Abstract
Langerhans cell histiocytosis (LCH) is a rare disease, especially when it involves the thyroid gland. Awareness of ultrasonic features will be helpful for a clinician who should consider this disease in the differential diagnosis from other more common thyroid disorders, especially prior to surgery. Here, we report two patients who have histologically confirmed LCH of the thyroid and summarize the reported cases with ultrasonographic scans from the last 10 years (n=10). Ultrasonograms showed isolated or multiple hypoechoic nodules in unilateral or bilateral thyroid gland. Internal acoustic features of most nodules was heterogeneous (n=5) or hypoechoic (n=2).
Keywords: Langerhans cell histiocytosis, thyroid, ultrasound
Introduction
Langerhans cell histiocytosis (LCH) is an uncommon disorder of unknown etiology characterized by monoclonal proliferation of Langerhans cells. It was first described in 1893 by Alfred Hand [1]. Its clinical presentation is highly variable because it can affect multiple organs, such as lung, bone, skin, lymph nodes, hypothalamopituitary axis and other multiple sites [2,3].
However, involvement of the thyroid either as an isolated mass or as part of multisystemic disease is extremely rare and treatment of such disease is still controversial. There are only less than 80 reported cases of LCH involving the thyroid gland. The imaging features just are mentioned in few cases and lacks detail. To the best of our knowledge, no previous studies have reported the ultrasonographic finding of LCH involving the thyroid in detail. The purpose of this essay was to investigate the ultrasonographic features of LCH involving the thyroid gland and review the literature of English reported cases which have reported the ultrasonographic features from the last 10 years.
Case report
Case 1
A 27-year-old Chinese male was referred for our hospital of a painless neck mass that had been present for more than 3 months. There were no other symptoms, such as dysphagia, dyspnea, hoarseness, appetite changes, weight changes or palpitations. He also denied a history of head or neck irradiation or family history of thyroid disease.
Thyroid function tests were as follows: thyroid stimulating hormone (TSH): 1.67 mIU/L (0.35-4.94 mIU/L); free triiodothyronine (FT3): 5.49 pmol/L (3.67-10.43 pmol/L); free thyroxine (FT4): 11.3 pmol/L (7.5-21.1 pmol/L). In addition, calcitonin, parathyroid hormone, thyroglobulin, antithyroglobulin and antimicrosomal antibodies were also within normal range.
Physical examination revealed the presence of a 2.5*2.0 cm mass on the right neck region and a 1.5*1.0 cm mass on the left. Both of them were painless and firm. Thyroid ultrasound showed diffuse hypoechogenicity and a 2.8*1.3*2.2 cm hypoechoic nodule on the right side of the thyroid and a 1.6*0.7*1.1 cm hypoechoic nodule on the left (Figure 1A and 1B). A fine-needle aspiration biopsy revealed proliferated Langerhans cells with heavy infiltrated eosinophils (Figure 1C). Thus, a diagnosis of LCH involving was made and the patient subsequently underwent subtotal thyroidectomy. Pathology findings revealed LCH (Figure 1D), because immunohistochemical staining for CD1α and S100 were positive (Figure 1E and 1F). Postoperatively, the results of thoracic computed tomography (CT) scan, abdominal ultrasonography, and whole-body bone scintigraphy were within normal range. There was not tumor recurrence in the patient after 6 months of follow-up.
Figure 1.
