Abstract
The atomic structure of a protein can greatly advance our understanding of molecular recognition and catalysis, properties of fundamental importance in signal transduction. However, a single structure is incapable of fully describing how a protein works, particularly when allostery is involved. Recent advances in the structure and function of G protein-coupled receptor (GPCR) kinases (GRKs) have concentrated on the mechanism of their inhibition by small and large molecules. These studies have generated a wealth of new information on the conformational flexibility of these enzymes, which opens new avenues for the development of selective chemical probes and provides deeper insights into the molecular basis for activation of these enzymes by GPCRs and phospholipids.
Introduction
G protein-coupled receptor (GPCR) kinases (GRKs) initiate the homologous desensitization of activated GPCRs through the phosphorylation of specific sites within the cytoplasmic loops and carboxy-terminal tails of the receptors [1]. These covalent modifications help to recruit arrestins, which uncouple the GPCRs from heterotrimeric G proteins and targets them for internalization. There are 7 mammalian GRKs grouped into 3 sub-families (GRK1, GRK2, and GRK4) [2] (Figure 1). Atomic structures representing each subfamily (GRK1 [3], GRK2 [4,5], and GRK6 [6,7]) in various ligand-bound states are now available. These structures establish that the conserved structural core of GRKs is comprised of a protein kinase domain inserted into a loop of a regulator of G protein signaling homology (RH) domain [8]. The RH domain serves as an intramolecular scaffold that maintains the small lobe of the kinase domain in a state that is competent to phosphorylate activated GPCRs. Consequently, the kinase domain, although closely related to those of protein kinases A, G and C (AGC kinases), does not require phosphorylation on its activation loop for full activity. GRKs, however, retain the C-terminal extension of the kinase domain characteristic of the AGC kinase family, which contributes residues to the active site cleft. Although this element is not fully ordered in most GRK structures, mutations in this region in GRK2 [9] and GRK1 [10] are known to dramatically inhibit the phosphorylation of receptor and soluble substrates, consistent with the idea that this element serves to regulate kinase activity as it does in other AGC kinases [11]. The first ~20 amino acids of GRKs are highly conserved and critical for GPCR and phospholipid-stimulated autophosphorylation. However, this region is disordered in most GRK structures reported to date, clouding interpretation of its molecular role.
Figure 1.

Domain structure of the three mammalian GRK subfamilies. The αN helix (red) is believed to engage the membrane and/or activated GPCRs. It has also proposed to simultaneously engage the kinase domain (yellow) and the C-terminal kinase extension (green) to stabilize the kinase domain in a more active conformation. The C-terminal region of all GRKs contributes to membrane localization, although in a subfamily-specific manner: it is prenylated in the GRK1 subfamily, it binds to Gβγ subunits in the GRK2 subfamily, and it has a basic amphipathic C-terminal helix (CT) and/or palmitoylation sites in the GRK4 subfamily. A black bar shows the region of GRK2 comprising the βARKct protein.
This review highlights recent advances in our molecular understanding of GRK function. The most recent structural studies have emphasized the conformational variability of the GRK kinase domain, an understanding of which will likely be key for the development of selective chemical probes. Some of the observed conformational changes observed have also provided much needed structural insight into how these enzymes might be recognized and activated by agonist occupied GPCRs and/or phospholipids.
Inhibiting the GRKs
Various GRKs are known to play roles in human disease [12]. GRK2 and GRK5 stand out due to their well characterized roles in heart failure and cardiac hypertrophy [13–17]. One of the most selective inhibitors of GRK2 known is βARKct (Figure 1), a fragment corresponding to the 222 C-terminal residues of GRK2 [13,18], which can be administered via adeno-associated virus gene delivery and improves contractile performance in both small and large animal models of heart failure [14,19]. This protein serves as a dominant negative because it blocks the recruitment of endogenous GRK2 to the membrane by heterotrimeric Gβγ subunits. Disadvantages of this approach are that all Gβγ signaling pathways would be impacted and humoral immunity may limit effectiveness. Clearly, small cell permeable molecules that can directly and selectively inhibit a specific GRK would be of great use in both the laboratory and clinic.
