Abstract
OBJECTIVE:
Atrial fibrillation is a common arrhythmia that increases the risk of stroke by four- to five-fold. We aimed to establish a profile of patients with atrial fibrillation from a population of patients admitted with acute ischemic stroke or transient ischemic attack using clinical and echocardiographic findings.
METHODS:
We evaluated patients consecutively admitted to a tertiary hospital with acute ischemic stroke or transient ischemic attack. Subjects were divided into an original set (admissions from May 2009 to October 2010) and a validation set (admissions from November 2010 to April 2013). The study was designed as a cohort, with clinical and echocardiographic findings compared between patients with and without atrial fibrillation. A multivariable model was built, and independent predictive factors were used to produce a predictive grading score for atrial fibrillation (Acute Stroke AF Score-ASAS).
RESULTS:
A total of 257 patients were evaluated from May 2009 to October 2010 and included in the original set. Atrial fibrillation was diagnosed in 17.5% of these patients. Significant predictors of atrial fibrillation in the multivariate analysis included age, National Institutes of Health Stroke Scores, and the presence of left atrial enlargement. These predictors were used in the final logistic model. For this model, the area under the receiver operating characteristic curve was 0.79. The score derived from the logistic regression analysis was
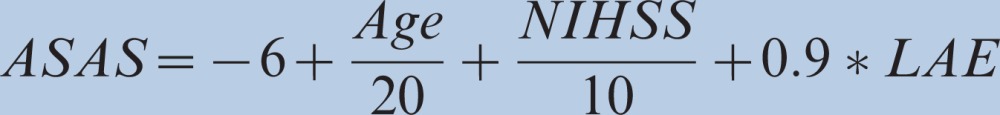 |
The model developed from the original data set was then applied to the validation data set, showing the preserved discriminatory ability of the model (c statistic = 0.76).
CONCLUSIONS:
Our risk score suggests that the individual risk for atrial fibrillation in patients with acute ischemic stroke can be assessed using simple data, including age, National Institutes of Health Stroke Scores at admission, and the presence of left atrial enlargement.
Keywords: Stroke, Atrial Fibrillation, Echocardiogram, Left Atrial Enlargement, Cardioembolic Stroke
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and it increases the risk of stroke by four- to five-fold (1). AF-related ischemic strokes have a high risk of recurrence and are associated with significant disability and mortality (2). In 50% of these patients, the AF pattern is paroxystic or asymptomatic, and although associated with the same risk of stroke it may not appear on standard electrocardiography (ECG) or even on the 24-hour ambulatory ECG recording (Holter) (3,4). Detecting this arrhythmia is essential because oral anticoagulation therapy is marked superior to antiplatelet drugs in reducing stroke risk (5,6).
Clinical features that predict AF occurrence in adults are well known, including increasing age, valvular heart disease, coronary artery disease, previous stroke or transient ischemic attack, diabetes, and history of hypertension or congestive heart failure (7,8). In patients with acute ischemic stroke, other features are also correlated with cardioembolic stroke related to AF, such as anterior circulation stroke, severe neurological deficit, and large infarct size. Still, most of these features are also associated with ischemic stroke from other causes, such as large vessel disease (8).
Previous studies have established clinical-radiological scores that predict AF in stroke patients. For example, the STAF score is calculated using the age of the patient, the National Institutes of Health Stroke Score (NIHSS) at admission, the presence of left atrial (LA) dilatation, and the absence of symptomatic intra- or extra-cranial stenosis and clinical-radiological lacunar syndrome (9,10). The STAF score has a sensitivity of 89% and a specificity of 88% for detecting AF. This score, however, requires information on the status of the intra- and extra-cranial vasculature, which is not always available, especially in services that do not specialize in stroke care (9,10). Actually, in a series of Brazilian patients with acute stroke consecutively admitted to general care hospitals, only 27% of the patients had a carotid ultrasonography, 3.5% had brain magnetic resonance imaging, and 2.9% had digital angiography (11). The exclusion of a lacunar syndrome generally also requires the expertise of a neurologist, as most general care physicians have little familiarity with stroke syndromes. The use of a simple score based on inexpensive investigation and clinical data would be of significant interest for everyday use in community hospitals and in low- and middle-income countries.
