Abstract
Hilus cell heterotopia is the presence of normal hilus cells in an abnormal site. It is rare and there are only a few case reports or case series. It has been reported in the fimbrial stroma of the fallopian tube, paratubal cyst wall and beneath ovarian capsule. Most cases are more than 40 years of age, and some of them are associated with other underlying pelvic pathology. Hilus cells are ovarian counterpart of testicular Leydig cells, carrying similar morphological and immunohistochemical findings. In this report, we described a patient having bilateral serous cystadenomas with an incidental finding of hilus cells in the fallopian tube.
Keywords: Hilus cell heterotopia, hilus cell rests
Introduction
The ovarian hilus cells are found in the ovarian hilum and mesovarium, often adjacent to nerves [1]. They resemble Leydig cells of the testis and contain lipofuscin pigments and occasional Reinke crystals. They have epithelioid morphology with round to oval, vesicular nuclei, prominent nucleoli, and abundant granular eosinophilic cytoplasm [2]. The histogenesis of hilus cells is unknown, which may be derived either from luteinized ovarian stromal cells that have wandered into the hilum or from perineural fibroblasts [3,4]. Hilus cell heterotopia is rare and often an incidental finding during pathological examination. In review of the literature, heterotopic hilus cells are identified in the fimbrial stroma of the fallopian tube, paratubal cyst wall or beneath ovarian capsule [5]. It often accompanies with other gynecologic abnormality, such as endometriosis, salpingitis isthmica nodosa and neoplasm. None of the cases reported in the literature were associated with a bilateral ovarian neoplasms. We present a patient having bilateral serous cystadenomas with an incidental finding of hilus cells heterotopia in the fallopian tube. Detailed morphological and immunohistochemical studies are also performed.
Case report
The patient was a 60 year-old woman who suffered from lower abdominal pain. The gynecologic ultrasound scan showed bilateral ovarian cysts measuring 2.6 cm in diameter in the right side and 9.5 cm in diameter in the left side, respectively. The tumor markers, including CEA, CA125 and CA199 were within normal range. She received bilateral salpingo-oophorectomy. And the specimens were sent for pathological examination.
Materials and methods
The specimen was fixed in 10% formalin solution and embedded in the paraffin block. Sections were cut and stained with hematoxylin and eosin for light microscopy. Immunohistochemical (IHC) stains were performed by using standard reagents and techniques on an BOND-MAX Automated Staining System (Leica Microsystems). Briefly, Sections were deparaffinized, hydrated, and subjected to heat-induced antigen retrieval with Bond epitope retrieval (EDTA based pH 9.0 solution, Leica Microsystems). The primary antibodies, including inhibin A (Clone AMY82, Leica, 1:100), Melan-A (Clone A103, Leica, 1:50), calretinin (Clone 5A5, Leica, 1:200), CD99 (Clone PCB1, Leica, 1:200) and WT1 (Clone 6F-H2, Genemed, 1:100) were applied for 30 minutes at room temperature followed by application of Biotin-free bond polymer refine detection (Leica Microsystems). Positive and negative controls were done according to manufacturer’s instruction. It was defined as positive when at least 20% of the malignant cells in the slides revealed positive staining.
Results
On the macroscopic examination, the bilateral ovaries were cystic and filled with pale yellow serous fluid. The right ovary measured 3.0 × 2.8 × 1.8 cm. The left ovary measured 10.0 × 7.5 × 7.0 cm. The right fallopian tube measured 5.0 cm in length and 0.5 cm in diameter. The left fallopian tube measured 5.5 cm in length and 0.6 cm in diameter. The bilateral fallopian tubes showed no gross abnormality.
Histologic examination of bilateral ovaries showed serous cystadenomas. In the left fallopian tube, there were clusters or cords of epithelioid cells bearing uniform round, vesicular nuclei and abundant granular eosinophilic cytoplasm, with the morphology of hilus cells, in the subepithelial stroma of the fimbrial area (Figure 1). No crystalloids of Reinke were identified in the hilus cells. In the immunohistochemical study, these hilus cells were strongly positive for inhibin A and Melan-A (Figure 2). Weak positivity for calretinin was also noted. There was no expression of CD99 and WT-1. In the bilateral ovaries, no hilus cell was found.
Figure 1.
