Abstract
Pulmonary enteric adenocarcinoma (PEAC), a extremely rare variant of primary invasive adenocarcinoma of the lung, was recognized by the international multidisciplinary classification of lung adenocarcinoma which was proposed by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) published in early 2011. Histologically, PEAC is considered to be mainly composed of tall columnar cells arranged in an irregular glandular cavity or cribriform pattern with extensive central necrosis which show high resemblance to that of intestinal epithelia and colorectal carcinomas. Immunohistochemically, PEAC can not only expresses typical proteins common to lung primaries but is positive for at least one intestinal markers, such as CDX2, cytokeratin (CK) 20, MUC2, therefore, the differentiation of primary PEACs from metastatic colorectal cancers can be challenging. In this study, we report 9 cases of PEAC and a panel of immunohistochemical protein markers of CK7, CK20, thyroid transcription factor 1 (TTF-1), Napsin A, MUC2 and villin was analyzed with the comparison of 20 metastatic colorectal carcinomas (MCRs), and 20 typical primary adenocarcinomas (tPACs). As was expected, CK7 expression was documented in all 9 PEACs and 20 tPCAs while CK20 was significantly more prevalent in adenocarcinoma that originated from colorectal. Additionally, we evaluate the classical mutations of EGFR, KRAS in the 9 cases of PEACs, it turned out that all tumors were EGFR-wild and KRAS-wild types, which confirmed that PEAC has a separate phenotype from usual pulmonary adenocarcinoma.
Keywords: Pulmonary adenocarcinoma, enteric, clinicopathologic features, immunohistochemistry, EGFR/KRAS mutations
Introduction
Primary adenocarcinoma of the lung is morphologically heterogeneous, showing a wide variety of histopathologic patterns including acinar, papillary, micropapillary, lepidic components or solid growth patterns with mucin-producing elements, among which the structures of acinar and papillary are the most common. Most cases share two or three even more of these growth patterns. Pulmonary enteric adenocarcinoma (PEAC) is a special type of primary adenocarcinoma which share some identical components with colorectal carcinoma. Due to its uncommon histopathologic feature, PEAC is barely recognized by most pathologists and most of the knowledge regarded is from case reports or small case series. To date, a total of fewer than 30 cases have been reported in the English literature [1-6]. Few documents may be the reason why it has not yet been separated in the current World Health Organization (WHO) classification of lung cancer.
However, a significant change occurred in 2011 with the publication of a new lung adenocarcinoma classification with the support from an international multidisciplinary team of experts assembled by The International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) [7]. In this most current classification, for the first time, PEAC, together with invasive mucinous adenocarcinoma (formerly mucinous bronchioloalveolar carcinoma), colloid adenocarcinoma (retained and expanded) and fetal adenocarcinoma (retained), is recognized as a rare variant of the pulmonary adenocarcinoma. It was defined as primary pulmonary adenocarcinoma with a predominant colorectal-like components (>50%) and the tumor cells must be positive for at least one immunohistochemical marker of enteric differentiation, such as CK20, CDX2, and mucin2 (MUC2). If the tumor cells are negative for any intestinal protein markers, it should be termed as “lung adenocarcinoma with enteric morphology” rather than as enteric carcinoma of the lung.
In the present study, nine cases of PEACs, characterized by the clinicopathologic features which similar to that of metastatic colorectal carcinomas (MCRs) were reported. We aimed to further explore the most likely multi-marker combinations of PEAC to increase accuracy in the diagnosis. Moreover, we analyzed EGFR, KRAS mutations in these rare tumors trying to figure out its molecular biologic changes, and to our best knowledge, this is the first analysis of molecular alteration in this kind of rare neoplasm, and literatures detailing these unusual neoplasms are also reviewed.