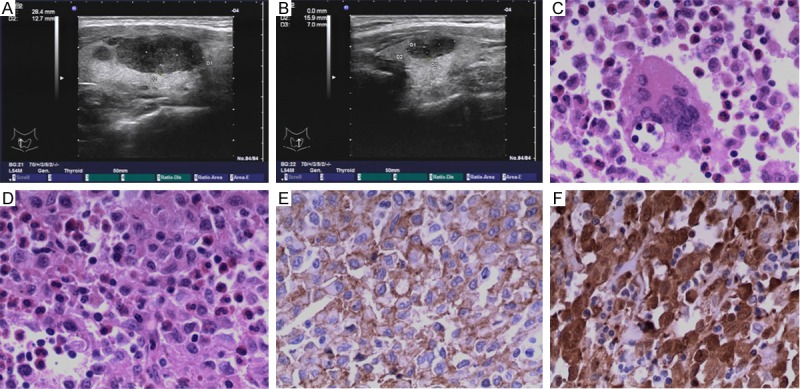
A: High-resolutions ultrasound image of the right thyroid lobe shows hypoechoic nodule. B: High-resolutions ultrasound image of the left thyroid lobe shows hypoechoic nodule. C: Cytologic smear from case 1 showing Langerhans cell with centrally or eccentrically located, oval to reniform “coffee bean” nuclei mixed with many mature eosinophils (×200). D: Hematoxylin and Eosin staining (×200) revealed Langerhans cells proliferated, with eosinophils infiltrated. E: Immunohistochemical staining showing strong reactivity of Langerhans cells for CD1α (×200). F: Immunohistochemical staining showing strong reactivity of Langerhans cells for S100 (×200).
Case 2
A 38-year-old Chinese female was referred for our hospital of two painless neck masses that had been present for more than 1 month. There were no other symptoms, such as dysphagia, dyspnea, hoarseness, appetite changes, weight changes or palpitations. She also denied a history of head or neck irradiation or family history of thyroid disease.
Thyroid function tests results were as follows: thyroid stimulating hormone (TSH): 0.22 mIU/L (0.35-4.94 mIU/L); free triiodothyronine (FT3): 4.16 pmol/L (3.67-10.43 pmol/L); free thyroxine (FT4): 9.9 pmol/L (7.5-21.1 pmol/L). In addition, calcitonin, parathyroid hormone, thyroglobulin, antithyroglobulin and antimicrosomal antibodies were also within normal range.
Physical examination revealed the presence of a 1.2*0.7*0.7 cm mass on the right neck region and a 4.0*2.8*3.3 cm mass on the left. Both of them were painless and firm. Ultrasound demonstrated bilateral multiple heterogeneous hypoechoic nodules with well-defined borders and several lymph nodes in the neck. The biggest one was 4.0*2.8*3.3 cm located on the left side and 1.2*0.7*0.7 cm on the right (Figure 2A and 2B). The result of the fine-needle aspiration biopsy showed proliferated Langerhans cells with heavy infiltrated eosinophils (Figure 2C), but the result is still controversial. Thus, a diagnosis of LCH involving was made and subtotal thyroidectomy was performed. Pathology findings revealed LCH (Figure 2D), because of positive immunohistochemical staining for CD1α and S100 (Figure 2E and 2F). Postoperatively, Thoracic computed tomography (CT) scan, abdominal ultrasonography, and whole-body bone scintigraphy showed no evidence of multifocal disease. The patient remains free of symptoms 3 months after surgery.
Figure 2.
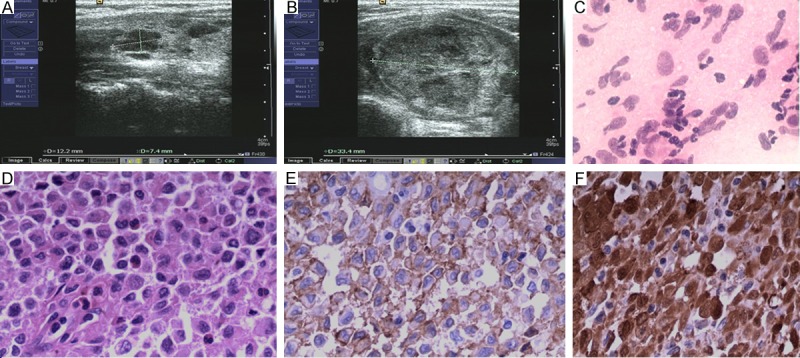
A: High-resolutions ultrasound image of the right thyroid lobe shows hypoechoic nodule. B: High-resolutions ultrasound image shows the biggest hypoechoic nodule on the left thyroid lobe. C: Cytologic smear from case 2 showing high cellularity consisting of many Langerhans cells, and a few eosinophils and lymphocytes (×200). D: Hematoxylin and Eosin staining (×200) revealed diffuse proliferation of Langerhans histiocytes with coffee bean-shaped and grooved nuclei and some eosinophils. E: Immunohistochemical staining showing strong reactivity of Langerhans cells for CD1α (×200). F: Immunohistochemical staining showing strong reactivity of Langerhans cells for S100 (×200).