We therefore aimed to establish a profile of patients with documented AF in a Brazilian population of patients consecutively admitted with ischemic stroke or transient ischemic attack (TIA) using only clinical and echocardiographic findings. The possibility of using simple echocardiographic and clinical parameters associated with an enhanced risk of AF in patients with acute ischemic stroke or TIA may provide a means for determining the need for further cardiac monitoring, with, for example, a loop event recorder, to exclude AF as a source of cerebral embolism in this population.
METHODS
We evaluated patients consecutively admitted to a tertiary hospital within 24 hours of symptom onset with acute ischemic stroke or TIA from May 2009 to April 2013. Subjects were divided into an original set (patients admitted from May 2009 to October 2010) and a validation set (patients admitted from November 2010 to April 2013). Patients not evaluated with transthoracic or transesophageal echocardiograms were excluded from the analyses. Clinical and echocardiographic characteristics were prospectively collected for each patient according to a standardized protocol from an institutional outcomes database, as part of a quality assurance program for stroke treatment. As part of our hospital stroke program, all patients admitted with a diagnosis of ischemic stroke or TIA were identified and followed daily by a case manager nurse. Data collected included demographics, the presence of stroke risk factors, the NIHSS score at admission, and the modified Rankin scale (mRs) score at discharge. Risk factors were considered if noted on the patient's chart or if medications for known risk factors were used either before hospital admission or at discharge. In our hospital, all patients admitted with acute stroke are placed under continuous telemetry for at least the first 24 hours. The definition of AF included a previous diagnosis based on patient recall confirmed by the attending physician or by documentation of the first episode of paroxystic, persistent, permanent, or long-standing AF during hospitalization through ECG, Holter monitoring, or cardiac telemetry.
Transthoracic and/or transesophageal echocardiograms were carried out by three board-certified cardiologists from our hospital during admission for acute ischemic stroke or TIA. A comprehensive transthoracic echocardiographic examination (iE33, Philips Ultrasound Systems, Andover, Massachusetts, USA) was performed in all patients, with measurements obtained within 3 [1-5] days of the ischemic episode. The LA anteroposterior diameter was obtained from the bidimensional parasternal long-axis view and analyzed as a dichotomous variable (<4.0 cm vs. ≥4.0 cm). The left ventricular ejection fraction (Teichholz formula) was calculated from M-mode–guided bidimensional measurements of end-diastolic and end-systolic diameters according to the American Society of Echocardiography guidelines (12). In case any regional contractile abnormalities were present, the left ventricular volume and ejection fraction were either calculated from Simpson's rule or estimated visually (12). The left ventricular ejection fraction was categorized as normal when >50%, mild dysfunction when 40% to 49%, moderate dysfunction when 30% to 39%, and severe dysfunction when <30%. The presence of wall motion abnormalities and valvular dysfunction was noted. Mitral valve prolapse was defined as leaflet displacement greater than 2 mm above the plane of the mitral annulus. Transesophageal echocardiography was performed using a multiplane probe (Philips Medical Systems, Andover, MA, USA) following transthoracic examination. Images of the LA appendage were obtained from the mid-esophagus with a continuous sweep from 0° to 180° at 10°–15° increments. LA thrombus was considered to be present if there was a well-circumscribed intra-cavitary echo-dense mass with acoustic characteristics distinct from the surrounding endocardium/pectinate muscles detected in more than one imaging plane. Spontaneous echocardiographic contrast was defined as a pattern of slowly swirling intracavitary echodensities imaged with gain settings adjusted to distinguish background noise and continuously present at standard gain. A patent foramen ovale was diagnosed if color flow was observed crossing the fossa ovalis region or if microbubbles were observed in the left heart up to three heartbeats after the injection of agitated saline. We considered atrial septal aneurysm to be present when the total excursion of the atrial septum within the left and/or right atrium was 11 mm or larger. The study was designed as a cohort, with clinical and echocardiographic findings analyzed and compared between patients with acute ischemic stroke or TIA with and without AF. The hospital's Institutional Review Board approved this study.