Materials and methods
Patients
A consecutive series of 400 patients who underwent resections for pulmonary adenocarcinoma during 2005 to 2012 diagnosed at the Department of Pathology of Nanjing Jingling Hospital (Nanjing, Jiangsu, China) were used for the present study, after obtaining the approval of patient’s written informed consent. To distinguish metastatic from primary adenocarcinoma of the lung, all patients in our study were routinely examined by abdominal computed tomography, video capsule endoscopy or other physical examinations, and further analysis was performed when abnormal findings were obtained. On the other hand, 20 samples from patients with lung metastasis of colorectal cancer and 20 from patients with primary lung adenocarcinoma were used as controls.
Each sample was histologically assessed by three experienced pathologists according to the classification criteria followed by WHO guidelines and pathological staging was determined based on the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual [8].
Immunohistochemistry
All tissues were fixed in 10% buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). For immunohistochemical staining, paraffin-embedded blocks were cut at a thickness of 4 µm, heated at 60°C for 1 hour, and then deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol and blocked using methanolic 3% hydrogen peroxide for 10 min. Each tumor section was subjected to antigen retrieval performed in citrate buffer (sodium citrate, pH 6.0) for 30 minutes. All tumor sections were incubated with antibodies specific to CK7 (OV-TL12/30; Zymed), CK20 (PW31; Novocastra, Newcastle, UK), CDX2 (AMT28; Novocastra, Newcastle, UK), TTF-1 (SpT24; Novocastra, Newcastle, UK), Napsin A (rabbit polyclonal; IBL, Gunma, Japan), MUC2 (Ccp58; Santa Cruz Biotechnology, UK), villin (CWWB1; Neomarkers, USA) at dilutions of 1:300, 1:100, 1:50, 1:200, 1:200, 1:300 and 1:100, respectively, for 12 h at 4°C; and they were then stained by avidin-biotin complex method using a Vcctastain Elite ABC kit (Vcctor Laboratories, Burlingame, CA). As clear cells were focally observed in one case, we also test the protein expression of EML4-ALK (D5F3; Ventana, USA). For the molecular analysis, MSH and MLH were studied as well. Appropriate positive and negative control samples were also used and the detection was performed with Iview DAB detection kit (Vantana, Tucson, AZ).
Tumors were considered as positive if staining was found more than 10% of neoplastic cells and negative if present fewer than 10%. Focal positivity will be classified if the positive tumor cells are less than 50% and diffuse positive while staining present in more than 50%. Cytoplasmic and/or membranous CK7 and CK20 were scored as the number of tumor cells. The results of immunostaining with Napsin A, villin were based on cytoplasmic staining of tumor cells whereas nuclear staining was used for CDX2 and TTF-1. EML4-ALK was considered positive if they were stained in cytoplasm and/or membrane.
DNA extraction and PCR amplification
Formalin-fixed, paraffin-embedded tumor sections, with area of tumor cells marked by comparison with the corresponding HE-stained slide, were deparaffinized and air dried. Genomic DNA was extracted and purified using standard Proteinase K digestion and a DNeasy minispin column (TIANamp Genomic DNAKit, Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. The amount of DNA obtained was quantified by spectrophotometry (Eppendorf, Hamburg, Germany). Extracted DNA was stored in -20°C until use. Real-time quantitative PCR amplification were performed in 50 µl volumes using 2 µl of the solution of the extracted DNA and 48 µl of a reaction mixture that contained 2 µM primers, 4 µM dNTPs mix, 1.5 mM MgCl2, PCR buffer source, and 0.25 U of Taq DNA polymerase (AmpliTaq Gold; Applied Biosystems, Foster City, CA, USA). Amplification was performed in a thermal cycler (Gene Amp PCR system 9700, Applied Biosystems) according to the manufacturer’s instruction. Wild type and no template samples were added in each assay as positive and negative controls.
EGFR and KRAS mutation detection
The presence of EGFR mutations which were frequently harbored in exons 18, 19, 20 and 21 were detected in the purified PCR products by direct sequencing using BigDyeTM Terminator Cycle Ready Reaction Mix (Applied Biosystems, Foster City, CA, USA). Data analysis was performed with an ABI PRISM 310 Genetic Analyser (Applied Biosystems, Foster City, CA, USA) using primers described by Paez et al. [9]. As KRAS point mutations in codon 12 and 13 on 1 exon and 61 codon were involved in a great majority of activating KRAS mutations, specific PCR primers were designed surrounding these codon. The sequences was amplified from the extracted DNA template and then the product were sequenced. Amplification of wide-type DNA in KRAS codon 12/13 and codon 61 were also used for comparison. The design of sequences of the TaqMan probes was kindly provided by Pr Laurent-Puig [10].