The literature review was performed using the internet PubMed and Google Scholar search engine from 2002 to 2012. There were less than 40 cases reported but only 10 cases mentioned ultrasonographic features in detail. We read all the articles, carefully reviewed clinical data and retrospectively analyzed the imaging findings for factors such as size, margin, echogenicity, internal acoustic features, and the presence of calcifications.
Results
We summarize the prior reported cases of LCH involving the thyroid with ultrasonographic features in detail (Table 1). In our review, there are 12 cases in total, and the male: female ratio is 3:1. The most common clinical feature is enlargement of thyroid or goiters as the first symptom (N=9). There are 2 patients presented with compressive symptoms such as hoarseness and dysphagia. No patient complained the pain of the enlarged mass of the thyroid. Other symptoms appeared first included polyuria, polydipsia, skin lesions, short stature, hearing loss and amenorrhea. All of 12 patients underwent thyroid function examinations. It is worth noting that thyroid hormone status in LCH of involving the thyroid is variable. There are 6 cases with euthyroid, 4 cases with hypothyroid, 2 cases with hyperthyroid and 1 case with no records. A fine needle aspiration biopsy (FNAB) was conducted in 10 patients. Only 3 cases were confirmed. One patient was underwent several times but failed due to inadequate specimen. Others were misdiagnosed as “atypical follicular epithelial cells”, “papillary carcinoma” or “hyperplasic nodule”. All patients received further investigations including CT, abdominal USS, and bone scintigraphy. 8 patients had isolated thyroid LCH in the cases we reviewed. Two patients first presented with polyuria and polydipsia, and then type 1 diabetes mellitus were detected. There is one patient presented with skin lesions and the hypacusis. Only one patient presented with thyroid LCH, papillary carcinoma and co-existing LCH of draining lymph nodes. It is interesting that one patient experienced thyroidectomy because of Graves’ disease and occasionally found a nodule which was confirmed by pathology located in the isthmus.
Table 1.
Reported cases of thyroid involvement of LCH
| Gender/Age | LCH | Other organ involvement | Thyroid | Treatment | Outcome | Author | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T3 | T4 | TSH | Clinical features | ||||||
| F/13 | D | NR | NL | NL | NL | Diffuse enlargement of the thyroid | Subtotal thyroidectomy; vinblastine, prednisolone | 8 yr remission | Yağcı et al [22] |
| F/26 | L | NR | ↑ | ↑ | ↓ | Goiter (with Graves’ disease) | Thyroidectomy | Well at 3 yr | Lassalle et al [12] |
| F/31 | L | NR | NL | NL | NL | An enlarged, diffusely firm, nontender, nonmobile, and not particularly nodular thyroid gland with mild compressive symptoms | Total thyroidectomy | NR | Lollar et al [20] |
| F/40 | D | Lymph nodes | NL | NL | NL | A swelling in the front area of the neck | Thyroidectomy | Died on the third postoperative day | Ramadas et al [23] |
| M/28 | D | Skin, mastoid, petrous bone | NR | ↓ | ↑ | Diffuse enlargement of the thyroid | Prednisolone, vinblastine, 6-mercaptopurine | NR | Wohlschlaeger [24] |
| F/13 | D | Hypothalamus, parotid gland, external acoustic meatus, cervical lymph node, thymus | ↓ | ↓ | ↑ | Diffuse goiter | Vincristine, cytosine arabinoside, prednisolone | 5 yr remission | Shima et al [25] |
| F/52 | L | NR | NL | NL | NL | An enlarging thyroid mass | Right hemithyroidectomy | Well at 1 yr | Ramon et al [26] |
| M/18 | D | DI | ↓ | ↓ | ↑ | A neck mass | Vincristine, prednisone | Alive | Xia et al [7] |
| F/44 | L | NR | NL | NL | ↓ | Left sided thyroid swelling and dysphonia | Hemithyroidectomy, levothyroxine hormone replacement therapy | Well at 9 mo | Patten et al [27] |
| F/53 | L | NR | NL | NL | NL | A progressively enlarging thyroid | Right thyroid lobectomy with right lymph node dissection | NR | Chung et al [28] |
| M/26 | L | None | NL | NL | NL | An enlarging thyroid mass | Subtotal thyroidectomy | Alive | Present case 1 |
| F/38 | L | None | NL | NL | ↓ | An enlarging thyroid mass | Subtotal thyroidectomy | Alive | Present case 2 |
M, male; F, female; D, diffuse; L, localized; DI, diabetes insipidus; NR, not reported; NL, normal level; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone.
