Multiple myeloma (MM) is predominantly composed of well-differentiated neoplastic plasma cells morphologically resemble non-neoplastic plasma cells. However, some rare cytological variants of MM, such as polymorphous, pleomorphic, plasmablastic, signet-ring cell, small-cell, histiocytoid cell, clear cell, and spindle cell variants, have been reported [1]. Plasmablastic myeloma is characterized by the presence of large-sized neoplastic plasma cells containing large hyperchromatic nuclei, single or multiple prominent centrally-located nucleoli, moderate rim of basophilic cytoplasm, high nuclear/cytoplasmic ratio, faint perinuclear hof, and increased number of mitoses [1,2]. This variant accounts for approximately 10% of all MM and shows an aggressive clinical course [3].
IgD MM is the rarest subtype of MM, accounting for less than 2% of all myeloma cases [4]. This variant also shows an aggressive clinical behavior and is associated with a relatively high rate of renal failure, extra-osseous lesion, hypercalcemia, and resistance to chemotherapy [4-8].
Only a few cytological reports of plasmablastic myeloma have been documented [9-12]. Herein, we describe an additional case of IgD plasmablastic myeloma with emphasis on the cytological features.
A 62-year-old Japanese male, who had been diagnosed with arteriovenous malformation of the brain at 44 years of age, presented with a painless nodule on the left side of his back. He experienced night sweating and weight loss of 2 kg body in the past 6 months, although fever was not present. Computed tomography revealed a relatively well-circumscribed tumorous lesion located between the left 10th to 11th costal bones, which had directly invaded into the parietal pleura, thoracic wall, and subcutaneous tissue. The tumorous lesion was also present between the right 8th to 9th costal bones and had also invaded into the thoracic wall. Positron emission tomography demonstrated accumulation in the bilateral humerus, scapula, costal, iliac, sacral, and pubic bones. Laboratory tests revealed an elevated soluble interleukin-2 receptor (red blood cells 4.96 x 1012/L (range 4.1-5.2), hemoglobin 14.7 g/L (range 13.4-17.0), white blood cells 7.3 x 109/L (3.0-8.0), platelets 237 x 109/L (150-400), lactate dehydrogenase 304 U/L (119-229), and soluble interleukin-2 receptor 1,220 U/mL (135-483)). Serum IgD level was markedly elevated (893 mg/dL (range <12.6)), however IgG, IgM, and IgA levels were within normal ranges (IgG 1,016 mg/dL (range 870-1,700), IgM 41 mg/dL (35-220), and IgA 175 mg/dL (110-410)). Immunofixation electrophoresis demonstrated the presence of a small amount of M-protein (IgD, lambda-type). Bence-Jones protein was negative.
He underwent a biopsy of the tumorous lesion located in the subcutaneous tissue adjacent to the left costal bone, and touch smear of the biopsy specimen was also performed. Bone marrow aspiration was performed as well.
Cytological analysis of the touch smear specimen demonstrated the presence of large dyscohesive polymorphous cells (Figure 1A, 1B). Most of these cells had large round to oval nuclei (the nuclear size of most of these cells was approximately four- to five-folds bigger than that of small lymphocytes), and the nuclear size of the largest cells was more than seven-folds bigger than that of small lymphocytes (Figure 1A, 1B). The nuclei had coarse granular chromatin with single or multiple conspicuous nucleoli (Figure 1A, 1B). The cytoplasm of these cells was variable in amount, however, some of these cells had relatively rich dark blue cytoplasm with poorly defined hof and eccentrically-located nuclei, and resembled plasma cells (Figure 1A, 1B). Mitotic figures were scattered (Figure 1B). Neither multinucleated cells nor intranuclear inclusion bodies were observed. Accordingly, a cytodiagnosis of plasmablastic myeloma was made.
Figure 1.
