Abstract
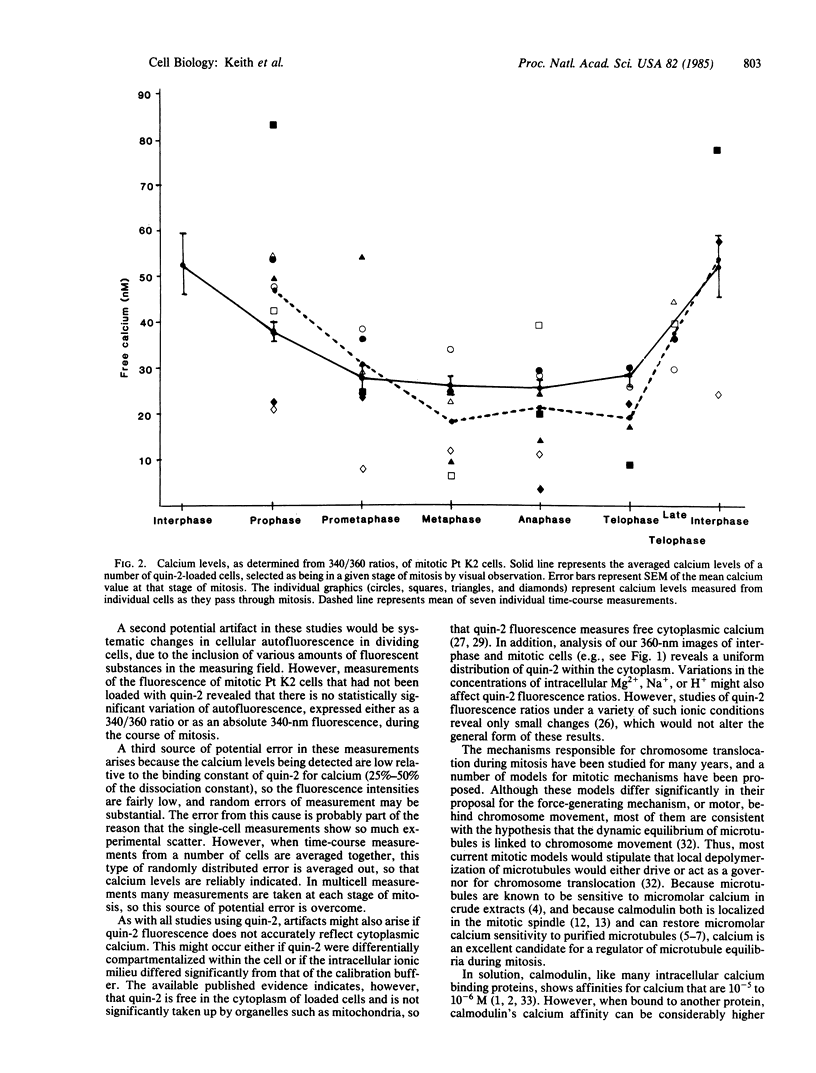
Using a fluorescence ratio method, we have studied the intracellular free calcium levels in individual quin-2-loaded mitotic cells under the microscope. We have found that intracellular free calcium concentrations in Pt K2 epithelial cells drop by approximately 50% as they pass through mitosis. Calcium levels in interphase cells were 53 +/- 7 nM. During prophase, free cytoplasmic calcium begins to decrease, reaching 28 +/- 3 nM in prometaphase. Calcium levels remain low until the nuclear envelope is re-formed in late telophase, when they increase again to interphase levels. This decrease in overall free calcium in mitosis suggests that the mitotic cell has mechanisms for the general sequestration, and perhaps local release, of calcium ions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E. Ca2+ transport and cell activation. Med Biol. 1982 Aug;60(4):168–182. [PubMed] [Google Scholar]
- Chai L. S., Sandberg A. A. Effect of divalent cations and chelators on metaphase to telophase progression and nuclear envelope formation in Chinese hamster cells. Cell Calcium. 1983 Oct;4(4):237–252. doi: 10.1016/0143-4160(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Cohen P., Klee C. B., Picton C., Shenolikar S. Calcium control of muscle phosphorylase kinase through the combined action of calmodulin and troponin. Ann N Y Acad Sci. 1980;356:151–161. doi: 10.1111/j.1749-6632.1980.tb29608.x. [DOI] [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K. Membranes in the mitotic apparatus of barley cells. J Cell Biol. 1980 Aug;86(2):490–499. doi: 10.1083/jcb.86.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G. The role of calcium ions during mitosis. Calcium participates in the anaphase trigger. Chromosoma. 1983;88(1):1–10. doi: 10.1007/BF00329497. [DOI] [PubMed] [Google Scholar]
- Jackson W. T., Doyle B. G. Membrane distribution in dividing endosperm cells of Haemanthus. J Cell Biol. 1982 Sep;94(3):637–643. doi: 10.1083/jcb.94.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984 Jan;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C., DiPaola M., Maxfield F. R., Shelanski M. L. Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol. 1983 Dec;97(6):1918–1924. doi: 10.1083/jcb.97.6.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Jemiolo D. K., Burgess W. H., Rebhun L. I. Strongylocentrotus purpuratus spindle tubulin. II. Characteristics of its sensitivity to Ca++ and the effects of calmodulin isolated from bovine brain and S. purpuratus eggs. J Cell Biol. 1982 Jun;93(3):797–803. doi: 10.1083/jcb.93.