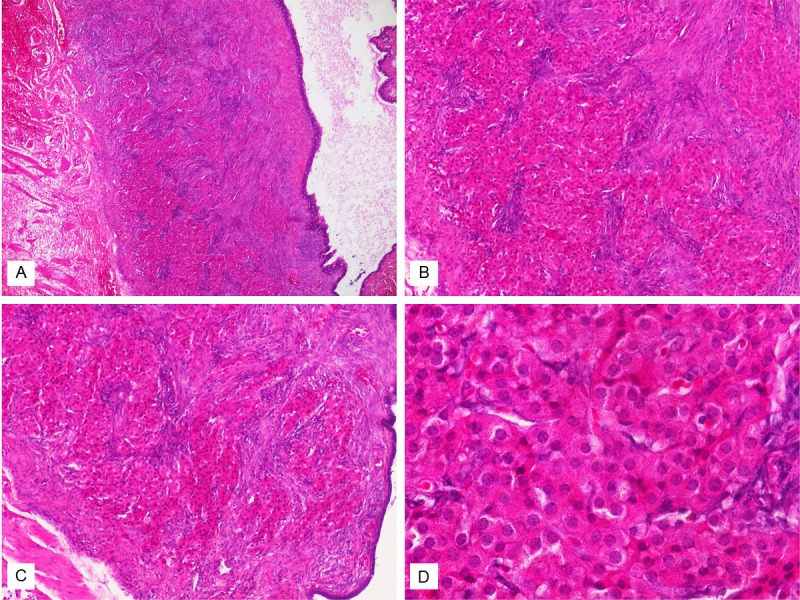
A. On hematoxylin and eosin-stained sections, there were clusters of eosinophilic epithelioid cells in the subepithelial stroma of the fallopian tube (× 40). B and C. They were arranged in clusters or cords (× 100). D. The cells were composed of uniform round, vesicular nuclei, occasional prominent nucleoli and abundant granular eosinophilic cytoplasm (× 400).
Figure 2.
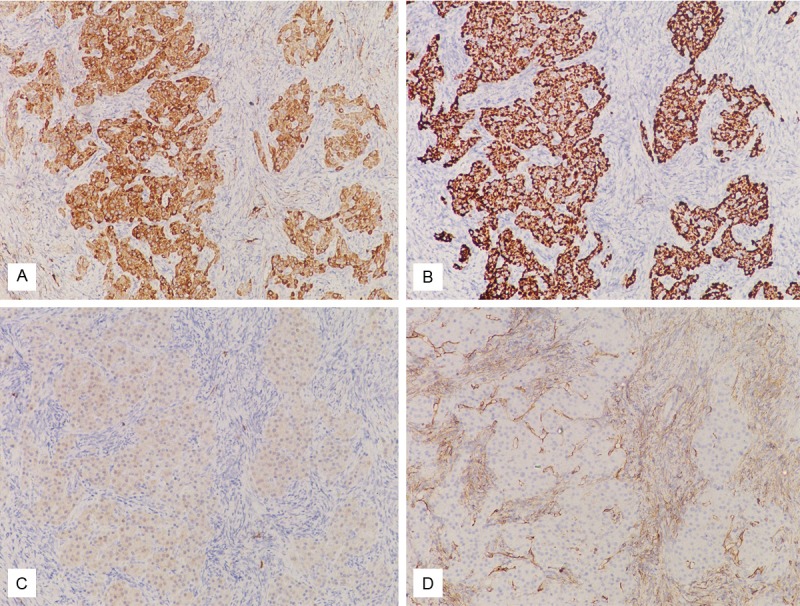
The hilus cells were immunohistochemically stained with inhibin A (A), Melan-A (B) and weakly stained with calretinin (C), but not stained with CD99 (D).
Discussion
It may be a diagnostic challenge to discriminate between hilus cell heterotopia and ectopic adrenal rests. Ectopic adrenal rests usually occur in the mesovarium or broad ligament, but they may be found in the fallopian tube occasionally. Ectopic adrenal nests usually grow in an organoid architecture, similar to a normal adrenal gland. The groups of ectopic hilus cells are usually smaller than the ectopic adrenal nests. Hilus cells are ovarian counterpart of testicular Leydig cells, having a similar immunoprofile to Leydig cells. The hilus cells are positive for inhibin, Melan-A, calretinin and vimentin. Although adrenal nest cells may have similar immunoprofile to hilus cells in these four markers, they also show immunoreactivity for neuroendocrine markers [6].