Results
Clinical features
Most patients were elderly with the age ranged from 34 to 74 years (Mean age: 60 years) and in general, cases of males slightly outnumber those in females with the male/female ratio of 4:5. Of the 9 patients with PEAC in this study, 6 were heavy cigarette smokers with average pack-years of 20 and none had a history of exposure to wood dust. Three patients presented with symptoms of nonproductive cough and chest pain, two with fever, and two with hemoptysis, whereas one with no significant symptoms is perceived in daily activities were discovered on routine medical examination. As spiculated masses can only be depicted in the lobe lesion by a computed tomographic (CT) scan, fluorine 18-labeled fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan or colonoscopy before surgical excision, all patients in our study underwent an attentive laboratory and iconography examinations, and none of them showed any clinical evidence of any malignancy other sites or gastrointestinal tumors. Interestingly, eight of tumors were localized to right lobe, including seven to right upper lobe, one to right lower lobe and one to left lower lobe, at the time of diagnosis. Lobectomies were underwent in five cases, pneumonectomies were performed in two cases and segmental resection in the remaining two cases. Of these patients who went directly to surgical intervention, 2 (22.2%) were confirmed as pathologic T2N0, 3 (33.3%) were downstaged, and 4 (44.4%) were upstaged at the operation. Distant metastasis in our study were all absent in the diagnosis.
Clinical follow-up information was obtained over a period of 6 to 43 months, with a average period time of 23.9 months for all patients. Among the 9 cases discussed, 2 died as a result of recurrent and 1 died for the metastasis, and 6 were still alive and well at the last follow-up visit. The clinical findings were briefly summarized in Table 1. Additionally, no evidence of primary or metastatic gastrointestinal tumors were identified by gastroscopy, colonoscopy, video capsule endoscopy or PET-CT scan during their follow-up period after surgery in all patients, confirming that tumors were all initially localized.
Table 1.
Clinical Data
| NO. | Age/Sex | Location | Surgery | Smoking | p-Stage | Follow-up (M) |
|---|---|---|---|---|---|---|
| 1 | 65/M | RLL | L | No | IIA (T1N1M0) | D (36) |
| 2 | 56/F | RUL | L | No | IA (T1N0M0) | A (27) |
| 3 | 60/M | RUL | S | Yes | IB (T2N0M0) | A (39) |
| 4 | 63/F | RUL | L | Yes | IA (T1N0M0) | A (43) |
| 5 | 65/F | RUL | P | Yes | IIB (T2N1M0) | D (12) |
| 6 | 74/M | LLL | L | Yes | IA (T1N0M0) | A (23) |
| 7 | 61/M | RUL | P | Yes | IIIB (T2N3M0) | D (7) |
| 8 | 34/F | RUL | L | No | IIB (T2N1M0) | A (22) |
| 9 | 63/F | RUL | S | Yes | IB (T2N0M0) | A (6) |
M, male; F, female; RLL, right lower lobe; RUL, right upper lobe; LLL, left lower lobe; L, lobectomy; S, segmentectomy; P, pneumonectomy; mo, month; D, died; A, alive.