Statistical analysis
Means and standard deviations or medians and interquartile intervals were used to describe the patients' characteristics. The independent samples t test was used to compare means between patients with and without AF. Nonparametric data were compared using the Mann-Whitney test. Categorical variables were compared with the χ2 test. Variables that had an association with AF with a p-value ≤0.1 were selected for multivariable analysis. A multivariable model was built using the likelihood ratio test for comparison between nested models. Independent predictive factors were then used to produce a predictive grading score for AF, derived by logistic regression analysis (Acute Stroke AF Score-ASAS). To check the sensitivity against the false-positive rate of the score, evaluation of discrimination was performed using the receiver operating characteristic (ROC) curve. Goodness-of-fit was assessed with the Hosmer-Lemeshow test. The internal validity of the prediction model was evaluated by bootstrapping. One thousand samples of equal size were drawn at random and with replacement from the complete dataset. In these bootstrap samples, coefficients of the final regression model were estimated and tested in the original sample (13). The amount of “optimism” was measured by calculating the difference between the area under the ROC curve in the original sample and bootstrap samples.
Validation of score reproducibility
The reproducibility of the discriminatory power of the ASAS was assessed by comparing the results of the score performance in the original set with its performance in the validation set. The areas under the curve (the c statistic) were presented for comparison among the prediction model in the original and validation datasets. Statistical analysis was performed with SPSS 20.0 software (Chicago, Ill., USA), and bootstrapping was performed in R version 2.15.1 for Mac OS X.
RESULTS
A total of 321 patients with acute ischemic stroke (226) or TIA (95) were evaluated from May 2009 to October 2010. Transthoracic (168 patients) or both transthoracic and transesophageal echocardiograms (89 patients) were performed in 257 patients. AF was diagnosed in 17.5% of the patients. This group was significantly older, had higher NIHSS scores at presentation, and was less frequently independent (modified Rankin scores ≤2) at discharge. Before the stroke or TIA admission index, the median CHADS2 score was 2 (1,4), and the median CHADSVAsc score was 5 (4,7). AF was more common in patients with ischemic stroke than in patients with TIA. Five patients had a previous history of AF and were on anticoagulants at the time of hospital admission (Table 1.
Table 1.
Demographics, clinical presentation, and risk factors between patients with and without atrial fibrillation.
| Atrial Fibrillation (n = 45) | No Atrial Fibrillation (n = 212) | p-value | |
| Mean age ± SD (years) | 80.4±14.3 | 69.9±9.6 | <0.01 |
| Male | 51.1% | 62.7% | 0.14 |
| TIA | 15.6% | 33.4% | 0.02 |
| NIHSS [median, IQ 25, 75] | 11 (2-17) | 2 [0-6] | <0.01 |
| Hypertension | 56.9% | 59.6% | 0.70 |
| Diabetes | 34.8% | 31.4% | 0.80 |
| Dyslipidemia | 23.5% | 30.4% | 0.40 |
| CAD | 7.8% | 11.5% | 0.60 |
| Smoking (current or previous) | 8.9% | 15.6% | 0.55 |
| Previous Stroke | 37.8% | 24.1% | 0.13 |
| Heart failure | 6.7% | 1.4% | 0.09 |
| CHADS2 Score [median, IQ 25, 75] | 2 (1,4) | - | |
| CHA2DS2-VASc | 5 (4,7) | - | |
| Anticoagulation prior to the stroke * | 11.1% | 0.9% | <0.01 |
| mRs ≤2 at discharge | 60.8% | 81% | <0.01 |
NIHSS: National Institutes of Health stroke scale; mRs: modified Rankin score; CAD: coronary artery disease. *Two patients without atrial fibrillation were on anticoagulants at admission due to a history of deep venous thrombosis. P-values lower than 0.05 were considered statistically significant.