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P. Studies on the in vivo sensitivity of spindle microtubules to calcium ions and evidence for a vesicular calcium-sequestering system. J Cell Biol. 1981 Mar;88(3):604–617. doi: 10.1083/jcb.88.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. The informational role of calcium in the cytosol. Adv Cyclic Nucleotide Res. 1979;11:1–26. [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Petzelt C., Williams R. O., Meza I. A Ca-activated ATPase in the mitotic apparatus of the sea urchin egg (isolated by a new method). Exp Cell Res. 1972 Feb;70(2):325–332. doi: 10.1016/0014-4827(72)90143-7. [DOI] [PubMed] [Google Scholar]
- Nishida E., Sakai H. Calcium-sensitivity of the microtubule reassembly system. Difference between crude brain extract and purified microtubular proteins. J Biochem. 1977 Jul;82(1):303–306. doi: 10.1093/oxfordjournals.jbchem.a131685. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Beaven M. A., Metcalfe J. C., Johnson P., Garland P. B. Intracellular pH and free calcium changes in single cells using quene 1 and quin 2 probes and fluorescence microscopy. FEBS Lett. 1983 Sep 5;161(1):21–27. doi: 10.1016/0014-5793(83)80722-4. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Metcalfe J. C. Intracellular pH of stimulated thymocytes measured with a new fluorescent indicator. J Biol Chem. 1983 May 25;258(10):5994–5997. [PubMed] [Google Scholar]
- Salmon E. D., Segall R. R. Calcium-labile mitotic spindles isolated from sea urchin eggs (Lytechinus variegatus). J Cell Biol. 1980 Aug;86(2):355–365. doi: 10.1083/jcb.86.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Schatten H., Simerly C. Detection of sequestered calcium during mitosis in mammalian cell cultures and in mitotic apparatus isolated from sea urchin zygotes. Cell Biol Int Rep. 1982 Aug;6(8):717–724. doi: 10.1016/0309-1651(82)90163-1. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Cole R. D., Cande W. Z. Isolation of mitotic apparatus containing vesicles with calcium sequestration activity. Cell. 1980 Feb;19(2):505–516. doi: 10.1016/0092-8674(80)90525-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Ionic changes in the mitotic apparatus at the metaphase/anaphase transition. J Cell Biol. 1983 Mar;96(3):598–605. doi: 10.1083/jcb.96.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavortink M., Welsh M. J., McIntosh J. R. The distribution of calmodulin in living mitotic cells. Exp Cell Res. 1983 Dec;149(2):375–385. doi: 10.1016/0014-4827(83)90350-6. [DOI] [PubMed] [Google Scholar]