In the reviewed cases, the treatment for LCH involving the thyroid gland included surgery and chemotherapy. There are 9 patients underwent surgical resection by hemi-, subtotal or total thyroidectomy. 6 patients received chemotherapy and the agents included vinblastine, prednisolone, 6-mercaptopurine, and cytosine arabinoside. There is only patient who died of the complication of surgery. 3 patients are surviving for alive till now, 3 patients’ outcomes are not reported and other 5 patients are followed up for 9 months to 8 years.
Ultrasonographic features
All the ultrasonographic features are summarized in Table 2. Most nodules located on the both lobes of the thyroid gland (n=8). The size of the nodule was from 0.3 cm to 4.0 cm. Two patients showed the nodules within the unilateral lobe of thyroid. Echogenicity of most nodules were hypoechoic (n=9). Others were not reported in detail. The internal acoustic features of the nodules were heterogenous (n=5) and hypoechogenous (n=2). Most patients showed no calcification within the nodule. Only one patient revealed three calcified masses within the right lobe of the thyroid.
Table 2.
Ultrasonographic features of thyroid involvement of LCH
| Size and location | Echogenicity | Internal acoustic features | Calcification | Enlarged lymph nodes in the neck | Author |
|---|---|---|---|---|---|
| Multiple in both lobes | Hypo | heterogeneity | - | N | Yağcı et al [22] |
| 1.5*0.8*0.7 cm on the isthmus | Hypo | hypoechogenicity | - | N | Lassalle et al [12] |
| 3.6*2.0*1.6 cm on the right; 3.6*1.6*1.7 cm on the left | Hypo | hypoechogenicity | - | N | Lollar et al [20] |
| Solid nodules in both lobes | NR | NR | NR | NR | Ramadas et al [23] |
| Multiple in both lobes | Hypo | heterogeneity | - | N | Wohlschlaeger [24] |
| Diffuese thyroid enlargement | Hypo | NR | - | N | Shima et al [25] |
| 3.6*2.0*1.6 cm on the right; 3.6*1.6*1.7 cm on the left | Hypo | NR | - | N | Ramon et al [26] |
| Diffuse thyroid enlargement | Hypo | heterogeneity | - | N | Xia et al [7] |
| 3 cm in diameter on the left thyroid lobe | NR | NR | NR | N | Patten et al [27] |
| Three masses measuring 0.6, 0.4 and 0.3 cm | NR | NR | + | N | Chung et al [28] |
| 2.8*1.3*2.2 cm on the right; 1.6*0.7*1.1 cm on the left | Hypo | heterogeneity | - | Y | Present case 1 |
| Multiple in both lobes | Hypo | heterogeneity | - | Y | Present case 2 |
NR, not reported; N, none; Y, yes.
Discussion
LCH is an uncommon disease with an incidence rate of 4.0-4.5 per 1 million individuals [4]. It can be confirmed to one organ or a systemic disease with lung, bone, skin and central nervous system being the most favored sites of involvement [5,6]. The incidence of LCH involving the thyroid gland either as an isolated manifestation or as part of multisystemic disease is extremely low. According to the report of Lieberman et al, thyroid LCH is seen in only 1 case of 238 LCH cases [5]. Although LCH is most commonly a pediatric disease, thyroid LCH is more common in adults than in children, and it may coexist with a thyroid carcinoma [7].