There were no differences in the frequency of evaluation with transesophageal echocardiogram in patients with and without AF (Table 2. LA enlargement was more frequent in patients with AF than without (28.9% vs. 11.3%, p<0.01, respectively). Similarly, the presence of spontaneous LA contrast, mitral valve prolapse, and mitral regurgitation or stenosis was also significantly more frequent in patients diagnosed with AF (Table 2. Patients with AF showed a trend of increased wall motion abnormalities compared with those without AF (11.1% vs. 5.9%, p = 0.13, respectively), whereas patients without AF had a trend to more patent foramen ovale than those with AF (6.6% vs. 0%, p = 0.06, respectively). There were no differences in left ventricular function, the presence of atrial septal aneurysm, or the presence of intramural thrombi between the two groups (Table 2. Significant predictors of AF in the multivariate analysis included the following (Table 3): age (adjusted OR: 1.04 95% CI 1.02-1.08), NIHSS (adjusted OR: 1.10 95% CI 1.05-1.16), and the presence of LA enlargement (adjusted OR: 2.5 95% CI 1.01-6.29). All of these predictors were used in the final logistic model.
Table 2.
Echocardiographic predictors of atrial fibrillation in patients admitted with acute ischemic stroke or TIA.
| Atrial Fibrillation (n = 45) | No atrial Fibrillation (n = 212) | p-value | |
| Transesophageal echocardiogram performed | 28.5% | 36.3% | 0.48 |
| LVEF*) | 87.8% | 89.7% | 0.76 |
| Normal | 7.3% | 4.9% | |
| Mild dysfunction | 4.9% | 3.8% | |
| Moderate dysfunction | 0.0% | 1.6% | |
| Severe dysfunction | |||
| Segmental wall motion abnormalities | 11.1% | 5.9% | 0.13 |
| Patent foramen ovale | 0% | 6.6% | 0.06 |
| Atrial septal aneurysm | 2.2% | 0.9% | 0.44 |
| Intramural thrombi | 4.4% | 1.9% | 0.28 |
| Left atrial enlargement | 28.9% | 11.3 | <0.01 |
| Spontaneous left atrial contrast | 4.4% | 0% | 0.03 |
| Mitral regurgitation or stenosis | 11.1% | 0.9% | <0.01 |
| Mitral valve prolapse | 4.4% | 0% | 0.03 |
LVEF: left ventricle ejection fraction. Normal LVEF>0.50; mild dysfunction = 0.40–0.49; moderate dysfunction = 0.30–0.39; severe dysfunction<0.30. LVEF = left ventricular ejection fraction.
Table 3.
Results of the multivariable logistic regression analyses for Atrial Fibrillation.
| Variable | OR | 95% CI | p-value |
| Age ( per year) | 1.04 | 1.02-1.08 | <0.01 |
| NIHSS (per point) | 1.10 | 1.05-1.16 | <0.01 |
| Left atrial enlargement | 2.5 | 1.01-6.29 | 0.04 |
NIHSS: National Institutes of Health stroke scale.
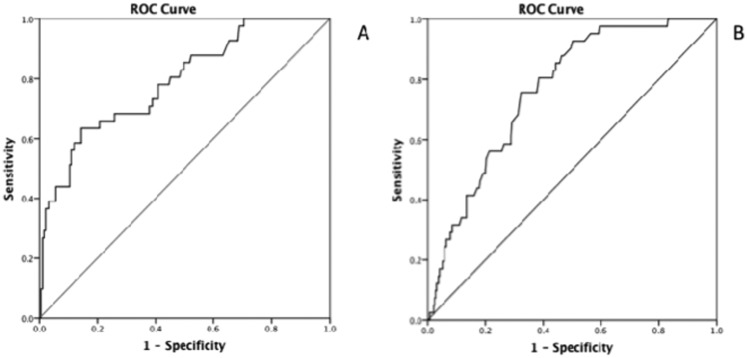
The Hosmer-Lemeshow test revealed a goodness-of-fit of 10.82, suggesting that the model was well calibrated (p = 0.21). The area under the ROC curve (AUC) for this model was 0.79 (95% confidence interval [95% CI 0.71-0.86]), indicating that the discrimination of the model was high (Figure 1). The mean area under the 1000 boot- strapped ROC was 0.79 (95% CI 0.72-0.87). The prediction rule was then simplified using integer scores based on the regression coefficients obtained from the logistic regression model. The score derived from the logistic regression analysis is shown below, where LAE means the presence of left atrial enlargement:
 |
Figure 1.
A) Receiver–operator curve for the ASAS in the original set. ASAS = the acronym for the scoring system incorporating age, National Institutes of Health Stroke Scale (NIHSS) at admission, and the presence of left atrial enlargement. B) Receiver–operator curve for the ASAS in the validation set.