Morphology
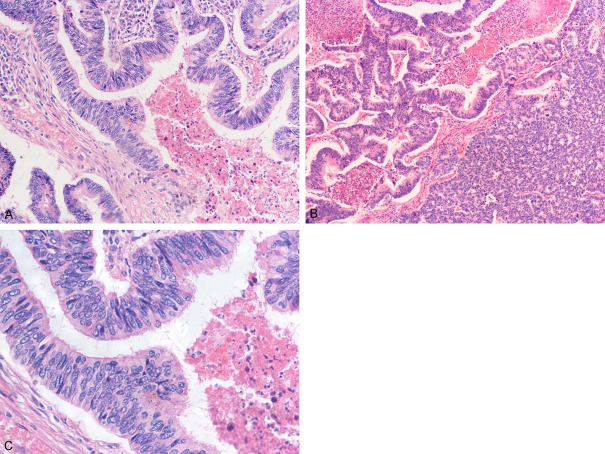
Grossly, the tumors ranged in size from 1.5 to 6.0 cm (average 32.3 cm) and on the cut surface, they were described as whitish or grey-white with some accompanied by focal areas of hemorrhage and necrosis. Histologically, all nine tumors consisted of prominent components of colorectal carcinoma-like glands varying range from 50% to 85%, mixed up with typical acinar or papillary structures (Table 2). All or less glands were medium-to-large sized, with lumina filled with extensive nuclear and cellular debris, resulting in an identical morphological feature of the so-called “dirty necrosis” (Figure 1A). Some of the tumor cells appeared in micropapillary architecture and in two cases, cribriform “gland in gland” patterns were arranged (Figure 1B). Solid growth pattern can barely observe in the present study. Therefore, they were all classified to be well or moderately differentiated and poorly differentiated adenocarcinoma can be excluded on the basis of the Japanese Lung Cancer Society criteria [11]. On higher magnification, the tumor cell population was composed of large-to-medium sized, cuboidal to tall columnar absorptive-like cells with abundant eosinophilic cytoplasm, stratified ovoid nuclei, prominent nucleoli, and occasionally brush borders. Nuclei arranged in a palisading pattern and the polarity of nuclei is well preserved (Figure 1C). All cases showed features of high mitotic activities and varied from 3 to 10 per 10 high-power fields (HPF). Focal goblet cells or signet ring cells were noted in one case, and abundant mucin production in the extracellular spaces of the tumor was observed in one instance. Inflammatory cells, such as lymphocytes and neutrocyte, were infiltrated in the mesenchyma of almost each tumor. 7 had areas of confluent necrosis, with 3 of obvious areas of hemorrhage, while 2 showed vascular invasion and 2 with visceral pleural invasion.
Table 2.
Pathological Features Studies
| NO. | Size (mm) | Necrosi/ Hemorrhages | Mitoses (10 HPF) | Mucinous change | Visceral pleural invasion | Vascular invasion | Histologic patterns (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CR-like | Acinar | Papillary | Micropapillary | Solid | |||||||
| 1 | 23 | +/+ | 6-7 | - | - | - | 50 | 20 | 15 | 15 | 0 |
| 2 | 30 | +/- | 4-5 | - | - | - | 70 | 20 | 10 | 0 | 0 |
| 3 | 35 | -/- | 8-10 | - | + | - | 60 | 30 | 0 | 0 | 10 |
| 4 | 27 | -/- | 3-4 | - | - | - | 55 | 30 | 5 | 0 | 0 |
| 5 | 20 | +/+ | 9-10 | + | + | + | 85 | 10 | 0 | 5 | 0 |
| 6 | 15 | +/- | 2-3 | - | - | - | 70 | 15 | 15 | 0 | 0 |
| 7 | 60 | +/+ | 7-8 | - | - | + | 65 | 15 | 10 | 10 | 0 |
| 8 | 48 | +/- | 4-5 | - | - | - | 80 | 20 | 0 | 0 | 0 |
| 9 | 33 | +/- | 3-4 | - | - | - | 55 | 25 | 20 | 0 | 0 |
HPF, high-power fields; CR-like, the colorectal carcinoma-like component.
Figure 1.
Histological features. A: Glands are medium-to-large sized, with lumina filled with extensive nuclear and cellular debris resulting in “dirty necrosis” (HE, ×200). B: Unlike MCRs, which are largely monotonous in histology, pulmonary enteric adenocarcinoma exhibits heterogeneous features, such as cribriform growth pattern (HE, ×100). C: Tall-columnar tumor cells with eosinophilic cytoplasm and tall or ovoid nuclei. Nuclei arrange in a palisading or pseudostratified pattern (HE, ×400).