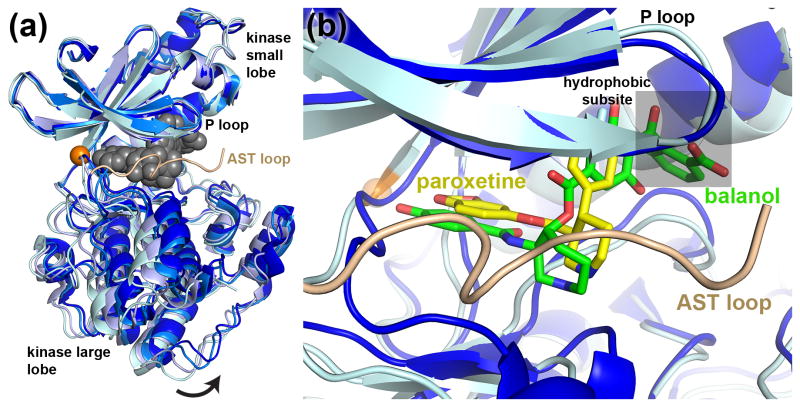
In the last three years there has been a dramatic expansion of our molecular understanding of how GRKs interact with inhibitors. Crystal structures of GRK2 in complex with an RNA aptamer that inhibits GRK2 with high affinity (3.3 nM) and selectivity (60- and 180-fold versus GRK6 and GRK1, respectively) were recently reported [20,21]. In these structures, a hairpin loop of the aptamer mimics the interactions of ATP in the active site (Figure 2a), and the RNA phosphodiester backbone forms extensive electrostatic interactions that remodels the large lobe of the kinase domain. The latter interaction may emulate how polyanionic macromolecules such as heparin inhibit GRK function [22,23]. The kinase domain of GRK2 in complex with the aptamer adopts an unusually “open” conformation that is likely enforced by the large size of the RNA hairpin.
Figure 2.
Conformational states of the GRK2 kinase domain as revealed by inhibitor binding studies. (a) Superposition of the GRK2 kinase domain using the small lobe for alignment, excluding the hinge region (orange sphere) and P-loop, illustrating movement of the large lobe in response to inhibitor binding. The most open conformation is found in its complex with the RNA aptamer C13.28 (PDB entry 3UZS, lightest-cyan). Gray spheres correspond to the hairpin loop of the aptamer, which fills the active site cleft. More typical, intermediate conformations are illustrated by the apo structure of GRK2 (PDB entry 3PSC, light blue) and the balanol complex (PDB entry 3KRX, blue). The most closed state of GRK2 available is its complex with paroxetine (PDB entry 3V5W, dark blue). (b) The binding of paroxetine (stick model with yellow carbons) to GRK2 induces a conformation that orders residues in the AST loop, which is part of the AGC kinase C-terminal extension. The natural product balanol binds similarly to paroxetine [24,29], but stabilizes a different conformation of the kinase domain and has an additional ring system that extends into the so-called hydrophobic subsite (grey transparent box).
More drug-like small molecules have also been structurally characterized in complex with GRK2, including the natural product balanol (Figure 2b), which exhibits an IC50 of 50 nM for GRK2, an order of magnitude lower than for GRK5 and GRK1 [24]. Remarkably, a series of related compounds developed by Takeda Pharmaceuticals demonstrate both high potency (50–300 nM IC50 values) and greater than 2000-fold selectivity for GRK2 over other GRKs [25,26]. However, balanol is a pan-AGC kinase inhibitor and represents a relatively difficult chemical synthesis, and the Takeda compounds have not progressed to clinic trials. Both classes of molecules consist of a linear arrangement of four ring systems that bind in the active site and trap the kinase domain in conformation that is thought to be the apo state of GRK2. The last ring system of these inhibitors extends into a region called the hydrophobic subsite [27] formed between the small and large lobes of the kinase domain (Figure 2b). A major conclusion from these structures and associated biochemical studies is that selectivity for GRK2 is likely driven more by the conformation of the kinase domain than the identity of residues in the active site, which are highly conserved among GRKs and other AGC kinases. The differential affinity of GRKs for ATP and ADP may also contribute to selectivity in kinetic assays [28].