The relationship between hilus cell heterotopia and other specific pathologic process in the gynecologic tract is a mystery. In all cases of hilus cell heterotopia reported earlier, most of the cases were associated with other gynecologic pathologic process, such as ovarian endometriosis, mature cystic teratoma, mucinous ovarian tumor, primary peritoneal carcinoma, salpingitis isthmica nodosa and endometrial carcinoma [1,5,7,8]. In this report, we described a case of ectopic hilus cells in the fallopian tube with concurrent bilateral ovarian serous cystadenomas.
The origin of hilus cells in the fallopian tube or other ectopic site is still unknown. It was hypothesized that ovarian hilus cells originated from undifferentiated ovarian mesenchyme, non myelinated nerve or perineural fibroblast. Lewis suggested that during embryologic development some ovarian mesenchymal cells might migrate into the müllerian ducts [3]. In a case series published by Lynn Hirschowitz et al, two cases were associated with hilus cell hyperplasia [5]. In 1949, Sternberg suggested that increased gonadotrophin levels had a role in not only the development but also hyperplasia of hilus cell [4]. Lynn Hirschowitz et al also found concurrent hilus cell heterotopia and stromal luteinization in 3 cases, indicating that the same stimulus (probably hormonal effect) that cause hyperplasia of hilus cells may also cause nearby fibroblasts or undifferentiated cells to differentiate into hilus cells [5].
Hilus cells are steroid hormone-producing cells, although to what extent hilus cells contribute to the steroid hormone pool is unknown. In vitro incubation studies indicate that the major product of hilus cells is the androstenedione, but small amounts of estradiol and progesterone are also produced [9]. It is difficult to carry out hormonal studies preoperatively since hilus cell heterotopia is always an incidental finding during the pathological examination. When the endometrial tissue is available, we may obtain some information from the change in the endometrium. In our case, the endometrium was in the proliferative phase without any other abnormality. Indeed, more studies are needed to further clarify the role of hilus cell heterotopia in hormonal effect and its association with other pelvic pathology.
In summary, we reported a case of hilus cell heterotopia in the fallopian tube with simultaneous bilateral ovarian serous cystadenomas. It is important to differentiate between hilus cell heterotopia and ectopic adrenal rest. Immunohistochemical study may be a useful tool to differentiate the extra-ovarian hilus cells from their mimics. The origin, pathogenesis and association with other underlying pelvic pathology are still not well elucidated.
References
- 1.Palomaki JF, Blair OM. Hilus Cell Rest of the Fallopian Tube. A Case Report. Obstet Gynecol. 1971;37:60–62. [PubMed] [Google Scholar]
- 2.Clement PB. Histology of the ovary. In: Sternberg SS, editor. Histology for Pathologists. Philadelphia: Lippincott-Raven; 1997. pp. 929–959. [Google Scholar]
- 3.Lewis JD. Hilus-Cell Hyperplasia of Ovaries and Tubes; Report of a Case. Obstet Gynecol. 1964;24:728–731. [PubMed] [Google Scholar]
- 4.Sternberg WH. The Morphology, Androgenic Function, Hyperlasia, and Tumors of the Human Ovarian Hilus Cells. Am J Pathol. 1949;25:493–521. [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschowitz L, Salmons N, Ganesan R. Ovarian hilus cell heterotopia. Int J Gynecol Pathol. 2011;30:46–52. doi: 10.1097/PGP.0b013e3181eaa1ff. [DOI] [PubMed] [Google Scholar]
- 6.Zhang PJ, Genega EM, Tomaszewski JE, Pasha TL, LiVolsi VA. The role of calretinin, inhibin, melan-A, BCL-2, and C-kit in differentiating adrenal cortical and medullary tumors: an immunohistochemical study. Mod Pathol. 2003;16:591–597. doi: 10.1097/01.MP.0000073134.60541.E8. [DOI] [PubMed] [Google Scholar]
- 7.Honore LH, Ohara KE. Ovarian hilus cell heterotopia. Obstet Gynecol. 1979;53:461–464. [PubMed] [Google Scholar]
- 8.Usubütün A. Hilus cells in tuba uterina; an uncommon localisation and coincidence with endometrial carcinoma: A case report and review of the literature. Turkish Journal of Pathology. 2007;23:52–55. [Google Scholar]
- 9.Clement PB. Anatomy and Histology of the Ovary. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. New York: Springer-Verlag; 2002. pp. 649–673. [Google Scholar]