Immunohistochemistry
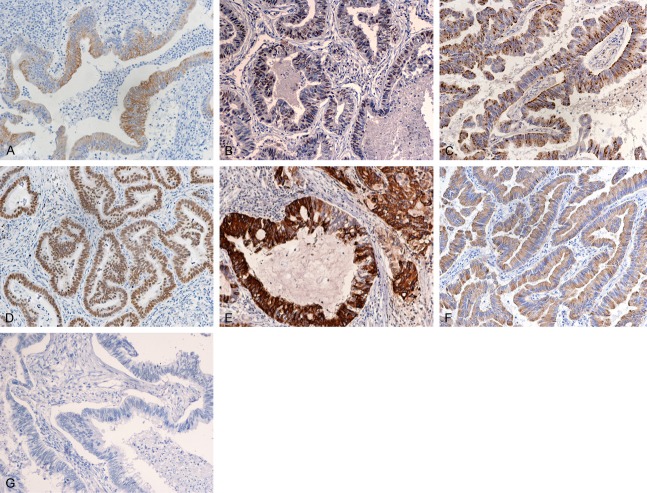
The immunohistochemical results of the 9 PEACs and the compared group of the 20 patients with metastatic colorectal adenocarcinoma as well as 20 primary classic lung adenocarcinomas are demonstrated in Table 3. We found that the PEACs showed positive staining for CK7, CK20, TTF-1, Napsin A, CDX2, MUC2 and villin in 9 (100.0%), 2 (22.2%), 4 (44.4%), 3 (33.3%), 6 (66.7%), 4 (44.4%), 6 (66.7%) of 9 cases, respectively (Figure 2A-G); MCCs in resected specimen were positive in 1 (5%), 16 (80%), 0 (0%), 2 (22.2%), 18 (90%), 10 50%), 17 (85%) of 20 cases, respectively and tPACs expressed were in 20 (100.0%), 1 (5.0%), 18 (90.0%), 17(85.0%), 0 (0.0%), 0 (0.0%), 2 (10%) of cases tested, respectively. All samples were EML4-ALK negative. Of the colonic markers (CK20, CDX2, MUC2 and villin) enrolled in the diagnosis of PEAC, the present study revealed that only one case (case 6) showed positive expression of all four, three cases (case 4, 8, 9) were positive for three markers, two (case 2, 3) were positively stained with two and three cases have only one colonic differential marker positively expressed. Among these PEACs, CDX2 was all strongly and diffusely stained, CK20 reaction was focally expressed in one of them, half of the MUC2 were partial and villin was evident in 5 cases.
Table 3.
Immunomarkers Profiles
| Case No. | CK7 | CK20 | TTF-1 | Napsin A | CDX2 | MUC2 | Villin |
|---|---|---|---|---|---|---|---|
| 1 | + | - | f+ | + | + | - | - |
| 2 | + | - | + | - | + | + | - |
| 3 | + | - | + | - | + | - | + |
| 4 | + | - | - | + | - | f+ | + |
| 5 | + | - | + | - | - | - | + |
| 6 | + | - | - | + | - | + | - |
| 7 | + | f+ | - | - | + | - | + |
| 8 | + | - | - | - | + | f+ | + |
| 9 | + | + | - | - | + | - | f+ |
f+, focal positive.
Figure 2.
Pulmonary enteric adenocarcinoma (PEAC) exhibit characteristic immunohistochemical features. A-F: It can show immunoreactivity for CK7 (A: Case 2), TTF-1 (B: Case 3), napsin A (C: Case 4), CDX2 (D: Case 8), MUC2 (E: Case 6) and villin (F: Case 5). G: Most PEAC are CK20 negative (case 2).
EGFR and KRAS mutations analysis
None of the specific EGFR mutations in exons 18 through 21 that were tested for were detected in PEACs and MCCs, while overall 6 (30%) of all 20 tPACs showed an activating EGFR mutation (0/6 mutations in exon 18; 1/6 mutations in exon 19; 2/mutations in exon 20; 3/6 in exon 21). The mutation in exon 19 was delE746-A750 and in exon 21 was located on L858R in all detected three mutations. As for the typical mutations in KRAS gene, none were found in 9 cases of PEACs but were seen in two patients of MCCs and one of tPAC, all of which appeared in codon 12 (two G12D and one G12V).