Most recently, the selective serotonin reuptake inhibitor paroxetine was identified as a low μM inhibitor of GRK2, with similar selectivity as balanol [29]. Remarkably, the drug inhibited GRK2-dependent GPCR phosphorylation in living cells and improved heart contractility in live mice. Like balanol and the Takeda compounds, paroxetine binds in a manner that overlaps with the ATP binding site, but it is a shorter molecule with only three ring systems and does not extend into the hydrophobic subsite. Because it exhibits a lower potency of inhibition than balanol and the Takeda compounds, occupation of this subsite may be a perquisite for high-affinity binding. Interestingly, the kinase domain of GRK2 adopts a novel, relatively closed conformation when bound to paroxetine that promotes order in the C-terminal extension of the kinase domain (Figure 2). This state may correspond to the ADP-bound conformation of GRK2 because it most closely resembles those exhibited by kinase domains in GRK1·ADP complexes [3,28]. Consequently, there are now three distinct conformations of the GRK2 kinase domain that have been revealed by crystallographic analysis of its complexes with inhibitors (Figure 2a). These models should prove to be fertile grounds for the development of more potent and selective inhibitors of GRK2 via structure-based drug design.
The N-terminal Conundrum
The importance of the extreme N-terminal region of GRKs for function was first recognized when antibodies directed against the N-terminus of GRK1 blocked the phosphorylation of rhodopsin but not peptide substrates [30]. Deletion of the first 14 amino acids or mutations in this span of GRK5 leads to loss of phospholipid-dependent autophosphorylation, and the corresponding 14-mer peptide from GRK5 could inhibit GRK5 but not GRK2-dependent phosphorylation of rhodopsin. Phosphorylation of tubulin, a soluble substrate, was unaffected [31]. More recently, mutation of conserved residues in the N-terminus of GRK2 were shown to diminish activity against receptors and eliminated receptor-stimulated GRK activity, but did not lead to loss of receptor binding or phosphorylation of soluble substrates. Furthermore, the N-terminal peptide from GRK2, but not GRK5, could inhibit GRK2-dependent phosphorylation of the β2-adrenergic receptor, and promote association of GRK2 with liposomes [32]. These results led to a model in which the N-terminus forms a helix that can interact with the acyl phase of the membrane via its hydrophobic residues, and with the kinase domain via its more polar residues, thereby stabilizing the active configuration of the GRK. In this model, receptors would presumably bind to a different region of the GRK and indirectly promote formation of the same or similar catalytically active state.