The etiology of LCH remains under investigation. To date, there is no study has definitively connected this disease to a viral etiology, cytogenic abnormality, or specific HLA genotype [8-10]. In the meantime, there were some reported cases of LCH involving the thyroid gland associated with lymphocytic thyroiditis [5,11]. Coexistence of thyroid LCH and thyroid carcinoma is not rare [12-16]. Meanwhile, thyroid carcinoma developed years after thyroid LCH has been made [11]. Therefore, in some extend, LCH involving the thyroid gland may raise the possibility of other associated thyroid diseases.
The first symptom of LCH involving the thyroid gland usually presents as the enlargement of neck mass or a goiter. Thyroid function may be variable depending on the degree of thyroid involvement [13]. But there is another possibility that means primary LCH and other thyroid disease synchronously occurred in one patient [12].
Although there are many methods of examination, diagnosing these patients is still a challenging, especially before the surgery. It is mainly due to the variable clinical presentation and confusing imaging characteristics. In fact, LCH involving the thyroid gland can mimic other common thyroid disease such as thyroid neoplasm. Therefore, imaging, cytology, pathology and immunohistochemistry of the thyroid LCH should be known well.
Thyroid fine needle aspiration is useful in establishing the diagnosis. However, it can be confused with other far more common thyroid disease and the misdiagnosis rate is not low. In our review, there are only 5 patients confirmed by FNAB (5/12). Accordingly, the diagnosis of LCH involving thyroid can be a challenge for pathologist. Meanwhile, FNAB, as an invasive examination method, probably has some risk when repeat several times just due to inadequate specimen.
Ultrasonography is first-line noninvasive modality for the workup of thyromegaly [17]. Familiarity with the ultrasonographic features of LCH involving the thyroid gland is helpful for crucial to a clinician. A comprehensive review of the literature revealed that lesions of LCH involving the thyroid usually showed multiple bilateral hypoechoic nodules and parenchymal heterogeneity. Although there is no obvious specificity of the ultrasonographic features in thyroid LCH, especially compared with common thyroid neoplasm, the finding still can reminds us to keep primary thyroid LCH in mind.
Nonetheless, histology remains the gold standard for the diagnosis of LCH. Pathological features usually revealed an infiltration of the Langerhans histocytes with eosinophils and lymphocytes. Immunohistochemical study is usually performed to diagnose LCH and can distinguish LCH from other lesions. Langerhans cells usually reactive to CD1α and S-100 protein. All of the 12 patients we reviewed here underwent an immunohistochemical examination and stained positively for S100 and/or CD1α.
Treatment of choice for LCH includes surgery and chemotherapy. Surgical treatment seems to be the suitable option, especially for localized LCH of the thyroid [18,19]. However, for aggressive disease, combination chemotherapy is highly recommended [2]. Although thyroid involvement is more common in adults, it has a indolent course [20]. The prognosis of LCH is still related the number of involved organs, degree of organ dysfunction and the response to treatment [7,21]. Hence, further investigations and prolonged follow-up are highly recommended.
In conclusion, LCH involving the thyroid gland is rare. We reported two cases of thyroid LCH and reviewed clinical manifestation and ultrasonographic features of cases reported from last 10 years. Although the prognosis is very good of the thyroid LCH, the findings remind us to keep it in mind for differential diagnosis of other thyroid diseases.
Acknowledgements
This work was supported by the Wenzhou City Science and Technology Bureau (NO. Y20120190).
Disclosure of conflict of interest
The authors declare that they have no competing interests.
