Abstract
Objectives
The molecular mechanism of postoperative cognitive dysfunction is largely unknown. Isoflurane has been shown to promote Alzheimer’s disease neuropathogenesis. We set out to determine whether the effect of isoflurane on spatial memory is associated with amyloid-beta (A-beta) levels and tau phosphorylation in aged rats.
Methods
Eighteen-month-old male Sprague–Dawley rats were randomly assigned as anesthesia group (n = 31, received 1.4% isoflurane for 2 hours and had behavioral testing), training group (n = 20, received no anesthesia but had behavioral testing), and control group (n = 10, received no anesthesia and had no behavioral testing). Spatial memory was measured before and 2 days after the anesthesia by the Morris water maze. We divided the anesthesia group into an isoflurane-induced severe memory impairment group (SIG, n = 6) and a no severe memory impairment group (NSIG, n = 25), according to whether the escape latency was more than 1.96 stand deviation of that from the training group. Levels of A-beta and tau in the hippocampus were determined by enzyme-linked immunosorbent assay and quantitative western blot at the end of behavioral testing.
Results
We found that isoflurane increased the escape latency in the SIG as compared to that in the training group and NSIG without affecting swimming speed. However, there were no differences in the levels of A-beta and tau among SIG, NSIG, training, and control groups.
Conclusions
Isoflurane may induce spatial memory impairment through non-A-beta or tau neuropathogenesis mechanisms in aged rats.
Keywords: Amyloid-beta, Hippocampus, Isoflurane, Morris water maze, Tau
Introduction
Postoperative cognitive dysfunction (POCD) was first reported by Bedford in 1955,1 and defined as a condition which could reduce patients’ quality of life, stress family members, and increase risk of mortality. In two international studies of POCD, 25.8 and 41.4% of elder patients suffered from POCD a week after anesthesia and surgery.2,3 Age appears to be the primary risk factor for POCD,4,5 but recently exposure to anesthetics is frequently cited as a potential cause.6
The molecular mechanisms underlying POCD have not been well understood so far. Its clinical manifestations are similar to those observed in early Alzheimer’s disease (AD), and some patients with POCD may progress to AD.7 Current evidence suggests that anesthesia might be associated with cognitive impairment in elderly patients and increase risk for AD.8 It seems that risk factors for POCD may overlap with those for AD, although shared mechanisms remain unclear.
AD is characterized by initial mild cognitive impairment that progressively develops into a loss of higher cognitive functions resulting in severe dementia. Senile plaques and intraneuronal neurofibrillary tangles play fundamental roles in the AD brain.9 Senile plaques are composed of extracellular aggregates of the amyloid-beta (A-beta) and neurofibrillary tangles are composed of abnormally hyperphosphorylated tau protein. Disturbances in the production or clearance of these two proteins form the pathologic basis of AD. Earlier in vitro and in vivo studies showed that inhaled anesthetics promoted oligomerization of A-beta, increased its level,8,10–12 and induced tau phosphorylation,13,14 although some other investigators revealed a lack of association between exposure to general anesthesia and AD.15 It is not clear whether POCD patients undergo AD-like neuropathogenesis.
These questions prompted us to test whether changes in A-beta levels and tau protein metabolism are involved in isoflurane-induced cognitive dysfunction in an aged-rat model. In the present study, we subjected aged rats to 1.4% isoflurane for 2 hours and studied subsequent behavior using the Morris water maze (MWM). We then measured the protein expression of A-beta, total tau protein (T-Tau), p-Tau-Thr231 (pT231), p-Tau-Ser396 (pS396), and their regulating enzymes including beta-secretase (BACE), insulin degrading enzyme (IDE), neprilysin (NEP), glycogen synthase kinase 3-beta (GSK-3-beta), and protein phophatase-2A (PP2A). At last, we studied the relationship between cognitive function and changes in these biomarkers.
Methods
Animals
The animal protocol was approved by the Standing Committee on Animals at Capital Medical University. We acquired 18- to 19-month-old male Sprague–Dawley rats from a commercial supplier (Institute of Experimental Animal of Medical Scientific Academy in Sichuan, Chengdu, China). All rats were bred under controlled laboratory conditions; temperature: 22 ± 2°C; humidity: 55 ± 5%; 12 hours/12 hours light/dark cycle. Food and water were available ad libitum. Animals were allowed to acclimate to their new environment for 2 weeks. Sixty-one rats were randomly separated into three groups: (1) anesthesia group (n = 31); (2) training group (n = 20); and (3) control group (n = 10).