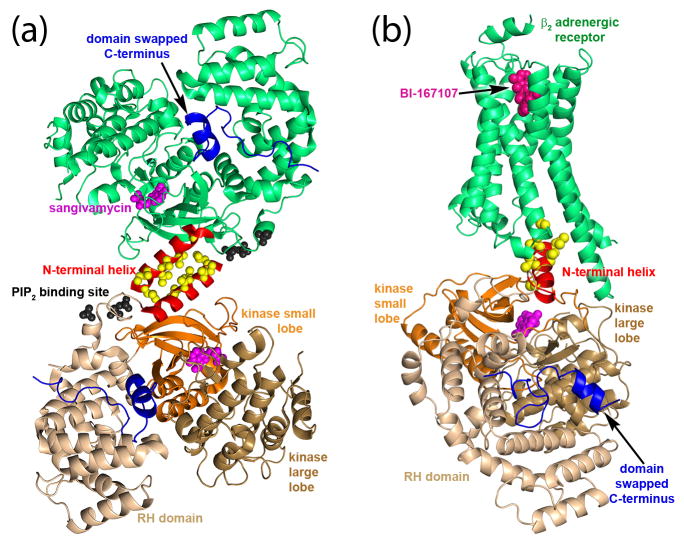
Recent structural studies of a GRK6·inhibitor complex have led to an alternative hypothesis for the role of the N-terminus. In the crystal structure of the GRK6 in complex with sangivamycin, the kinase domain adopts a relatively closed conformation mimicking that of active PKA [7]. The N-terminal region forms an extended helix that packs against the small lobe and a section of the C-terminal extension of the kinase domain called the active site tether (AST), forming a stabilizing bridge between the large and small lobes that spans the active site. Conserved hydrophobic residues at the end of the N-terminal helix point outward from the core of the molecule (Figure 3a). Mutation of these outward facing hydrophobic residues diminished receptor phosphorylation but not peptide phosphorylation, whereas mutation of residues in the N-terminal helix that interact with the small lobe and C-terminal kinase extension significantly reduced both activities. Analogous functional studies of GRK1 [10,33] and GRK2 [9,34] generated similar results. Thus, it was proposed that the N-terminal helix together with the C-terminal extension form the docking site for activated GPCRs, with outward facing hydrophobic residues forming direct contacts with receptors. Mg2+·ATP-induced order in the N-terminal region of GRK1 has been observed by deuterium exchange mass spectrometry, consistent with a functional linkage between binding substrates and formation of a receptor docking site that bridges the active site [35].
Figure 3.
Structural insights into the role of the extreme N-terminus of GRKs. (a) In the GRK6·sangivamycin structure (PDB entry 3NYN), the N-terminal helix (red), and the closed conformation of the kinase domain, is stabilized by a crystal contact from a symmetry related molecule. This interaction may serve as a surrogate for interactions that would be made by an activated GPCR or by the plasma membrane. Yellow spheres indicate the position of conserved hydrophobic residues that either interact with activated GPCRs (see model in panel b) or with the acyl phase of the phospholipid bilayer. The C-terminus of the enzyme (blue), which contains an amphipathic helix that is also known to be a membrane-binding determinant, is observed to dock between the RH domain (wheat) and kinase domain (large lobe in sand and small lobe in orange) via a domain swap from a symmetry related molecule. Magenta spheres depict sangivamycin bound in the active site and black spheres depict sulfate anions that likely demark one or more PIP2 binding sites. (b) Theoretical model of the interaction between GRK6 and the β2 adrenergic receptor (green). To create this model, conserved hydrophobic residues (yellow spheres) in the N-terminal helix of GRK6 (red cartoon) were aligned with those present in the C-terminal helix of Gαs in the structure of the receptor–Gs complex (PDB entry 3SN6): (α-carbons of Val10, Val9, and Leu6 aligned with those of Leu388, His387, and Tyr391, respectively). The ligands sangivamycin and the agonist BI-167107 (hot pink) are shown as spheres.
It remains an open question as to whether loss of activity resulting from mutation of hydrophobic residues at the extreme N-termini of GRKs results from a defect in binding phospholipid membranes or receptors. It is possible that both are true given experimental context. However, both models are consistent in their prediction that the N-terminus is critical for stabilizing the kinase domain of the GRK in an active conformation. This configuration is likely similar to that seen in the GRK6·sangivamycin structure. Models of GRK–GPCR complexes have been generated using the recently solved Gs–β2-adrenergic receptor crystal structure, assuming that the N-terminal helix of GRKs plays a similar role to the C-terminal helix of Gα subunits (Figure 3) [36–38]. Such theoretical models at minimum represent a starting point for the design of new experiments geared at testing the receptor docking site hypothesis.