Discussion
PEAC is characterized by the features of growth architectures of moderate-to-well differentiated glands, sometimes with a cribriform pattern formed, lined by tall columnar tumor cells with nuclear pseudostratification, dirty necrosis in the lumen and occasionally, prominent nuclear debris. However, some of these morphologic features such as the tall columnar cells with brush-border and eosinophilic cytoplasm arranged in garland-like patterns can also be seen in MCR. Besides, the lung presents a common site of metastasis for colorectal carcinoma, and the treatment strategies and prognosis outcomes differ considerably for patients with these malignancies, so, it is of vital importance for pathologists and clinicians to make a clear distinguish between these entities [12]. Initially, PEAC was described by Tsao and Fraser in 1991 as a primary lung adenocarcinoma with enteric differentiation that had partial component closely resembling colorectal carcinomas, composed of brush-border and eosinophilic cytoplasm with dirty necrosis [13]. Since then, a few cases have been reported continuously, and to date, there have been 17 reports in the English literature including two groups of PEACs reported by Yousem and Inamura et al. in 2005 [1,3]. All PEACs reported (26 cases, including 9 cases in present study; Table 4) mostly occurred in patients with the ages available ranging from 34 to 74 years. The male/female ratio is 6:7. Of all 26 cases reported, 20 patients were heavy smokers, suggesting that smoking being likely an causative factor for PEAC; 18 masses were detected in the right lung, but much larger number of cases will be required to determine whether PEACs are more likely to occur in the right lung; eight died within a follow-up period of 2 to 60 months, however, the associated prognosis underlie this rare variant of adenocarcinoma is still unclear for its limited data base.
Table 4.
Review of Literature ever reported for PEACs
| Study | No. Of Cases | Sex/Age (y) | Size (mm)/Site | Smoking | Immunohistochemical Combination | Follow-up (mo) |
|---|---|---|---|---|---|---|
| Inamura et al. [2] | 7 | 6, M; 1, F/NA | 17-50//3, RLL; 2, LUL; 1, LLL; 1, RUL | 7, Yes | CK7/CK20/TTF-1/CDX2/SPA/Napsin A/MUC2 | 12-60/5, A; 2, D |
| Yousem [8] | 6 | 1, M; 5, F/57-74 | 15-70/5, RUL; 1, LLL | 6, Yes | CK7/CK20/TTF-1/CDX2/SPA/MUC1/MUC2/MUC5A | 2-26/3, A; 3, D |
| Maeda et al. [3] | 1 | M/69 | 25/RLL | NA | CK7/CK20/TTF-1 | NA |
| Li HC et al. [10] | 1 | F/51 | 33/LLL | Yes | CK7/CK20/TTF-1/CDX2 | 10/A |
| Hatanaka et al. [9] | 1 | F/51 | 30/LLL and 10/RUL | No | CK7/CK20/TTF-1/CDX2/Napsin A/MUC2/MUC5AC | 48/A |
| Lin D et al. [22] | 1 | F/61 | 50/RML | No | CK7/CK20/TTF-1/Villin | 6/A |
M, male; F, female; A, alive; D, dead; CK, cytokeratin; LLL, left lower lobe; RLL, right lower lobe; LUL, left upper lobe; RUL, right upper lobe; RML, right middle lobe; NA, not avaiable.