Anesthetic exposures
Rats in the anesthesia group received 1.4% isoflurane (NO26C818A; Baxter Healthcare, Deerfield, IL, USA) in 100% oxygen (anesthesia machine: Sulla 909V; Drägger, Luebeck, Germany) for 2 hours in an anesthetizing chamber. They breathed spontaneously, and the temperature was controlled using a heating pad (CMA150; CMA/Microdialysis AB company, Sweden) to maintain the rectal temperature at 37.5 ± 0.5°C. Arterial blood pressure was measured non-invasively using a tail cuff (non-invasive sphygmomanometers: ZH-HX-Z; Huaibei Zhenghua Biologic Apparatus Facilities Ltd, Huaibei, China). Arterial blood gas was measured intermittently by the blood gas analyzer (i-STAT; Abbott, Princeton, NJ, USA). Rats received 100% O2 after anesthesia and returned right reflex in a few minutes. Rats in the training group were exposed to air for 2 hours in the identical chamber.
Behavioral testing
The MWM consisted of a circular pool of 150 cm in diameter and 50 cm in height, and the water was 31 cm deep and 22 ± 1°C. The pool area was divided into four equal quadrants: SW, NW, SE, and NE. A colorless and transparent platform, which was 9 cm in diameter and 2 cm below the water level, was located in the center of the SW quadrant. The visual cues and illumination remained unchanged. In reference memory testing, rats were trained to find the escape platform. They were trained for eight trials every day with an interval of 30 minutes between each trial. The maximum swimming time in each trial was 120 seconds, and trials were ended with the animal finding the platform. If the time limit was exceeded, the animal was gently guided to platform where it remained for 30 seconds. The time to reach the platform (escape latency), swimming track, and swimming speed were recorded by a video track system.16,17 The spatial probe test was performed after the last reference memory test. The platform was removed, and then the rats were placed into the water from a random quadrant. The swimming time was 120 seconds. The swimming track and the time spent in the target quadrant were recorded.16,17 The MWM investigators were blind to the animal grouping.
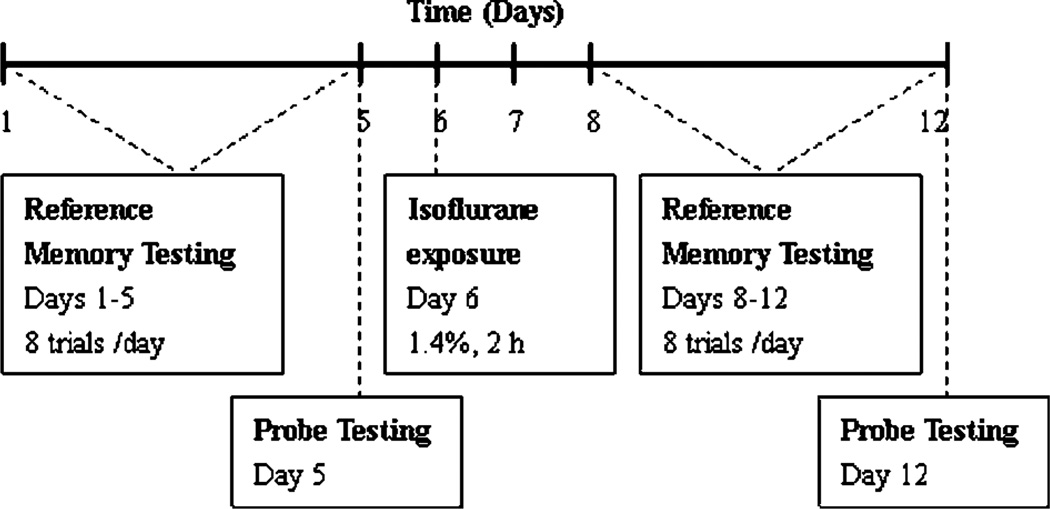
A schematic of the experimental design is shown in Fig. 1. For the rats in the anesthesia group, reference memory tests were performed before and 2 days after anesthetic exposures, and the trainings lasted for 5 days. Probe tests were performed on day 5 and day 12 after the last reference memory test. For the rats in the training group, behavioral tests were performed at the same times as the anesthesia group. For the rats in the control group, anesthesia and behavioral tests were not performed.
Figure 1.
Schematic time-line of the experimental design.