Structure and Location of the Anionic Phospholipid Binding Site
Each GRK sub-family is localized to the membrane through different mechanisms (Figure 1): GRK1 subfamily members are prenylated, GRK2 subfamily members bind to Gβγ, which is itself prenylated, and GRK4 subfamily members contain two basic regions: immediately following the N-terminal helix and at their C-terminus, which in some isoforms is also palmitoylated [36]. In addition to membrane localization, anionic phospholipids are required for full activity of GRK2 and GRK4 sub-family members [36]. The PH domain of GRK2 mediates both PIP2 and Gβγ interactions [39] but as of yet there is no well-defined structural binding site for PIP2. An expansive, basic surface present on the GRK2–Gβγ complex has been proposed to interact with the negatively-charged inner leaflet of the membrane, an idea that is supported experimentally by sum-frequency generation spectroscopy of the GRK2–Gβγ complex on model membranes [40]. In the GRK6·sangivamycin structure [7], bound sulfate anions are thought to mimic phosphates in PIP2 and interact with residues immediately adjacent to the N-terminal helix (Figure 3). If these anions represent the PIP2 binding site, then GPCR and PIP2 binding is likely highly cooperative. In the same structure, the C-terminal basic region, expected to form an amphipathic helix when bound to membranes [41], docks at a site far removed from the predicted membrane surface (Figure 3b). This was unanticipated because the C-terminus of GRK6 is disordered in the crystal structure of the GRK6·AMPPNP complex [6], which would permit the C-terminal helix to bind to the membrane at the same time as the N-terminal basic region. Although the position of the C-terminal helix in the GRK6·sangivamycin structure may simply reflect a crystallographic artifact, there are a number of alternative interpretations [36], the most intriguing of which is that the C-terminal helix switches from a membrane-binding determinant to a structural element that stabilizes the active conformation of the kinase domain when the enzyme is productively engaged with an GPCR.
Conclusions
Recent structural analyses of GRKs have greatly expanded our molecular understanding of this family of enzymes. These atomic models serve as templates for structure-based rational drug design and have spawned new hypotheses regarding the molecular nature of their interactions with activated receptors and phospholipids. The identification of paroxetine as an effective inhibitor of GRK2 in vitro, in cells, and presumably in live animals strongly suggests that more potent GRK2-selective molecules with drug-like properties can be identified and/or developed. The fact that some small molecules exhibit nanomolar potency with high selectivity indicates that off-target effects can be minimized despite the close homology of GRKs to other AGC kinases. Such compounds would be important tools for deciphering GRK function in living cells and in some cases may serve as therapeutic leads. The most pressing question regarding GRK function at the structural level, however, remains the molecular basis of its interaction with activated GPCRs. Although the analogy between GRKs and Gα subunits in terms of their receptor interactions is attractive, it is not easy to interpret existing functional data because mutations in the N-terminus of GRKs could lead to defects in either receptor or phospholipid binding, either of which could lead to loss of activity. The crystal structure of a GRK–GPCR complex would give much needed clarity.