As a primary pulmonary adenocarcinoma, PEAC more or less contained typical pulmonary adenocarcinoma histologic components, including lepidic growth and features of clear cytoplasm which MCRs were largely monotonous in histology. However, on occasional situations, when the tumor is exclusively composed of glands closely resembling MCR, it is difficult to make a discriminating diagnosis based solely on their morphologic features; then, an immunostaining profile would be required. It is generally known that primary pulmonary adenocarcinomas are supposed to be positive for CK7, TTF-1, Napsin A but negative for CK20, CDX2, MUC2, while MCR was reversed repressed. As a result, they were all for the differentiation between PAC and MCR. However, PEAC can exhibit immunoreactivity for both pulmonary- and intestinal-type markers, so a immunohistochemical stains, namely CK7, CK20, TTF-1, CDX2 and MUC2, were usually selected by previous studies to help diagnosis of PEAC. Here, markers of Napsin A and villin were added. To our knowledge, nearly all cases of PEAC reported were immunoreactive for CK7 (24/26, 92.3%) and a majority expressed TTF-1 (14/26, 53.8%). Eight cases were diffusely positive for CK20 (6/26, 23.1%), with partial immunoreactivity in four and five being positive for CK7 and CK20 coexpression. Thirteen cases showed immunoreactivity for CDX2 (13/26, 50%) while MUC2 enrolled in twenty-three cases were expressed in eight patients (8/23, 34.8%). Napsin A, which is seldom adopted by former researches, positively staining is closely associated with pulmonary origin and villin, a cytoskeletal actin-binding protein (Mr 95 kDa), is universally expressed in small intestinal microvilli and serves as an early marker of intestinal differentiation [14]. It is not expressed diffusely in the brush border in primary pulmonary adenocarcinoma except for a few cases of bronchioalveolar carcinoma [15,16], however, including 66.7% of PEAC in the present study. Hence, it can be used as markers to determine the presence of enteric differentiation in the cases of PEAC. Coordinate expression of all these intestinal differential markers in the current study were detected in only one case; of three markers were found in three cases; of two in two patients and one in three case.
To be mentioned, cases of CK7-negative, CK20-positive and TTF-1-negative PEAC were also noted [4,5]. Therefore, physical examinations, such as CT, FDG-PET and fiberoptic colonoscopy, are important for the diagnosis of primary PEAC and should be performed to rule out the possibility of metastatic colorectal cancer. Careful long-term follow-up is required in the presence of this rare adenocarcinoma, because no one know how this variant will behave in this cohort. In this study, all final diagnoses depended on the evaluation of clinical examination and natural history.
In fact, we also stained the PEACs with immunohistochemical markers of MLH1, MSH2. As is known, MLH1 and MSH2, the DNA mismatch repair genes, are associated with microsatellite instability (MSI) status and several pathologic features such as mucinous or signet ring differentiation which occasionally present in PEACs are correlated with MSI [17,18]. However, it turned out those 8 in 9 cases of PEACs show positive nuclear staining, indicating that MSI may not play a key role in the molecular mechanism of PEAC. As is reported, signet ring cell features are present in up to 56% of tumors with echinoderm microtubule-associated EML4-ALK gene fusion. The negative results of the present study indicated that the origin of PEAC may be poorly associated with echinoderm microtubule. Additionally, molecular analysis of the EGFR genomic mutations were performed in this study, because the EGFR pathway has been proposed to be important for cancer pathophysiology and EGFR gene mutations have been reported in patients with non-small cell lung cancer, and these mutations have been considered to have a significant association with nonmucinous pulmonary adenocarcinomas, especially predominant subtypes of acinar or micropapillary [19,20-22]. Nevertheless, no EGFR mutations were observed in our series of cases, indicating that PEAC, which consist of a predominant component of colorectal like patterns, might have no correction with EGFR mutations.
Regarding the KRAS alternations, which were proved to have a more frequency in solid predominant subtype, especially those with mucinous changes [23,24], did not found in the nine cases of PEACs, inclusive of the one with mucinous changes, suggesting that KRAS mutations may be rare in PEAC and it was highly unlikely to be a genetic driver in the molecular mechanism of PEAC.
To be concluded, the characteristic histology, immunoprofile, and total lack of EGFR/KRAS or ALK mutations, may suggest that PEAC forms a histologically or molecularly coherent subgroup of adenocarcinoma. And the separation from the usual PAC by the IASLC/ATS/ERS classification is quite reasonable. Due to its low incidence, larger series of reports are required to have the clinical and pathologic features of this rare tumor be well documented.