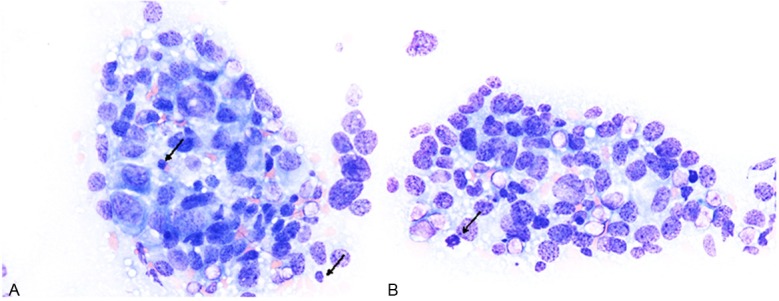
Cytological features of the touch smear specimen of the tumor. (A, B) Large-sized polymorphous cells (size of the nucleus is four- to five-folds bigger than that of small lymphocytes (A, arrows). These cells have large round to oval nuclei with coarse chromatin and nucleoli. Some of these cells have eccentrically-located nuclei and rich cytoplasm, and resemble plasma cells. Mitotic figure is noted (B, arrow). Giemsa stain, x 400.
Histopathological study of the biopsy specimen revealed diffuse proliferation of neoplastic cells containing rich slightly eosinophilic cytoplasm and large round to oval nuclei with single or multiple conspicuous nucleoli (Figure 2). Some of these neoplastic cells had large eccentrically-located nuclei and poorly defined perinuclear hof, and resembled plasma cells (Figure 2, arrows). The size of the nuclei was variable, and the nuclear size of the largest neoplastic cells was approximately three- to four-folds than that of the small ones. Mitotic figures were scattered (15/10 high power fields).
Figure 2.
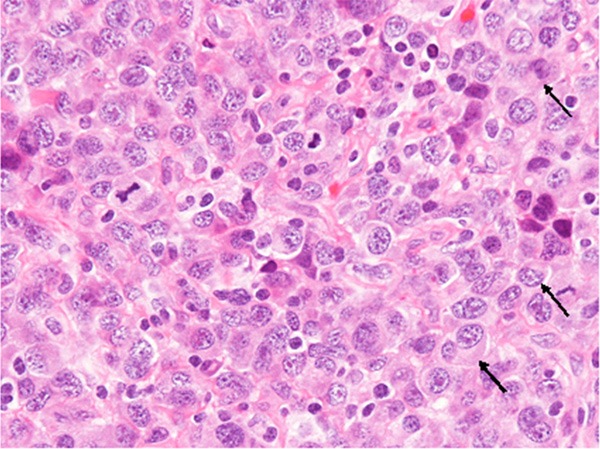
Histopathological features of the tumor. Proliferation of large-sized neoplastic cells with large round to oval nuclei containing conspicuous nucleoli. The tumor cells have relatively rich eosinophilic cytoplasm, and some of them have eccentrically-located nuclei and poorly defined perinuclear hof (arrows). Mitotic figures are scattered. HE, x 400.
Immunohistochemical and in situ hybridization studies were performed using an autostainer (Ventana) by the same method as previously reported [13-17]. The neoplastic cells were diffusely positive for CD38 (Figure 3A) and MUM-1 (Figure 3B), but negative for CD3, CD10, CD15, CD19, CD20, CD30, CD79a, CD138, bcl-2, bcl-6, and ALK. Some of the neoplastic cells showed weak positive immunoreactivity for cyclin D1. IgD was diffusely positive in the cytoplasm of the tumor cells (Figure 3C), but IgG, IgM, and IgA were negative. In situ hybridization analyses clearly demonstrated that all neoplastic cells had lambda-chain, but no kappa-chain-positive neoplastic cells were observed (Figure 3D). Moreover, no EBER-positive cells were present.
Figure 3.
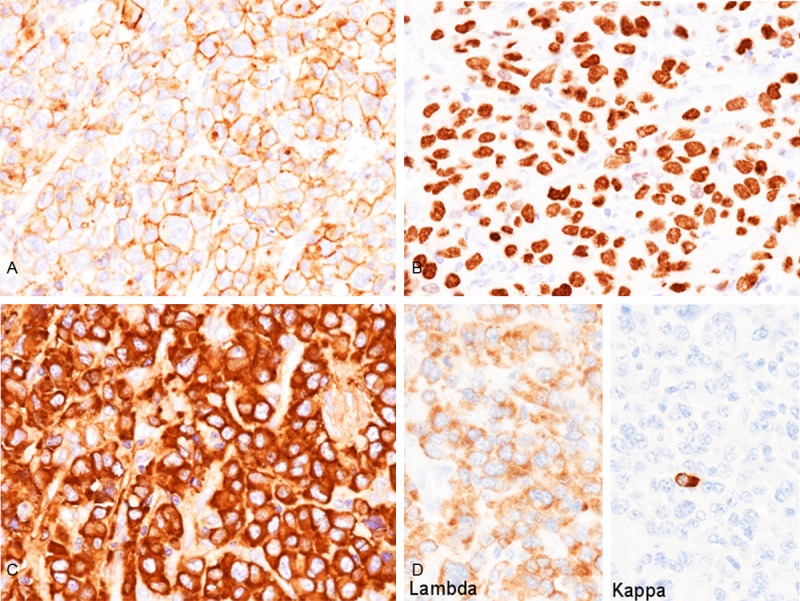
Immunohistochemical and in situ hybridization findings of the tumor. A. CD38 is diffusely expressed in the cell membrane of the tumor cells, x 400. B. MUM-1 is diffusely expressed in the nuclei of the tumor cells, x 400. C. IgD is diffusely expressed in the cytoplasm of the tumor cells, x 400. D. Lambda-chain is diffusely expressed, but kappa-chain is absent (note that non-neoplastic plasma cells are kappa-chain positive), x 400.