Acknowledgments
This work was supported by the National Institute of Health grants HL071818 & HL086865 (to J.J.G.T.) and the American Heart Association grant N014938 (to K.T.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mushegian A, Gurevich VV, Gurevich EV. The origin and evolution of G protein-coupled receptor kinases. PloS one. 2012;7:e33806. doi: 10.1371/journal.pone.0033806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Wang B, Maeda T, Palczewski K, Tesmer JJ. Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J Biol Chem. 2008;283:14053–14062. doi: 10.1074/jbc.M708974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 5.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 6.Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem. 2006;281:16785–16793. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- **7.Boguth CA, Singh P, Huang CC, Tesmer JJ. Molecular basis for activation of G protein-coupled receptor kinases. EMBO J. 2010;29:3249–3259. doi: 10.1038/emboj.2010.206. Describes the first structure of a GRK in what is believed to be an active configuration. A putative GPCR docking site is revealed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesmer JJ. Structure and function of regulator of G protein signaling homology domains. Prog Mol Biol Transl Sci. 2009;86:75–113. doi: 10.1016/S1877-1173(09)86004-3. [DOI] [PubMed] [Google Scholar]
- 9.Sterne-Marr R, Leahey PA, Bresee JE, Dickson HM, Ho W, Ragusa MJ, Donnelly RM, Amie SM, Krywy JA, Brookins-Danz ED, et al. GRK2 activation by receptors: role of the kinase large lobe and carboxyl-terminal tail. Biochemistry. 2009;48:4285–4293. doi: 10.1021/bi900151g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Yoshino-Koh K, Tesmer JJ. A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J Biol Chem. 2009;284:17206–17215. doi: 10.1074/jbc.M809544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannan N, Haste N, Taylor SS, Neuwald AF. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc Natl Acad Sci U S A. 2007;104:1272–1277. doi: 10.1073/pnas.0610251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metaye T, Gibelin H, Perdrisot R, Kraimps JL. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 14.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Lefkowitz RJ, Koch WJ. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-κB-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 16.Gold JI, Gao E, Shang X, Premont RT, Koch WJ. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ Res. 2012;111:1048–1053. doi: 10.1161/CIRCRESAHA.112.273367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, et al. Nuclear translocation of cardiac G protein-coupled receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One. 2013;8:e57324. doi: 10.1371/journal.pone.0057324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 19.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, et al. AAV6βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer G, Wulffen B, Huber C, Brockmann J, Flicke B, Neumann L, Hafenbradl D, Klebl BM, Lohse MJ, Krasel C, et al. An RNA molecule that specifically inhibits G-protein-coupled receptor kinase 2 in vitro. RNA. 2008;14:524–534. doi: 10.1261/rna.821908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Tesmer VM, Lennarz S, Mayer G, Tesmer JJ. Molecular mechanism for inhibition of G protein-coupled receptor kinase 2 by a selective RNA aptamer. Structure. 2012;20:1300–1309. doi: 10.1016/j.str.2012.05.002. Describes the atomic structure of GRK2 in complex with a potent and selective RNA aptamer. High affinity binding seems to be achieved via emulating the binding of ATP in the active site and interactions with the large lobe, which is remodeled by the aptamer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benovic JL, Stone WC, Caron MG, Lefkowitz RJ. Inhibition of the β-adrenergic receptor kinase by polyanions. J Biol Chem. 1989;264:6707–6710. [PubMed] [Google Scholar]
- 23.Palczewski K, McDowell JH, Hargrave PA. Rhodopsin kinase: substrate specificity and factors that influence activity. Biochemistry. 1988;27:2306–2313. doi: 10.1021/bi00407a010. [DOI] [PubMed] [Google Scholar]
- 24.Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem. 2010;53:1867–1870. doi: 10.1021/jm9017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda S, Keneko M, Fujiwara S. Cardiotonic agent comprising GRK inhibitor. US Patent. 2007
- *26.Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol. 2011;80:294–303. doi: 10.1124/mol.111.071522. Characterizes the molecular interaction of potent and selective small molecule probes produced by Takeda Pharmaceuticals with GRK2. Functional studies show that selectivity is more likely dicated by kinase domain conformation than by identity of residues in the active site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LN. Protein kinase inhibitors: contributions from structure to clinical compounds. Q Rev Biophys. 2009;42:1–40. doi: 10.1017/S0033583508004745. [DOI] [PubMed] [Google Scholar]