Subdivision
We found that there was individual difference of prolonged escape latency in aged rats. According to clinical diagnosis of POCD, a rat was considered to have severe memory impairment if its prolonged escape latency of each day after anesthesia was more than 1.96 standard deviation (SD) of the mean escape latency in the training group. The method of clinical diagnosis of POCD is ‘Z score’ which was used to sieve POCD patients in two large clinical studies in 1998 and 2008.2,3 There were six rats which exhibited severe post-anesthesia memory impairments. The incidence of isoflurane-induced severe memory impairment was 19.35%. The other 25 rats in the anesthesia group had no severe memory impairments, defined as their prolonged escape latency of each day after anesthesia were equal or less than 1.96 SD. Consequently, we divided the rats from the anesthesia group into an isoflurane-induced severe memory impairment group (SIG, n = 6) and a no severe memory impairment group (NSIG, n = 25). We described the animal grouping in Table 1.
Table 1.
Animal grouping
| MWM (days 1–5) |
Iso (day 6) |
MWM (days 8–12) |
Subdivision | |
|---|---|---|---|---|
| Con (n = 10) | No | No | No | No |
| Tra (n = 20) | Yes | No | Yes | No |
| Ane (n = 31) | Yes | Yes | Yes | SIG (n = 6) NSIG (n = 25) |
Note: Ane, anesthesia group; Con, control group; Iso, isoflurane exposure; MWM, Morris water maze; NSIG, no severe memory impairment group; SIG, severe memory impairment group; Tra, training group.
After behavioral tests, all of rats were sacrificed by decapitation and their hippocampi were dissected for biochemical study.
Enzyme-linked immunosorbent assay (ELISA)
We measured soluble A-beta in the hippocampus using ELISA kits (Human/Rat beta Amyloid 40 ELISA Kit Wako, 294-64701 and Human/Rat beta Amyloid 42 ELISA Kit Wako, 292-64501). The tissues were homogenized in 70% formic acid, and the homogenates were centrifuged at 100 000g for 1 hour. The supernatants were diluted 20-fold with 1M Tris Base to neutralize the acid. Then the samples were diluted with the Standard Diluent in the kit, and the protein concentration was measured as instructed. Monoclonal antibody coated 96-well separable micro-plates were used to capture A-beta. Captured A-beta was recognized by another antibody labeled with horseradish peroxidase. After addition of tetramethyl benzidine solution, positive samples developed blue. The reaction was terminated by the addition of the Stop Solution, which produced a yellow color. The absorbance was then measured at 450 nm. The A-beta concentrations for unknown samples and controls were read from the standard curve.
Western blot analysis
Hippocampi were immediately removed on ice and frozen in liquid nitrogen. Frozen hippocampi were weighed and homogenized in epoxydicarboxylic fatty acid buffer [50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM dithiothreitol, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, 1% TritonX-100, 0.01% sodium dodecyl sulfate, 50 µg/ml phenylmethylsulfonyl fluoride, and protease inhibitors (1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 µg/ml pepstatin)] with a handheld homogenizer. The homogenates were then centrifuged for 10 minutes at 12 000g at 4°C. Protein content of the supernatants was quantified using a Coomassie Blue Fast Staining Solution (sc215013; Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacture’s instructions. Equal amounts of protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk in Tris-buffered saline Tween-20 for 1 hour, the membranes were probed with primary antibodies: BACE (1 : 500; MAB5308; Chemicon, Temecula, CA, USA), IDE (1 : 800; ab32216; Abcam, Cambridge, MA, USA), NEP (1 : 1000; AB5458; Millipore), T-Tau (1 : 1000; sc-32274; Santa Cruz Biotechnology, Santa Cruz, CA, USA), GSK-3-beta (1 : 2000; sc-9166; Santa Cruz Biotechnology), PP2A (1 : 600; sc-13600; Santa Cruz Biotechnology), pT231 (1 : 500; ab30665; Abcam), and pS396 (1 : 2500; ab32057; Abcam). Primary antibodies were incubated overnight at 4°C and beta-actin (1 : 4000; sc-1616-R; Santa Cruz Biotechnology) was used as the loading control. The proteins were detected using horseradish peroxidase-conjugated anti-rabbit (1: 4000; sc2004; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or antimouse (1: 2000; sc2005; Santa Cruz Biotechnology, Santa Cruz, CA, USA) secondary antibodies at room temperature. The membranes were then treated with an enhanced chemiluminescence kit (Santa Cruz Biotechnology), and the image developed on film and the films were scanned. We analyzed the blots using Image-Pro Plus 6.0 software. The relative expression of the protein was normalized to beta-actin.