The bone marrow aspiration specimen revealed that the proliferating neoplastic cells shared the same histopathological features as those in the biopsy specimen of the tumorous lesion adjacent to the costal bone. Immunohistochemical and in situ hybridization features of the bone marrow were also identical to those of the biopsy specimen.
Accordingly, an ultimate diagnosis of IgD plasmablastic myeloma was made.
Bartl et al. analyzed the cell size, cytoplasmic structure, and nuclear configuration of the neoplastic cells of 674 cases of MM, and classified them into six subtypes, namely Marschalko, small cell, cleaved, polymorphous, asynchronous, and blastic [2]. Marschalko type is the most common subtype (comprising 59% in their series) and is characterized by the presence of neoplastic cells that are morphologically indistinguishable from non-neoplastic plasma cells. Blastic type is the rarest subtype, accounting for 2% of their series, and is characterized by the presence of neoplastic plasmablasts with large nuclei, prominent centrally-located (immunoblast-like) nucleoli, moderate rim of basophilic cytoplasm, and faint perinuclear hof. Sixty percent of the blastic type had IgG type M protein, 20% contained IgA type, and the remaining 20% with light chain only, while no IgD type was present in their series [2]. This subtype showed the most aggressive clinical course among all subtypes [2]. The histopathological features of the present case corresponded to the blastic type of MM according to the classification by Bartl et al [2].
Only a small number of cytological reports on plasmablastic myeloma have been documented [9-12]. The characteristic cytological features of this variant of MM are as follows: i) discohesive polymorphous population ranging from intermediate- to very large-sized neoplastic cells; ii) most of the tumor cells have large centrally-located nuclei with coarse chromatin and nucleoli; iii) some of the neoplastic cells have large eccentrically-located nuclei and poorly defined perinuclear hof, and resemble plasma cells; iv) mitotic figures and apoptotic bodies are scattered; and v) binucleated cells are frequently observed [9]. Although binucleated cells were not observed in the present case, the other above-mentioned cytological features were present. While the presence of neoplastic cells with large eccentrically-located nuclei and poorly differentiated perinuclear hof that resemble plasma cells is an important and characteristic feature of plasmablastic myeloma, this feature is also observed in ALK-positive large B-cell lymphoma and plasmablastic lymphoma [9].
Differential diagnostic considerations of plasmablastic myeloma include lymphoid neoplasms with plasmablastic, immunoblastic, or plasmacytoid features, such as diffuse large B-cell lymphoma (DLBCL), ALK-positive large B-cell lymphoma, and plasmablastic lymphoma, because histopathologically and cytologically the neoplastic cells of plasmablastic myeloma show blastic features, and typical plasmacytic differentiation is usually scant [9]. DLBCL typically shows positive immunoreactivity for CD20 and CD79a, and may or may not express CD138, while ALK-positive large B-cell lymphoma can lack expression of CD20, CD79a, and CD138, although most cases are positive for cytoplasmic IgA [9,17]. Neoplastic cells in the latter may also have pseudopodial cytoplasmic projections such that they resemble “flaming plasma cells”, which has been recognized as the characteristic finding of IgA myeloma [17]. Plasmablastic lymphoma is usually associated with HIV, and most cases have EBV-infected neoplastic cells. Further, the neoplastic cells of plasmablastic lymphoma show plasma cell phenotype (CD38+, CD138+, CD20-). This type of malignant lymphoma is classified as a variant of DLBCL. However, it has been recognized that plasmablastic lymphoma shares many cytomorphological and immunophenotypical features of plasmablastic myeloma. The only significant difference is the presence of EBV, which is positive in plasmablastic lymphoma, but negative in plasmablastic myeloma [18]. Differential diagnosis may be difficult if only the cytomorphological and/or histopathological features were analyzed, therefore, detailed immunohistochemical analyses, in situ hybridization for EBER, and clinical information are needed for correct diagnosis. Moreover, recognition of scant plasmacytic differentiation, such as a few neoplastic cells showing perinuclear hof and eccentrically-located nuclei, is a key for cytological diagnosis of plasmablastic myeloma.