- 28.Homan KT, Wu E, Wilson MW, Singh P, Larsen SD, Tesmer JJG. Structural and Functional Analysis of G Protein-Coupled Receptor Kinase Inhibition by Paroxetine and a Rationally Designed Analog. Molecular Pharmacology. 2013 doi: 10.1124/mol.113.089631. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, et al. Paroxetine is a direct inhibitor of G protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7:1830–1839. doi: 10.1021/cb3003013. Identifies the drug paroxetine as a relatively selective inhibitor of GRK2 in vitro, in cells, and presumably in live animals. Structural analysis shows that the drug binds in the active site cleft of the enzyme and stabilizes the kinase domain in a conformation that may resemble an ADP-bound state (see also Ref. 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palczewski K, Buczylko J, Lebioda L, Crabb JW, Polans AS. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J Biol Chem. 1993;268:6004–6013. [PubMed] [Google Scholar]
- 31.Noble B, Kallal LA, Pausch MH, Benovic JL. Development of a yeast bioassay to characterize G protein-coupled receptor kinases. Identification of an NH2-terminal region essential for receptor phosphorylation. J Biol Chem. 2003;278:47466–47476. doi: 10.1074/jbc.M308257200. [DOI] [PubMed] [Google Scholar]
- *32.Pao CS, Barker BL, Benovic JL. Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry. 2009;48:7325–7333. doi: 10.1021/bi900408g. Provides data to support an alternative hypothesis in which the N-terminus of GRK2 interacts directly with lipid bilayers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Huang CC, Orban T, Jastrzebska B, Palczewski K, Tesmer JJ. Activation of G protein-coupled receptor kinase 1 involves interactions between its N-terminal region and its kinase domain. Biochemistry. 2011;50:1940–1949. doi: 10.1021/bi101606e. Following up Boguth et al. (2010), this paper provides additional evidence that the N-terminal helix of GRKs interacts directly with the kinase domain. Functional analysis of the N-terminus of GRK1 suggests, as in the case of GRK6, that its conserved hydrophobic residues interact directly with GPCR substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalski KR, Beautrait A, Lopez TS, Mannix KM, McDonald DJ, Cutter AR, Medina CB, Charnelle JF, Bouvier M, Tesmer JJG, et al. Functional Analysis of the G Protein-Coupled Receptor (GPCR) Docking Site of GPCR Kinase 2: Requirement for N-terminal Helix, Active Site Tether, and Kinase Small Lobe Collaboration. J Biol Chem. 2013 In review. [Google Scholar]
- 35.Orban T, Huang CC, Homan KT, Jastrzebska B, Tesmer JJ, Palczewski K. Substrate-induced changes in the dynamics of rhodopsin kinase (G protein-coupled receptor kinase 1) Biochemistry. 2012;51:3404–3411. doi: 10.1021/bi300295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homan KT, Glukhova A, Tesmer JJ. Regulation of G protein-coupled receptor kinases by phospholipids. Curr Med Chem. 2013;20:39–46. [PubMed] [Google Scholar]
- **37.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. This remarkable atomic structure serves as a model for how heterotrimeric G proteins recognize an activated GPCR. Other protein families that interact selectively with activated GPCRs, such as GRKs and arrestins, may use a similar strategy to that of Gs. See Ref. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CC, Tesmer JJ. Recognition in the face of diversity: interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. J Biol Chem. 2011;286:7715–7721. doi: 10.1074/jbc.R109.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carman CV, Barak LS, Chen C, Liu-Chen LY, Onorato JJ, Kennedy SP, Caron MG, Benovic JL. Mutational analysis of Gβγ and phospholipid interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2000;275:10443–10452. doi: 10.1074/jbc.275.14.10443. [DOI] [PubMed] [Google Scholar]
- *40.Boughton AP, Yang P, Tesmer VM, Ding B, Tesmer JJ, Chen Z. Heterotrimeric G protein β1γ2 subunits change orientation upon complex formation with G protein-coupled receptor kinase 2 (GRK2) on a model membrane. Proc Natl Acad Sci U S A. 2011;108:E667–673. doi: 10.1073/pnas.1108236108. This study used an unusual approach called sum frequency generation spectroscopy to experimentally determine the orientation of Gβγ and its complex with GRK2 on model membranes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiyagarajan MM, Stracquatanio RP, Pronin AN, Evanko DS, Benovic JL, Wedegaertner PB. A predicted amphipathic helix mediates plasma membrane localization of GRK5. J Biol Chem. 2004;279:17989–17995. doi: 10.1074/jbc.M310738200. [DOI] [PubMed] [Google Scholar]