Disclosure of conflict of interest
None.
Abbreviations
- WHO
World Health Organization
- IASLC
International Association for the Study of Lung Cancer
- ATS
American Thoracic Society
- ERS
European Respiratory Society
- HE
hematoxylin and eosin
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- KRAS
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
References
- 1.Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, Ishikawa Y. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. 2005;29:660–665. doi: 10.1097/01.pas.0000160438.00652.8b. [DOI] [PubMed] [Google Scholar]
- 2.Maeda R, Isowa N, Onuma H, Miura H. Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc Thorac Surg. 2008;7:349–351. doi: 10.1510/icvts.2007.168716. [DOI] [PubMed] [Google Scholar]
- 3.Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol. 2005;18:816–821. doi: 10.1038/modpathol.3800358. [DOI] [PubMed] [Google Scholar]
- 4.Hatanaka K, Tsuta K, Watanabe K, Sugino K, Uekusa T. Primary pulmonary adenocarcinoma with enteric differentiation resembling metastatic colorectal carcinoma: a report of the second case negative for cytokeratin 7. Pathol Res Pract. 2011;207:188–191. doi: 10.1016/j.prp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Li HC, Schmidt L, Greenson JK, Chang AC, Myers JL. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol. 2009;131:129–133. doi: 10.1309/AJCPB04XWICTFERL. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, Zhao Y, Li H, Xing X. Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: a case report and literature review. J Thorac Dis. 2013;5:E17–20. doi: 10.3978/j.issn.2072-1439.2012.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K, Austin JH, Rusch VW, Hirsch FR, Jett J, Yang PC, Gould M American Thoracic Society. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edn. New York: Springer-Verlag; 2010. [Google Scholar]
- 9.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 10.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 11.Japan Lung Cancer Society. General Rules for Clinical and Pathologic Record of Lung Cancer [in Japanese] 5th ed. Tokyo: Kanahara; 1999. [Google Scholar]
- 12.Sauter ER, Bolton JS, Willis GW, Farr GH, Sardi A. Improved survival after pulmonary resection of metastatic colorectal carcinoma. J Surg Oncol. 1990;43:135–138. doi: 10.1002/jso.2930430303. [DOI] [PubMed] [Google Scholar]
- 13.Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. 1991;68:1754–1757. doi: 10.1002/1097-0142(19911015)68:8<1754::aid-cncr2820680818>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Moll R, Robine S, Dudouet B, Louvard D. Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54:155–169. doi: 10.1007/BF02899208. [DOI] [PubMed] [Google Scholar]
- 15.Tan J, Sidhu G, Greco MA, Ballard H, Wieczorek R. Villin, cytokeratin 7, and cytokeratin 20 expression in pulmonary adenocarcinoma with ultrastructural evidence of microvilli with rootlets. Hum Pathol. 1998;29:390–396. doi: 10.1016/s0046-8177(98)90121-6. [DOI] [PubMed] [Google Scholar]
- 16.Nambu Y, Iannettoni MD, Orringer MB, Beer DG. Unique expression patterns and alterations in the intestinal protein villin in primary and metastatic pulmonary adenocarcinomas. Mol Carcinog. 1998;23:234–242. [PubMed] [Google Scholar]
- 17.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J. Clin. Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 19.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 2007;75:770–787. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 21.Shim HS, Lee da H, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med. 2011;135:1329–1334. doi: 10.5858/arpa.2010-0493-OA. [DOI] [PubMed] [Google Scholar]
- 22.Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, Jheon S, Lee CT, Lee JS, Chung JH. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323–330. doi: 10.1097/JTO.0b013e3182381515. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, Luo X, Ye T, Wang R, Hu H, Li H, Wang L, Pao W, Chen H. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ang DC, Zakowski MF, Ladanyi M, Moreira AL, Rekhtman N. Characteristic morphology and immunoprofile of lung adenocarcinoma with KRAS mutations: propensity for solid growth pattern and correlation with TTF-1 expression. Mod Pathol. 2010;23:396A. [Google Scholar]