Abstract
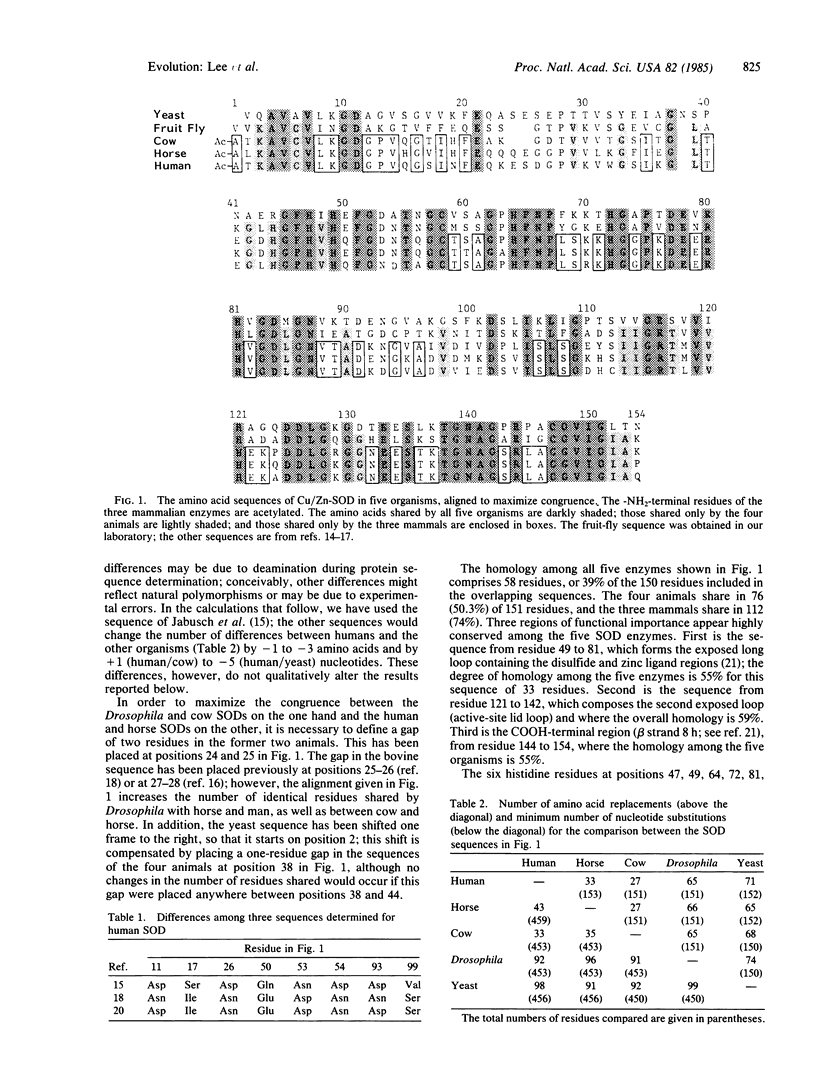
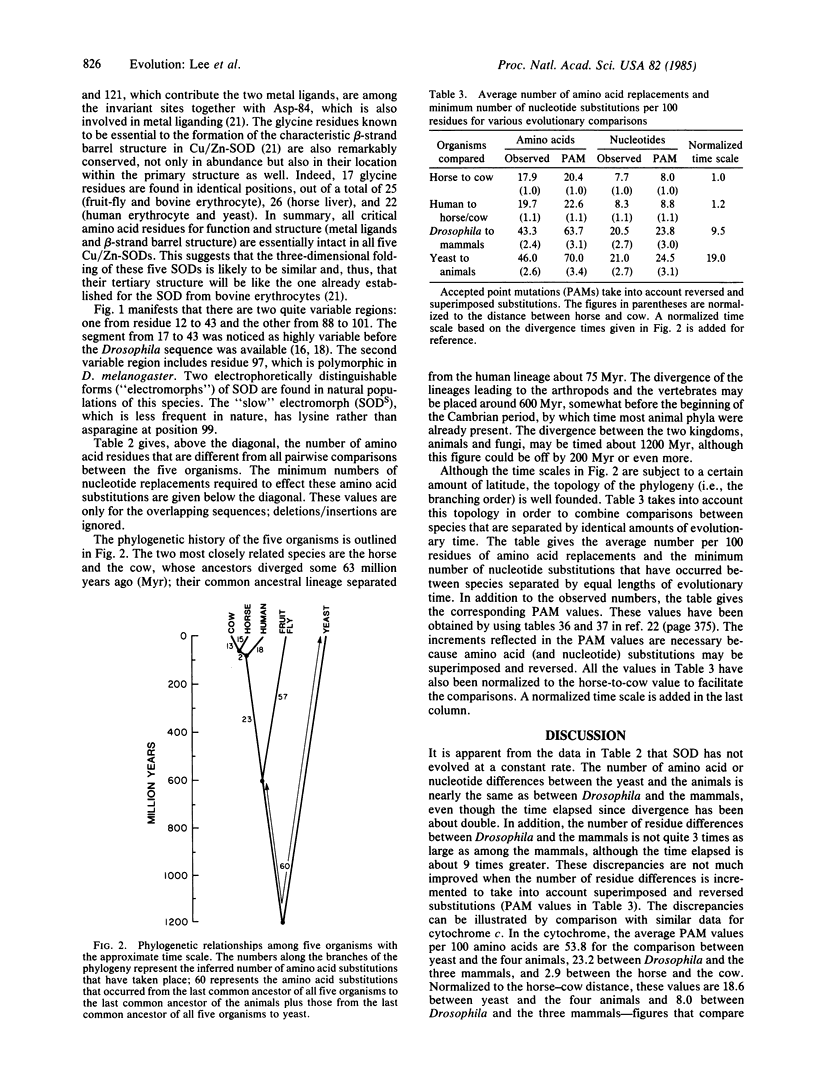
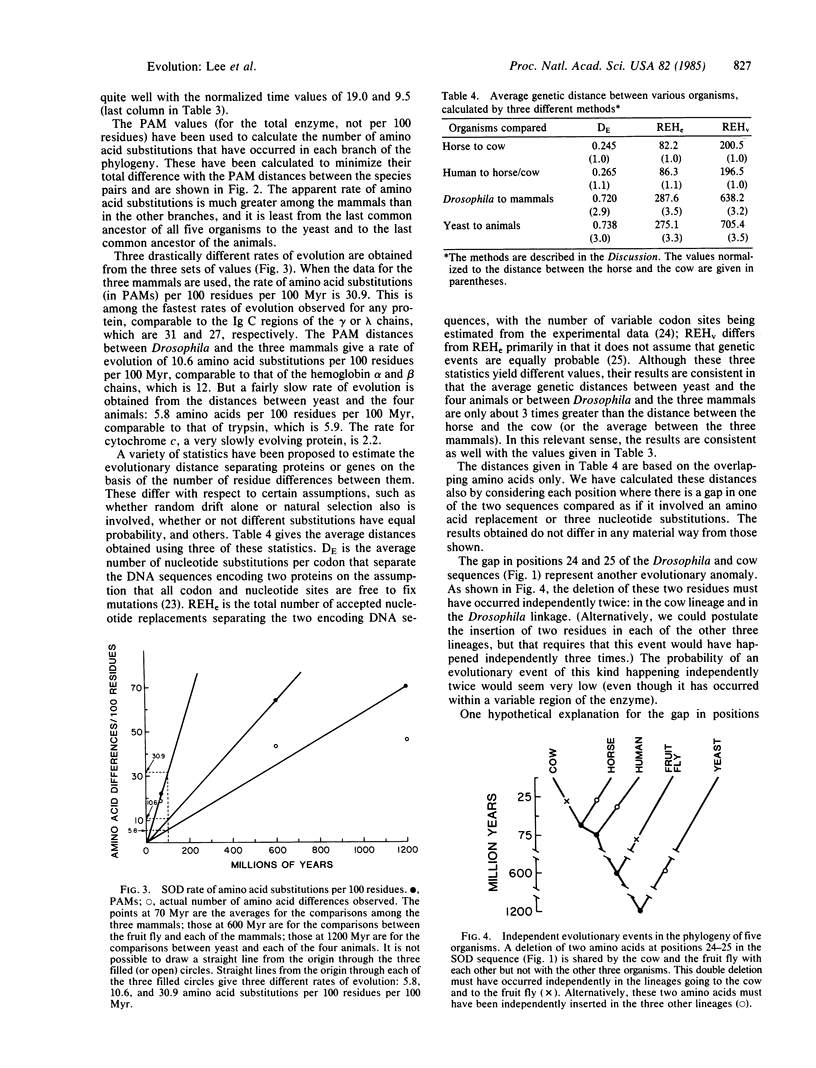
We have obtained the complete amino acid sequence of copper/zinc-containing superoxide dismutase (SOD, superoxide:superoxide oxidoreductase, EC 1.15.1.1) from Drosophila melanogaster. The sequence of this enzyme is also known for man, horse, cow, and the yeast Saccharomyces cerevisiae. The rate of evolution of this enzyme is far from constant. The number of amino acid substitutions per 100 residues per 100 million years is 30.9 when the three mammals are compared to each other, 10.6 when Drosophila is compared to the three mammals, and 5.8 when the yeast is compared to the four animals. The first value represents one of the fastest evolutionary rates for any protein, the second is similar to the globin rate, and the third is similar to some cytochromes and other slowly evolving proteins. Hence, SOD is not an acceptable evolutionary clock. Another peculiarity of this enzyme is that a two-amino-acid deletion must have occurred independently in the lineages going to the cow and to Drosophila. We conclude that using the primary structure of a single gene or protein to time evolutionary events or to reconstruct phylogenetic relationships is potentially fraught with error.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra D., Martini F., Bannister J. V., Schininà M. E., Rotilio G., Bannister W. H., Bossa F. The complete amino acid sequence of human Cu/Zn superoxide dismutase. FEBS Lett. 1980 Oct 20;120(1):53–56. doi: 10.1016/0014-5793(80)81044-1. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. The structures of cytochrome c and the rates of molecular evolution. J Mol Evol. 1971;1(1):26–45. doi: 10.1007/BF01659392. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Langley C. H. Protein evolution and the molecular clock. Fed Proc. 1976 Aug;35(10):2092–2097. [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Barnabas J., Matsuda G. The phylogeny of human globin genes investigated by the maximum parsimony method. J Mol Evol. 1974 Feb 28;3(1):1–48. doi: 10.1007/BF01795974. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Holmquist R. The REH theory of protein and nucleic acid divergence: a retrospective update. J Mol Evol. 1978 Oct 6;11(4):361–374. doi: 10.1007/BF01733843. [DOI] [PubMed] [Google Scholar]
- Jabusch J. R., Farb D. L., Kerschensteiner D. A., Deutsch H. F. Some sulfhydryl properties and primary structure of human erythrocyte superoxide dismutase. Biochemistry. 1980 May 27;19(11):2310–2316. doi: 10.1021/bi00552a005. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ota T. On the stochastic model for estimation of mutational distance between homologous proteins. J Mol Evol. 1972 Dec 29;2(1):87–90. doi: 10.1007/BF01653945. [DOI] [PubMed] [Google Scholar]
- Langley C. H., Fitch W. M. An examination of the constancy of the rate of molecular evolution. J Mol Evol. 1974;3(3):161–177. doi: 10.1007/BF01797451. [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Ayala F. J., Misra H. P. Purification and properties of superoxide dismutase from Drosophila melanogaster. J Biol Chem. 1981 Aug 25;256(16):8506–8509. [PubMed] [Google Scholar]
- Lee Y. M., Misra H. P., Ayala F. J. Superoxide dismutase in Drosophila melanogaster: biochemical and structural characterization of allozyme variants. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7052–7055. doi: 10.1073/pnas.78.11.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Ammer D. Amino acid sequence of copper-zinc superoxide dismutase from horse liver. J Biol Chem. 1981 Nov 25;256(22):11545–11551. [PubMed] [Google Scholar]
- Martin J. P., Jr, Fridovich I. Evidence for a natural gene transfer from the ponyfish to its bioluminescent bacterial symbiont Photobacter leiognathi. The close relationship between bacteriocuprein and the copper-zinc superoxide dismutase of teleost fishes. J Biol Chem. 1981 Jun 25;256(12):6080–6089. [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M., Naik V. R., Abernethy J. L., Hill R. L. Bovine erythrocyte superoxide dismutase. Complete amino acid sequence. J Biol Chem. 1974 Nov 25;249(22):7326–7338. [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of copper-zinc superoxide dismutase from bakers' yeast. J Biol Chem. 1980 Jul 25;255(14):6758–6765. [PubMed] [Google Scholar]