Abbreviations
- LCH
Langerhans cell histiocytosis
- TSH
Thyroid stimulating hormone
- FT3
Free triiodothyronine
- FT4
Free thyroxine
- FNAB
Fine needle aspiration biopsy
References
- 1.Hand AJ. Polyuria and tuberculosis. Arch Pediatr. 1893;10:673–675. [Google Scholar]
- 2.Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278–2290. doi: 10.1002/(sici)1097-0142(19990515)85:10<2278::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Kleinjung T, Woenckhaus M, Bachthaler M, Wolff JE, Wolf SR. Langerhans’ cell histiocytosis with bilateral temporal bone involvement. Am J Otolaryngol. 2003;24:265–270. doi: 10.1016/s0196-0709(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 4.Donadieu J, Rolon MA, Thomas C, Brugieres L, Plantaz D, Emile JF, Frappaz D, David M, Brauner R, Genereau T, Debray D, Cabrol S, Barthez MA, Hoang-Xuan K, Polak M French LCH Study Group. Endocrine involvement in pediatric-onset Langerhans’ cell histiocytosis: a population-based study. J Pediatr. 2004;144:344–350. doi: 10.1016/j.jpeds.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman PH, Jones CR, Steinman RM, Erlandson RA, Smith J, Gee T, Huvos A, Garin-Chesa P, Filippa DA, Urmacher C, Gangi MD, Sperber M. Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol. 1996;20:519–552. doi: 10.1097/00000478-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Malpas JS. Langerhans cell histiocytosis in adults. Hematol Oncol Clin North Am. 1998;12:259–268. doi: 10.1016/s0889-8588(05)70509-8. [DOI] [PubMed] [Google Scholar]
- 7.Xia CX, Li R, Wang ZH, Lv FJ, Tang XQ, Li QF, Zhang SH. A rare cause of goiter: Langerhans cell histiocytosis of the thyroid. Endocr J. 2012;59:47–54. doi: 10.1507/endocrj.ej11-0243. [DOI] [PubMed] [Google Scholar]
- 8.McClain K, Jin H, Gresik V, Favara B. Langerhans cell histiocytosis: lack of a viral etiology. Am J Hematol. 1994;47:16–20. doi: 10.1002/ajh.2830470104. [DOI] [PubMed] [Google Scholar]
- 9.McClain KL, Laud P, Wu WS, Pollack MS. Langerhans cell histiocytosis patients have HLA Cw7 and DR4 types associated with specific clinical presentations and no increased frequency in polymorphisms of the tumor necrosis factor alpha promoter. Med Pediatr Oncol. 2003;41:502–507. doi: 10.1002/mpo.10366. [DOI] [PubMed] [Google Scholar]
- 10.Shimakage M, Sasagawa T, Kimura M, Shimakage T, Seto S, Kodama K, Sakamoto H. Expression of Epstein-Barr virus in Langerhans’ cell histiocytosis. Hum Pathol. 2004;35:862–868. doi: 10.1016/j.humpath.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Egeler RM, Neglia JP, Puccetti DM, Brennan CA, Nesbit ME. Association of Langerhans cell histiocytosis with malignant neoplasms. Cancer. 1993;71:865–873. doi: 10.1002/1097-0142(19930201)71:3<865::aid-cncr2820710334>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Lassalle S, Hofman V, Santini J, Sadoul JL, Hofman P. Isolated Langerhans cell histiocytosis of the thyroid and Graves’ disease: an unreported association. Pathology. 2008;40:525–527. doi: 10.1080/00313020802198002. [DOI] [PubMed] [Google Scholar]
- 13.Jamaati HR, Shadmehr MB, Saidi B, Khosravi A, Arab M, Mohammadi F. Langerhans cell histiocytosis of the lung and thyroid, co-existing with papillary thyroid cancer. Endocr Pathol. 2009;20:133–136. doi: 10.1007/s12022-009-9068-0. [DOI] [PubMed] [Google Scholar]