The relationship between the total ASAS score and AF was:
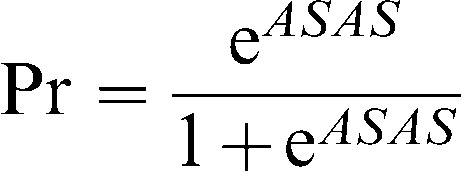 |
where Pr is the probability of AF in patients admitted with acute ischemic stroke or TIA. A Pr values ≥0.09 targets AF with a sensitivity of 82% and specificity of 52% in the secondary prevention of stroke. The AUC for the predicted probabilities of the ASAS was 0.78 [95% CI 0.70-0.86], excluding patients with a previous diagnosis of atrial fibrillation.
A total of 486 patients were admitted with acute ischemic stroke (339) or TIA (147) and evaluated with transthoracic and/or transesophageal echocardiograms from November 2010 to April 2013 (validation set). The model developed with the original data set was subsequently applied to the validation data set, showing the preserved discriminatory ability of the model (c statistic = 0.76, 95% CI 0.69-0.83) (Figure 1B).
DISCUSSION
In this study, we developed and validated a clinical and echocardiographic risk score for patients with acute ischemic stroke or TIA to identify individuals at risk for AF. This model may help emergency physicians and neurologists to detect patients with acute ischemic stroke or TIA at high risk for AF in daily clinical practice, even in the absence of vascular neuroimaging.
Although some previous studies have investigated the predictive value of echocardiographic parameters as possible risk factors for the occurrence of AF in adults, there is still no consensus on the best echocardiographic predictors of AF risk, particularly in patients with acute ischemic stroke (14,15). Two previous studies evaluated the ability of scores to help identify patients at risk for AF among patients with ischemic stroke or TIA (9,16). The STAF score was calculated from the sum of the points for 4 items: age, NIHSS, LA enlargement, and the absence of symptomatic intra- or extra-cranial stenosis or clinico-radiological lacunar syndrome. This score identified patients with AF with a sensitivity of 89% and a specificity of 88% (9,10). Although the sensitivity and specificity of the STAF score are high, this scoring system requires vascular imaging, which is not widely available in community hospitals (11). Furthermore, the value of the STAF score in patients with TIA is uncertain because such patients were not included in the study. The LADS score, in contrast, is based on simple criteria, including the presence of LA enlargement, age, and the diagnosis of stroke instead of TIA. This score had a sensitivity of 85.5% and a specificity of 53.1% for the diagnosis of AF (16). The LADS score, however, is based on a retrospective, single-center study and was not validated any further (16). Therefore, the broader applicability of this score is uncertain.
Our score was based on simple, widely available clinical and echocardiographic data and showed good calibration and discrimination. After internal validation, performed by bootstrapping, the performance of the model remained high, as evident by the area under the ROC = 0.79 (13). Furthermore, the ASAS score was developed and internally validated in a Brazilian population. Brazil has a continental dimension and a multiethnic population. Genetic studies have indicated that the Brazilian population has European, African, and Native Americans constituents (9,17). Therefore, the use of risk scores developed and validated in the Brazilian population is of significant importance.
Standard ECG has a poor sensitivity for detecting paroxysmal AF. Therefore, Holter monitoring is often used in patients with ischemic stroke, allowing for the detection of previously unrecognized AF in approximately 2% of stroke patients (3,4). Recent data suggest that following acute stroke or TIA, prolonged rhythm recording might help to identify patients with AF not detected by standard ECG and Holter (18). Our risk score may help to determine which patients should undergo more comprehensive screening with prolonged rhythm monitoring.
