lymphadenectomy was performed and total of 12 lymph nodes were isolated, none of which shows presence of metastatic carcinoma (0/12).
Materials and methods
Immunohistochemistry was performed using Dako Autostainer EnVisionTM (Dako North America Inc, Carpinteria, CA, USA). All of the following pre-made and ready-to-use monoclonal antibodies were purchased from Dako (Carpinteria) with the indicated titration as follows: ER (1:50-1:200), PR (1:50-1:200), CKp (1:50-1:100), EMA (1:50-1:100), 34βE12 (1:50-1:100), Ki-67 (1:50-1:100), p120 (1:50-1:100), Her-2, E-Cadehrin (1:25-1:50), HMB-45 (1:50-100), Melan A (1:50-100), S-100 (1:50-100), CD10 (1:20-40), SMA (1:25-50), CD34 (1:25-50), desmin (1:25-50), CD68, synaptophysin (1:50-100), and chromogranin (1:50-100).
Pathological findings and immunohistochemistry
The modified radical mastectomy specimen was measured 18 cm × 13 cm × 3 cm with 10 cm × 3 cm normal-appearing overlying skin and nipple without gross abnormalities (Figure 1A). The excised breast specimen contains a 3.8 cm × 3.0 cm × 1.4 cm mass lesion, which shows reddish to brown–black color with no obvious hemorrhage and necrosis (Figure 1B). On the cut surface, the tumor was solid with grayish color with a slightly irregular border measuring 3.5 cm × 3.0 cm (Figure 1C), again the dark color is easily appreciated. The remaining breast parenchyma is grossly unremarkable.
Figure 1.
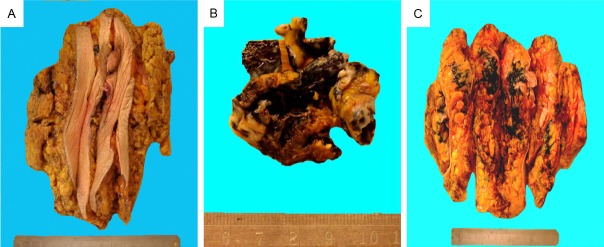
Gross appearance of the mastectomy specimen. (A) The color of the overlying skin is normal, and there is no invagination of the nipple; (B & C) The tumor is solid and shows black color with irregular borders but with no capsule (B), the cut surface also shows areas of darkness (C).
Microscopic examination of the mass with black color shows two distinct components. First the majority of the mass are composed of solid clusters and sheets of cohesive atypical epithelial cells consistent with poorly differentiated ductal carcinoma (Figure 2A). By immunohistochemistry, the carcinoma cells are positive for ER, PR, CKp, EMA, and 34βE12, but negative for E-Cadherin, HMB-45, Melan A, S-100, CD10, desmin, SMA, CD34, CD68, Her-2, synaptophysin, and chromogranin (data not shown). Ki-67 was positive in approximately 30% tumor cells; in addition, the tumor cell membrane is positive for p120. However, no brown to black cytoplasmic pigments were found (Figure 2A).
Figure 2.
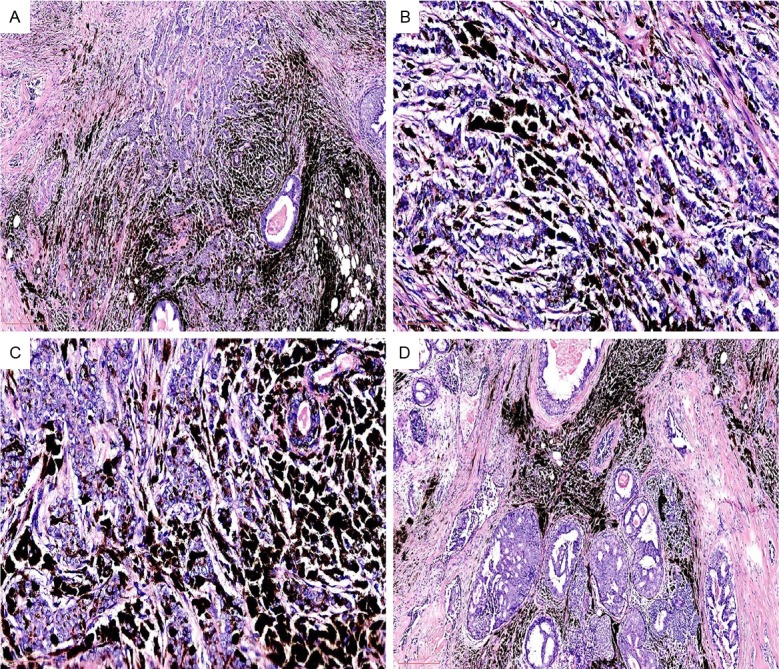
(A) Tumor was composed of clusters and sheets of atypical cohesive epithelial cells. No pigments are seen within the carcinoma cells (H&E, original magnification 4X); (B & C) Abundant pigments between tumor cells are easily appreciated (B & C: H&E, original magnifications 40X, 40X, respectively); (D) DCIS is present in the vicinity of the main invasive carcinoma (H&E, original magnification 4X).