- 14.Foulet-Roge A, Josselin N, Guyetant S, Gardet JJ, Besancon A, Saint-Andre JP, Fabiani B. Incidental langerhans cell histiocytosis of thyroid: case report and review of the literature. Endocr Pathol. 2002;13:227–233. doi: 10.1385/ep:13:3:227. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LD, Wenig BM, Adair CF, Smith BC, Heffess CS. Langerhans cell histiocytosis of the thyroid: a series of seven cases and a review of the literature. Mod Pathol. 1996;9:145–149. [PubMed] [Google Scholar]
- 16.Burnett A, Carney D, Mukhopadhyay S, Scalzetti EM, Leino D, Souid AK. Thyroid involvement with Langerhans cell histiocytosis in a 3-year-old male. Pediatr Blood Cancer. 2008;50:726–727. doi: 10.1002/pbc.21030. [DOI] [PubMed] [Google Scholar]
- 17.Farrag TY, Lin FR, Cummings CW, Sciubba JJ, Koch WM, Flint PW, Tufano RP. Importance of routine evaluation of the thyroid gland prior to open partial laryngectomy. Arch Otolaryngol Head Neck Surg. 2006;132:1047–1051. doi: 10.1001/archotol.132.10.1047. [DOI] [PubMed] [Google Scholar]
- 18.Behrens RJ, Levi AW, Westra WH, Dutta D, Cooper DS. Langerhans cell histiocytosis of the thyroid: a report of two cases and review of the literature. Thyroid. 2001;11:697–705. doi: 10.1089/105072501750362781. [DOI] [PubMed] [Google Scholar]
- 19.Hung CS, Yeh YC, Chen JC, Jung SM, Hung IJ, Lo FS. Isolated Langerhans cell histiocytosis of the thyroid in a female infant. Eur J Pediatr. 2007;166:1151–1153. doi: 10.1007/s00431-006-0397-4. [DOI] [PubMed] [Google Scholar]
- 20.Lollar K, Farrag TY, Cao D, Niparko J, Tufano RP. Langerhans cell histiocytosis of the thyroid gland. Am J Otolaryngol. 2008;29:201–204. doi: 10.1016/j.amjoto.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Favara BE, Jaffe R. Pathology of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1987;1:75–97. [PubMed] [Google Scholar]
- 22.Yagci B, Kandemir N, Yazici N, Yalcin B, Varan A, Akyuz C, Buyukpamukcu M. Thyroid involvement in Langerhans cell histiocytosis: a report of two cases and review of the literature. Eur J Pediatr. 2007;166:901–904. doi: 10.1007/s00431-007-0487-y. [DOI] [PubMed] [Google Scholar]
- 23.Ramadas PT, Kattoor J, Mathews A, Abraham EK. Fine needle aspiration cytology of Langerhans cell thyroid histiocytosis and its draining lymph nodes. Acta Cytol. 2008;52:396–398. doi: 10.1159/000325536. [DOI] [PubMed] [Google Scholar]
- 24.Wohlschlaeger J, Ebert S, Sheu SY, Schmid KW, Totsch M. Immunocytochemical investigation of Langerin (CD207) is a valuable adjunct in the cytological diagnosis of Langerhans cell histiocytosis of the thyroid. Pathol Res Pract. 2009;205:433–436. doi: 10.1016/j.prp.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Shima H, Inokuchi M, Shimada H. A case of multisystem Langerhans cell histiocytosis with primary hypothyroidism followed by type 1 diabetes mellitus. Pediatr Blood Cancer. 2009;53:232–234. doi: 10.1002/pbc.22066. [DOI] [PubMed] [Google Scholar]
- 26.Vilallonga R, Ciudin A, Fort JM, Baena JA, Gonzalez O, Armengol M, Mesa J, Ruiz Marcellan MC. Isolated langerhans cell histiocytosis of the thyroid in an adult female: one-year followup. Int J Endocrinol. 2011;2011:898302. doi: 10.1155/2011/898302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patten DK, Wani Z, Tolley N. Solitary langerhans histiocytosis of the thyroid gland: a case report and literature review. Head Neck Pathol. 2012;6:279–289. doi: 10.1007/s12105-011-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung DH, Ha SY, Cho HY, Kim NR, An JS, Lee YD, Park S. Langerhans Cell Histiocytosis in the Thyroid and Draining Lymph Nodes: A Case Report. Endocrinol Metab. 2012;27:138–141. [Google Scholar]