Statistical analysis
The results were expressed as mean ± SD. The escape latency and swimming speed of the 10 daily trials were averaged to give a mean value for each day of testing. The escape latency and swimming speed were analyzed by one-way analysis of variance with repeated measures. Time in the target quadrant and the relative expression of proteins were analyzed by one-way analysis of variance without repeated measures. When appropriate, post hoc testing was performed using the least significant difference procedure. All statistical data were analyzed using SPSS 11.5 for windows. Statistical significance was set at P<0.05.
Results
Reference memory testing
There were no significant differences in the escape latencies between the anesthesia group and the training group before and after anesthesia. Isoflurane did not affect motor function of rats, since the average swimming speed was the same for all groups during testing.
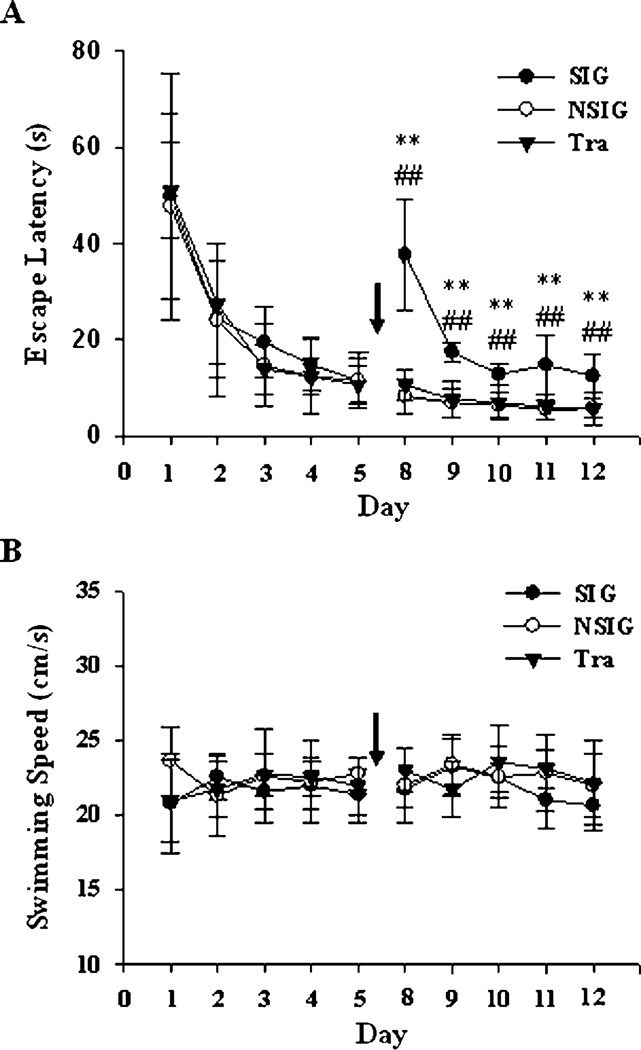
After subdivision, there were no significant differences in the escape latencies among the SIG and other groups before anesthesia. We found that isoflurane increased the escape latency in the SIG as compared to those in the training group (P<0.01) and NSIG (P<0.01) without affecting swimming speed (Fig. 2). And the physical sighs were normal during anesthesia (Table 2).
Figure 2.
Escape latency (A) and average swimming speed (B) during the reference memory testing. After subdivision, the average escape latency of SIG was longer significantly than those of NSIG and the training group on each day post-exposure to isoflurane. We described the analysis results of escape latency in Table 3 (df = 2). No significant difference in swimming speed was found between SIG and the other two groups. SIG: severe memory impairment group, black square and solid line, n = 6; NSIG: no severe memory impairment group, open square and solid line, n = 25; Tra: training group, black triangle and solid line, n = 20; black arrow: isoflurane exposure. Data are expressed as mean ± standard deviation, analysis of variance with least significant difference post hoc test. **P<0.01, SIG versus Tra; ##P<0.01, SIG versus NSIG.
Table 2.
Physical sighs during anesthesia of the SIG and NSIG
| HR (bpm) | MBP (mmHg) | SpO2 (%) | pH | pCO2 (mmHg) | pO2 (mmHg) | |
|---|---|---|---|---|---|---|
| SIG (n = 6) | 353.3 ± 35.3 | 100.1 ± 4.4 | 97.3 ± 1.4 | 7.432 ± 0.028 | 42.8 ± 1.8 | 322.7 ± 55.3 |
| NSIG (n = 25) | 365.7 ± 27.4 | 98.7 ± 3.3 | 96.8 ± 1.1 | 7.424 ± 0.02 | 42.0 ± 2.6 | 321.4 ± 61.2 |
Note: NSIG, no severe memory impairment group; SIG, severe memory impairment group; HR, heart rate; MBP, mean blood pressure.