The second component is large quantity of pigments within the desmoplastic fibrous tissue in the stroma (Figure 2B & 2C). These pigments are positive for Fontana-Masson (Figure 3A), but negative for PAS (Figure 3B) and Prussian blue (not shown).
Figure 3.
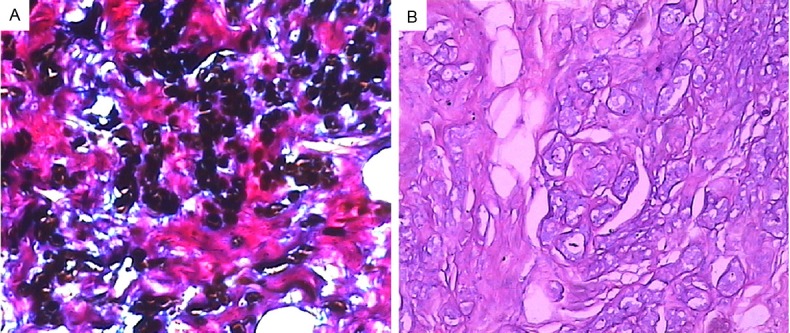
The stromal pigment is positive for Fontana-Masson (A) (original magnification 10X) but negative for PAS (B) (original magnification 10X).
In the vicinity of the tumor, a ductal carcinoma in situ (DCIS) component composed of irregular ducts layered by atypical carcinoma cells is easily appreciated (Figure 2D). The remaining breast tissue including overlying skin, the nipple, and 12 axillary lymph nodes are free of tumor infiltration or metastasis. The pigment cells of the basal lamina of nipple are also normal.
Discussion
Herein we described an interesting and unique case of extensive melanin melanosis from the desmoplastic stroma in a patient with invasive ductal carcinoma of the breast with no evidence of melanoma or melanocytic differentiation. The Melanosis exhibits not only microscopically, but also macroscopically. To the best of our knowledge, this is the first reported such case. While primary melanoma of the breast was initially highly suspicious based on the gross examination of the specimen due to its black color, melanoma is clearly ruled out in this case based on morphologic and immunohistochemical findings, so is carcinoma with melanocytic differentiation.
It is of paramount importance to distinguish invasive ductal/lobular carcinoma of the breast in association with melanin melanosis from melanoma [9,10] and carcinoma with melanocytic differentiation [11,12], which are extremely rare in the breast. In a reported case of primary melanoma of the breast by Tan et al [10], the patient had black skin and mass lesions of the breast. The histology showed spindle and epithelioid heavily pigmented malignant melanocytes with high mitotic rate. Atypical melanocytes eroded the epithelium of mammary skin and nipple. The tumor cells from the reported case were positive for S100 and HMB-45 [13]. Invasive ductal carcinoma with melanocytic differentiation is composed of intimately admixed ductal carcinoma cells and malignant melanoma cells with abundant melanin pigment [12], immunohistochemistry showed that the non-pigmented ductal carcinoma cells were positive for keratin but negative for S100 and HMB-45, and the reverse is true in the pigmented cells. Electron microscopy demonstrated melanosomes in both types of neoplastic cells.
Melanosis of the breast in association with carcinoma of the breast due to melanin or lipofusion in the absence of melanoma is a rare phenomenon, and there has been total of 21 reported cases so far in the English literature (see Table 1). Since the first report of 14 cases by Azzopardi and Eusebi in 1977 [12], there has been additional 7 cases up to year 2012 [11,13-16,18,19]. Among the reported 20 reported cases of breast carcinoma with melanin melanosis or with melanocytic differentiation [11-16,19] from (Table 1), the common theme is that most, if not all, of the reported cases showed deep and or superficial dermal infiltrate by the carcinoma cells with resultant breach of dermal-epidermal junction. However, there is clear difference in our case from the reported cases in that there is no dermal infiltrate by carcinoma cells in our case, and there is no Pagetoid disease of the nipple.
Table 1.
