Abstract
Activation of cellular protein kinase C appears to be involved in the mechanism by which phorbol diesters induce differentiation of human myeloid leukemia cells (HL-60). Protein kinase C is thought to be physiologically activated by diacylglycerol derived from receptor-mediated phosphatidylinositol hydrolysis. sn-1,2-diacylglycerols with short saturated acyl side chains (C4-C10) were synthesized and found to be potent activators of protein kinase C partially purified from HL-60 cells. These diacylglycerols were also competitive inhibitors of [3H]phorbol dibutyrate binding to the soluble phorbol diester receptor. The most potent diacylglycerol, sn-1,2-dioctanoylglycerol, displaced greater than 90% of [3H]phorbol dibutyrate from the phorbol diester receptor of intact HL-60 cells. Because of probable cellular metabolism of sn-1,2-dioctanoylglycerol, hourly doses were required to maintain persistent occupancy of the phorbol diester binding site. Treatment of HL-60 cells with either phorbol 12-myristate 13-acetate or sn-1,2-dioctanoylglycerol produced identical phosphoprotein changes. Finally, sn-1,2-dioctanoylglycerol induced differentiation of the HL-60 cells into cells with morphologic characteristics of macrophages. Substitution of the hydroxyl group at position 3 with a hydrogen, chloro, or sulfhydryl moiety inactivated sn-1,2-dioctanoylglycerol. These data strengthen the hypothesis that protein kinase C activation plays a role in macrophage differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L., Staller J. M., Boutwell R. K. Protein kinase activity associated with a phorbol ester receptor purified from mouse brain. Cancer Res. 1983 Sep;43(9):4333–4337. [PubMed] [Google Scholar]
- BERENBLUM I. A speculative review; the probable nature of promoting action and its significance in the understanding of the mechanism of carcinogenesis. Cancer Res. 1954 Aug;14(7):471–477. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cooper R. A., Braunwald A. D., Kuo A. L. Phorbol ester induction of leukemic cell differentiation is a membrane-mediated process. Proc Natl Acad Sci U S A. 1982 May;79(9):2865–2869. doi: 10.1073/pnas.79.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Cooper H. L. Rapid protein phosphorylation induced by phorbol ester in HL-60 cells. Unique alkali-stable phosphorylation of a 17,000-dalton protein detected by two-dimensional gel electrophoresis. J Biol Chem. 1983 Sep 10;258(17):10786–10793. [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger Z., Lapidot Y. Synthesis of fatty acid anhydrides by reaction with dicyclohexylcarbodiimide. J Lipid Res. 1966 Jan;7(1):174–175. [PubMed] [Google Scholar]
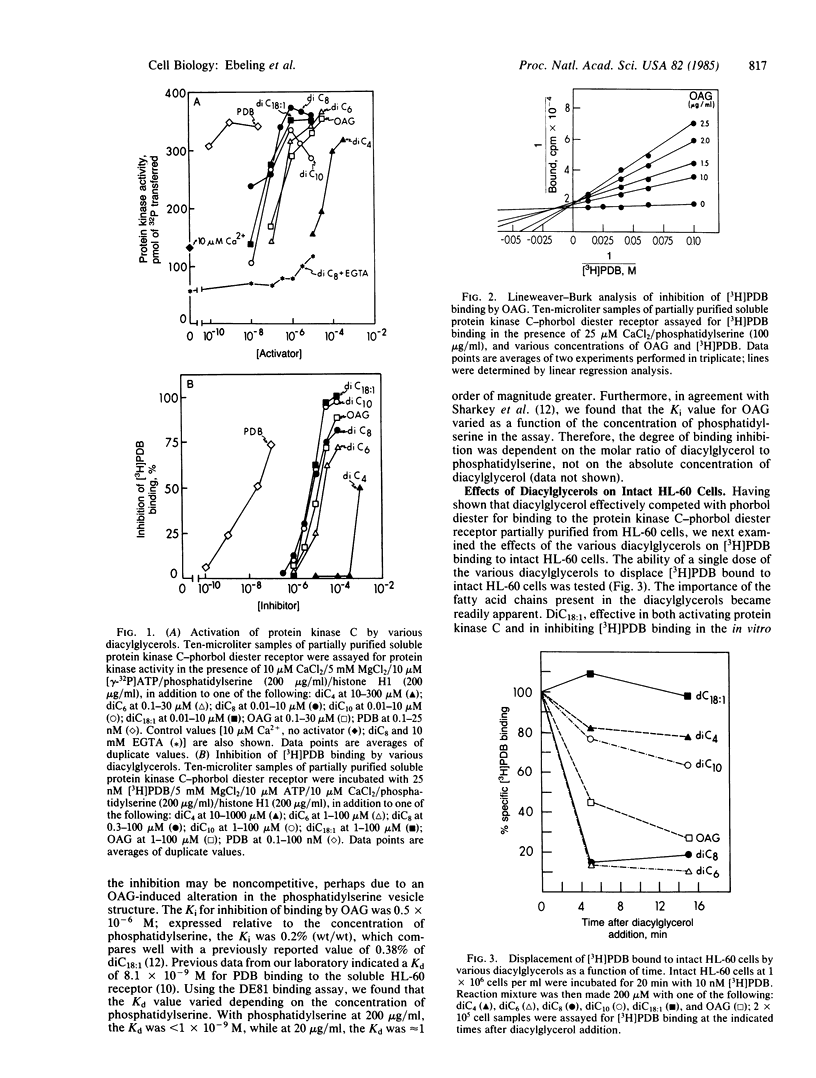
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]