Abstract
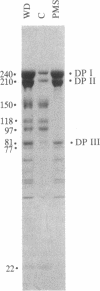
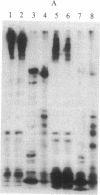
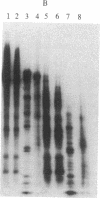
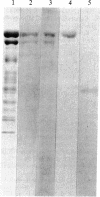
The cytoplasmic plaque of the spot desmosome or macula adhaerens mediates the attachment of bundles of intermediate filaments to the plasma membrane. We have isolated from a bovine epidermal desmosome preparation a fraction that is highly enriched in the non-glycosylated desmosomal proteins. Plastic-embedded and thin-sectioned high-speed pellets of this fraction reveal closely packed filaments that resemble plaque regions of the low pH whole desmosome preparation from which they are derived. NaDodSO4/polyacrylamide gel electrophoresis reveals four major, non-glycosylated proteins of 240, 210, 81, and 77 kDa. In agreement with a previous study, we find the 240- and 210-kDa proteins (desmoplakins I and II) to be closely related, whereas the 81- and 77-kDa proteins are unique. This is shown both immunologically and by one-dimensional proteolytic peptide mapping. Monospecific, polyclonal rabbit antibodies were prepared against the 81-kDa protein and used, in conjunction with protein A-complexed colloidal gold particles (PAG), to immunolocalize this antigen on ultrathin sections of bovine muzzle epidermis. On antibody-labeled sections, PAG particles were associated principally with the desmosomal cytoplasmic plaque. Sections exposed to preimmune serum showed little or no labeling. We conclude that the 81-kDa protein, like the 240/210-kDa protein family, is one of the major components of the desmosomal plaque. We designate it as "desmoplakin III." The location of the 77-kDa protein remains to be definitively established.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen S. M., Gorbsky G., Steinberg M. S. Immunochemical characterization of related families of glycoproteins in desmosomes. J Biol Chem. 1983 Feb 25;258(4):2621–2627. [PubMed] [Google Scholar]
- Cowin P., Garrod D. R. Antibodies to epithelial desmosomes show wide tissue and species cross-reactivity. Nature. 1983 Mar 10;302(5904):148–150. doi: 10.1038/302148a0. [DOI] [PubMed] [Google Scholar]
- Cowin P., Mattey D., Garrod D. Distribution of desmosomal components in the tissues of vertebrates, studied by fluorescent antibody staining. J Cell Sci. 1984 Mar;66:119–132. doi: 10.1242/jcs.66.1.119. [DOI] [PubMed] [Google Scholar]
- Drochmans P., Freudenstein C., Wanson J. C., Laurent L., Keenan T. W., Stadler J., Leloup R., Franke W. W. Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J Cell Biol. 1978 Nov;79(2 Pt 1):427–443. doi: 10.1083/jcb.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Kapprell H. P., Mueller H. Isolation and symmetrical splitting of desmosomal structures in 9 M urea. Eur J Cell Biol. 1983 Nov;32(1):117–130. [PubMed] [Google Scholar]
- Franke W. W., Mueller H., Mittnacht S., Kapprell H. P., Jorcano J. L. Significance of two desmosome plaque-associated polypeptides of molecular weights 75 000 and 83 000. EMBO J. 1983;2(12):2211–2215. doi: 10.1002/j.1460-2075.1983.tb01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Geiger B., Schmid E., Franke W. W. Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation. 1983;23(3):189–205. doi: 10.1111/j.1432-0436.1982.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Giudice G. J., Cohen S. M., Patel N. H., Steinberg M. S. Immunological comparison of desmosomal components from several bovine tissues. J Cell Biochem. 1984;26(1):35–45. doi: 10.1002/jcb.240260104. [DOI] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttermann A., Wendlberger-Schieweg G. Studies on metrizamide-protein interactions. Biochim Biophys Acta. 1976 Nov 26;453(1):176–184. doi: 10.1016/0005-2795(76)90261-0. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Franke W. W., Moser J. G., Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 1983;2(5):735–742. doi: 10.1002/j.1460-2075.1983.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Schwechheimer K., Moll R., Franke W. W. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J Cell Biol. 1984 Mar;98(3):1072–1081. doi: 10.1083/jcb.98.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. E., Kuda A. M. Traversing filaments in desmosomal and hemidesmosomal attachments: freeze-fracture approaches toward their characterization. Anat Rec. 1981 Jan;199(1):1–14. doi: 10.1002/ar.1091990102. [DOI] [PubMed] [Google Scholar]
- Kelly D. E., Shienvold F. L. The desmosome: fine structural studies with freeze-fracture replication and tannic acid staining of sectioned epidermis. Cell Tissue Res. 1976 Sep 20;172(3):309–323. doi: 10.1007/BF00399514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. Membrane ultrastructure at mammalian intercellular junctions. Prog Biophys Mol Biol. 1973;26:45–101. doi: 10.1016/0079-6107(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Mueller H., Franke W. W. Biochemical and immunological characterization of desmoplakins I and II, the major polypeptides of the desmosomal plaque. J Mol Biol. 1983 Feb 5;163(4):647–671. doi: 10.1016/0022-2836(83)90116-x. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Hell A., Birnie G. D., Gilhuus-Moe C. C. Reversible interaction of metrizamide with protein. Biochim Biophys Acta. 1974 Apr 11;342(2):367–371. doi: 10.1016/0005-2795(74)90092-0. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Jones C. Properties of ribosomes centrifuged in metrizamide gradients. Biochim Biophys Acta. 1981 Jun 26;654(1):26–30. doi: 10.1016/0005-2787(81)90132-5. [DOI] [PubMed] [Google Scholar]
- Skerrow C. J., Matoltsy A. G. Isolation of epidermal desmosomes. J Cell Biol. 1974 Nov;63(2 Pt 1):515–523. doi: 10.1083/jcb.63.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Schekman R., Singer S. J. Domains of receptor mobility and endocytosis in the membranes of neonatal human erythrocytes and reticulocytes are deficient in spectrin. J Cell Biol. 1979 Feb;80(2):481–486. doi: 10.1083/jcb.80.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]