Cases of melanosis in association with carcinoma of the breast in the absence of breast involvement by either primary or metastatic melanoma
| # of cases | Macroscopic melanosis of either overlying skin/tumor mass | Microscopic melanosis | Type of pigment | Diagnosis | Reference |
|---|---|---|---|---|---|
| 1 | Absent/Absent | Present | Melanin | Metaplastic CA with melanocytic differentiation | [11] |
| 14 | Absent/Absent | Present | Melanin | Infiltrating CA | [12] |
| 1 | Present/Absent | Present | Melanin | Infiltrating ductal CA | [13] |
| 1 | Absent/Absent | Present | Melanin | Papillary CA | [14] |
| 1 | Present/Not mentioned | Present | Melanin | Infiltrating ductal CA | [15] |
| 1 | Present/Not mentioned | Present | Melanin | Infiltrating ductal CA | [16] |
| 1 | Absent/Absent | Present | Lipofuscin | Invasive ductal CA | [18] |
| 1 | Present/Not mentioned | Present | Melanin | Infiltrating ductal CA | [19] |
Melanin pigments should be differentiated from other types of pigment depositions including but not limited to lipofuscin and hemosiderin. Breast carcinoma with prominent cytoplasmic lipofuscin granules has been reported [18], and it can mimic invasive carcinoma [20]. Lipofuscin deposits are referred to as lipofuscinosis or pseudomelanosis in order to separate it from true melanosis due to melanin deposition. Lipofuscin contains several varieties of lysosomal bodies or residual bodies. Lipofuscin exhibits brown fine granules usually perinuclear located and represents undigested material from lipid peroxidation, and it is more often associated with aging. Lipofuscin is positive for PAS, but negative for Fontana Mason and Prussian blue, and it does not disappear after bleaching treatment (bleaching resistant) [21].
Hemosiderin is a form of intracellular storage iron that is produced by phagocytic digestion of hemoglobin. Hemosiderin exhibits a golden yellow to brown either intracellularly or extracellularly under the light microscopy. Hemosiderin is refractile, and shows positivity by Gomori’s or Prussian blue (iron) stains. Excessive iron storage in tissues/organs is known as hemosiderosis [22] or hemochromatosis [23]. Hemosiderosis is a form of iron overload and can be encountered in many different medical conditions such as hemorrhagic conditions and multiple blood transfusions as part of treatment for anemia. Histologically, the subepidermis and internal organs show prominent hemosiderin deposits and hemosiderin-laden macrophages.
Melanosis of the skin or oral mucosa is a commonly encountered phenomenon. Mucocutaneous melanosis/hyperpigmentation can be seen in many conditions with a wide spectrum of disease entities [24]. Grossly melanosis of skin appears as gray or black macules; histologically, it shows melanin deposits in the basal layer of the squamous epithelium. Melanosis of oral mucosa can also be seen in smokers and in patients with Peutz-Jeghers syndrome [25].
The exact underlying mechanism of melanin melanosis in this case is not understood or known. In contrast to many other reported cases previously (Table 1) in which melanocyte colonization [12] was postulated as a cause due to breach of dermal-epidermal junction, there is clearly no evidence of dermal or epidermal infiltration by carcinoma cells in our case. Since there are no melanocytes normally present in the mammary ductal and acinus cells, melanocyte implant, ectopia or metaplasia is a pure speculation.
Disclosure of conflict of interest
None.
References
- 1.Maize JC. Mucosal melanosis. Dermatol Clin. 1988;6:283–293. [PubMed] [Google Scholar]
- 2.Li D, Browne LW, Ladabaum U. Melanosis coli. Clin Gastroenterol Hepatol. 2009;7:A20. doi: 10.1016/j.cgh.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Freeman HJ. “Melanosis” in the small and large intestine. World J Gastroenterol. 2008 Jul 21;14:4296–4299. doi: 10.3748/wjg.14.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin B, Zaidi SY, Hollowell M, Hollowell C, Saleh H. A unique case of urinary bladder simple melanosis: A case report and review of the literature. Diagn Pathol. 2009;4:24. doi: 10.1186/1746-1596-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt PF, Hohlbrugger G, Riedl CR. Melanosis of the urinary bladder-a case report with 10 years of follow up and review of the literature. Aktuelle Urol. 2006;37:222–224. doi: 10.1055/s-2005-919095. [DOI] [PubMed] [Google Scholar]