There are some limitations to our study. Our relatively small sample size may have prevented the detection of any association between other echocardiographic parameters and the diagnosis of AF. Additionally, we based our analyses on the use of both transthoracic and transesophageal echocardiography, which have different sensitivities for the detection of cardiac abnormalities. Only one-third of our patients had a TEE performed because the investigation of stroke etiology was performed at the discretion of the attending physician. However, the frequency of investigation with TEE was balanced in patients with and without AF. The LA volume was previously validated in multiple studies as the gold standard for evaluating prognosis in several situations, including AF (19). Unfortunately, the LA volume was not available in more than half of our patients. However, the LA volume is likely co-linear with the LA diameter, and the information provided by the LA volume is partially included in the regression analysis by using the LA diameter. Finally, because patients did not undergo further diagnostic monitoring (for example, with a loop event recorder or a 30-day event monitor), we may have missed the diagnosis of AF in some patients. Unfortunately, long-term monitoring is not covered by most health insurance providers in Brazil and therefore was not available for the patients included in our series. Nevertheless, our cohort was the first to specifically evaluate clinical and echocardiographic predictors of AF in the context of acute ischemic stroke and TIA in a Brazilian population and therefore serves as a hypothesis-generating study.
In conclusion, we developed and validated a risk score for patients with acute ischemic stroke or TIA to predict the risk of AF. The final model showed that the individual risk for AF in patients with acute ischemic stroke or TIA can be assessed using simple data including age, NIHSS at admission, and the presence of LA enlargement. The discriminatory ability of this model and the results of internal validation showed that the model performed well. The results of our study suggest that clinical and echocardiographic parameters can be used as a tool for screening patients with acute ischemic stroke to identify those at a higher risk of AF who should undergo further cardiac monitoring, to exclude AF as a source of cerebral embolism. Detection of this arrhythmia is essential because AF-related strokes have a poor prognosis and high risk of recurrence, yet anticoagulant treatment dramatically reduces these risks.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Kimura K, Minematsu K, Yamaguchi T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(5):679–83. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35(7):1647–51. doi: 10.1161/01.STR.0000131269.69502.d9. [DOI] [PubMed] [Google Scholar]
- 4.Sposato LA, Klein FR, Jauregui A, Ferrua M, Klin P, Zamora R, et al. Newly diagnosed atrial fibrillation after acute ischemic stroke and transient ischemic attack: importance of immediate and prolonged continuous cardiac monitoring. J Stroke Cerebrovasc Dis. 2012;21(3):210–6. doi: 10.1016/j.jstrokecerebrovasdis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Bugnicourt JM, Flament M, Guillaumont MP, Chillon JM, Leclercq C, Canaple S, et al. Predictors of newly diagnosed atrial fibrillation in cryptogenic stroke: a cohort study. Eur J Neurol. 2013;20(10):1352–9. doi: 10.1111/ene.12017. [DOI] [PubMed] [Google Scholar]
- 8.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 9.Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40(8):2866–8. doi: 10.1161/STROKEAHA.109.552679. [DOI] [PubMed] [Google Scholar]
- 10.Suissa L, Mahagne MH, Lachaud S. Score for the targeting of atrial fibrillation: a new approach to diagnosing paroxysmal atrial fibrillation. Cerebrovasc Dis. 2011;31(5):442–7. doi: 10.1159/000323852. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho JJ, Alves MB, Viana GA, Machado CB, Dos Santos BF, Kanamura AH, et al. Stroke Epidemiology, Patterns of Management, and Outcomes in Fortaleza, Brazil: A Hospital-Based Multicenter Prospective Study. Stroke. 2011;42(12):3341–6. doi: 10.1161/STROKEAHA.111.626523. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Steyerberg EW, Bleeker SE, Moll HA, Grobbee DE, Moons KG. Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J Clin Epidemiol. 2003;56(5):441–7. doi: 10.1016/s0895-4356(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 14.Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158(12):1316–20. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- 15.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998;128(8):639–47. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Malik S, Hicks WJ, Schultz L, Penstone P, Gardner J, Katramados AM, et al. Development of a scoring system for atrial fibrillation in acute stroke and transient ischemic attack patients: the LADS scoring system. J Neurol Sci. 2011;301(1-2):27–30. doi: 10.1016/j.jns.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Alves-Silva J, da Silva Santos M, Guimaraes PE, Ferreira AC, Bandelt HJ, Pena SD, et al. The ancestry of Brazilian mtDNA lineages. Am J Hum Genet. 2000;67(2):444–61. doi: 10.1086/303004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2012;14(1):8–27. doi: 10.1093/europace/eur241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467–75. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]