Disclosure of conflict of interest
None.
References
- 1.Banerjee SS, Verma S, Shanks JH. Morphological variants of plasma cell tumours. Histopathology. 2004;44:2–8. doi: 10.1111/j.1365-2559.2004.01763.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–355. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- 3.Greipp PR, Raymond NM, Kyle RA, O’Fallon WM. Multiple myeloma: significance of plasmablastic subtype in morphological classification. Blood. 1985;65:305–310. [PubMed] [Google Scholar]
- 4.Pisani F, Petrucci MT, Giannarelli D, Bongarzoni V, Montanaro M, De Stefano V, La Verde G, Gentilini F, Levi A, Za T, Moscetti A, Annino L, Petti MC Multiple Myeloma GIMEMA-Latium Region Working Group, Italy. IgD multiple myeloma a descriptive report of 17 cases: survival and response to therapy. J Exp Clin Cancer Res. 2012;31:17. doi: 10.1186/1756-9966-31-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimamoto Y, Anami Y, Yamaguchi M. A new risk grouping for IgD myeloma based on analysis of 165 Japanese patients. Eur J Haematol. 1991;47:262–267. doi: 10.1111/j.1600-0609.1991.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair D. IgD myeloma: clinical, biological and laboratory features. Clin Lab. 2002;48:617–622. [PubMed] [Google Scholar]
- 7.Blade J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J. Clin. Oncol. 1994;12:2398–2404. doi: 10.1200/JCO.1994.12.11.2398. [DOI] [PubMed] [Google Scholar]
- 8.Fibbe WE, Jansen J. Prognostic factors in IgD myeloma: a study of 21 cases. Scand J Haematol. 1984;33:471–475. doi: 10.1111/j.1600-0609.1984.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 9.Mettler TN, Holman CJ, McKenna RW, Pambuccian SE. Plasmablastic myeloma in ascitic fluid. Diagn Cytopathol. 2011;40:806–809. doi: 10.1002/dc.21759. [DOI] [PubMed] [Google Scholar]
- 10.Chang H, Chou WC, Lee SY, Huang JY, Hung YH. Myelomatous pleural effusion in a patient with plasmablastic myeloma: A case report. Diagn Cytopathol. 2009;37:205–207. doi: 10.1002/dc.21004. [DOI] [PubMed] [Google Scholar]
- 11.Khayyata S, Bentley G, Fregene TA, Al-Abbadi M. Retroperitoneal extramedullary anaplastic plasmacytoma masquerading as sarcoma: Report of a case with an unusual presentation and imprint smears. Acta Cytol. 2007;51:434–436. doi: 10.1159/000325761. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JM, Krishnamurthy S. Fine-needle aspiration cytology of a case of HIV-associated anaplastic myeloma. Diagn Cytopathol. 2002;27:218–222. doi: 10.1002/dc.10174. [DOI] [PubMed] [Google Scholar]
- 13.Ishida M, Fukami T, Nitta N, Iwai M, Yoshida K, Kagotani A, Nozaki K, Okabe H. Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2242–2246. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida M, Kodama N, Takemura Y, Iwai M, Yoshida K, Kagotani A, Matsusue Y, Okabe H. Primary bone carcinosarcoma of the fibula with chondrosarcoma and squamous cell carcinoma components. Int J Clin Exp Pathol. 2013;6:2216–2223. [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida M, Igarashi T, Teramoto K, Hanaoka J, Iwai M, Yoshida K, Kagotani A, Tezuka N, Okabe H. Mucinous brobchioloalveolar carcinoma with K-ras mutation arising in type 1 congenital cystic adenomatous malformation: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2597–2602. [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 18.Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, Lau CC, Finch CJ, Vilchez RA, McGregor D, Jorgensen JL. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18:806–815. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]