- 6.Davies JD. Pigmented periductal cells in normal and carcinomatous breasts. Arch Pathol. 1974;97:369–372. [PubMed] [Google Scholar]
- 7.Davies JD. Pigmented periductal cells (ochrocytes) in mammary dysplasias: their nature and significance. J Pathol. 1974;114:205–216. doi: 10.1002/path.1711140405. [DOI] [PubMed] [Google Scholar]
- 8.Varga Z, Kubik-Huch RA, Spycher M, Pok J, Caduff R. Melanosis arising in a lumpectomy scar, mimicking invasive carcinoma. Histopathology. 1999;35:279–287. doi: 10.1046/j.1365-2559.1999.0781f.x. [DOI] [PubMed] [Google Scholar]
- 9.Pressman PI. Malignant melanoma and the breast. Cancer. 1973;31:784–788. doi: 10.1002/1097-0142(197304)31:4<784::aid-cncr2820310404>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Tan M, Howard A, Cyr AE. Malignant melanoma of the breast: a case report and review of the literature. Tumori. 2013;99:e11–3. doi: 10.1177/030089161309900125. [DOI] [PubMed] [Google Scholar]
- 11.Ruffolo EF, Koerner FC, Maluf HM. Metaplastic carcinoma of the breast with melanocytic differentiation. Mod Pathol. 1997;10:592–596. [PubMed] [Google Scholar]
- 12.Azzopardi JG, Eusebi V. Melanocyte colonization and pigmentation of breast carcinoma. Histopathology. 1977;1:21–30. doi: 10.1111/j.1365-2559.1977.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 13.Marco V, Autonell J, Cirera L, Gay M. Breast cancer melanosis in a postmastectomy scar. Cancer. 1988;62:206–209. doi: 10.1002/1097-0142(19880701)62:1<206::aid-cncr2820620131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Romanelli R, Toncini C. Pigmented papillary carcinoma of the male breast. Tumori. 1986;72:105–108. doi: 10.1177/030089168607200116. [DOI] [PubMed] [Google Scholar]
- 15.Sau P, Solis J, Lupton GP, James WD. Pigmented breast carcinoma: a clinical and histopathologic simulator of malignant melanoma. Arch Dermatol. 1989;125:536–539. doi: 10.1001/archderm.125.4.536. [DOI] [PubMed] [Google Scholar]
- 16.Saitoh K, Saga K, Okazaki M, Maeda K. Pigmented primary carcinoma of the breast: a clinical mimic of malignant melanoma. Br J Dermatol. 1998;139:287–290. doi: 10.1046/j.1365-2133.1998.02368.x. [DOI] [PubMed] [Google Scholar]
- 17.Nobukawa B, Fujii H, Hirai S, Kumasaka T, Shimizu H, Matsumoto T, Suda K, Futagawa S. Breast carcinoma diverging to aberrant melanocytic differentiation: a case report with histopathologic and loss of heterozygosity analyses. Am J Surg Pathol. 1999;23:1280–1287. doi: 10.1097/00000478-199910000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Shin SJ, Kanomata N, Rosen PP. Mammary carcinoma with prominent cytoplasmic lipofuscin granules mimicking melanocytic differentiation. Histopathology. 2000;37:456–459. doi: 10.1046/j.1365-2559.2000.01013.x. [DOI] [PubMed] [Google Scholar]
- 19.Mele M, Laurberg T, Engberg Damsgaard T, Funder J, Jensen V. Melanocyte colonization and pigmentation of breast: pathological and clinical aspects. Case Rep Pathol. 2012;2012:427628. doi: 10.1155/2012/427628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga Z, Kubik-Huch RA, Spycher M, Pok J, Caduff R. Melanosis arising in a lumpectomy scar, mimicling invasive carcinoma. Histopathology. 1999;35:284–287. doi: 10.1046/j.1365-2559.1999.0781f.x. [DOI] [PubMed] [Google Scholar]
- 21.Jung T, Hohn A, Grune T. Lipofuscin: detection and quantification by microscopic techniques. Methods Mol Biol. 2010;594:173–193. doi: 10.1007/978-1-60761-411-1_13. [DOI] [PubMed] [Google Scholar]
- 22.Lancu TC. Biological and ultrastructural aspects of iron overload: an overview. Pediatr Pathol. 1990;10:281–296. doi: 10.3109/15513819009067114. [DOI] [PubMed] [Google Scholar]
- 23.Dever J, Kowdley KV. Iron metabolism and diagnosis of iron overload disorders. Expert Opin Med Diagn. 2010;4:67–77. doi: 10.1517/17530050903440138. [DOI] [PubMed] [Google Scholar]
- 24.Rigopoulos D, Larios G, Katsambas A. Skin signs of systemic diseases. Clin Dermatol. 2011;29:531–540. doi: 10.1016/j.clindermatol.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Keller JJ, Offerhaus GJ, Giardiello FM, Menko FH. Jan Peutz, Harold Jeghers and a remarkable combination of polyposis and pigmentation of the skin. Fam Cancer. 2001;1:181–185. doi: 10.1023/a:1021149327174. [DOI] [PubMed] [Google Scholar]