Probe testing
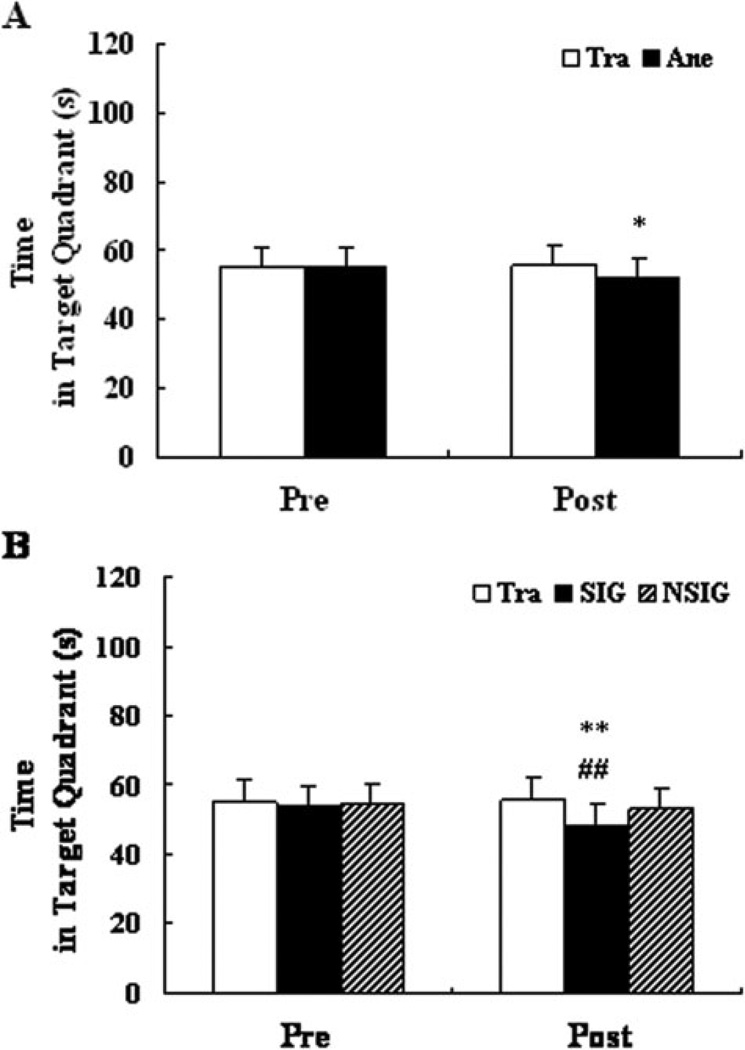
Probe tests were performed before and after anesthesia. There were no differences between the anesthesia group and the training group before anesthesia. However, after anesthesia, the rats in the anesthesia group spent less time in the target quadrant searching for the platform than those in the training group (Fig. 3A, P<0.05).
Figure 3.
The time that rats spent in the target quadrant during probe testing. Before subdivision (A), compared to the training group, the rats in the anesthesia group (52.74 ± 3.87) spent less time in the target quadrant than that in the training group (55.00 ± 3.28) after isoflurane exposure (F = 4.67, df = 1, P = 0.036). After subdivision (B), the rats in the SIG (49.07 ± 0.88) spent less time in the target quadrant than those in the training group (55.00 ± 3.28) and NSIG (53.62 ± 3.79) after isoflurane exposure (F = 7.062, df = 3, P = 0.02). Before isoflurane exposure, no significant differences in time in the target quadrant were found among groups before and after subdivision. Tra: training group, n = 20; Ane: anesthesia group, n = 31; SIG: severe memory impairment group, n = 6; NSIG: no severe memory impairment group, n = 25; Pre: pre-exposure to isoflurane; Post: post-exposure to isoflurane. Data are expressed as mean ± standard deviation. Analysis of variance with least significant difference post hoc test. *P<0.05, Ane versus Tra; **P<0.01, SIG versus Tra; ##P<0.01, SIG versus NSIG.
After subdivision, there were no differences among the SIG and other two groups before anesthesia. After anesthesia, the rats in the group SIG spent less time in the target quadrant than those in the training group (P<0.01) and NSIG (P<0.01, Fig. 3B).
Protein expression of A-beta, tau, and regulating enzymes
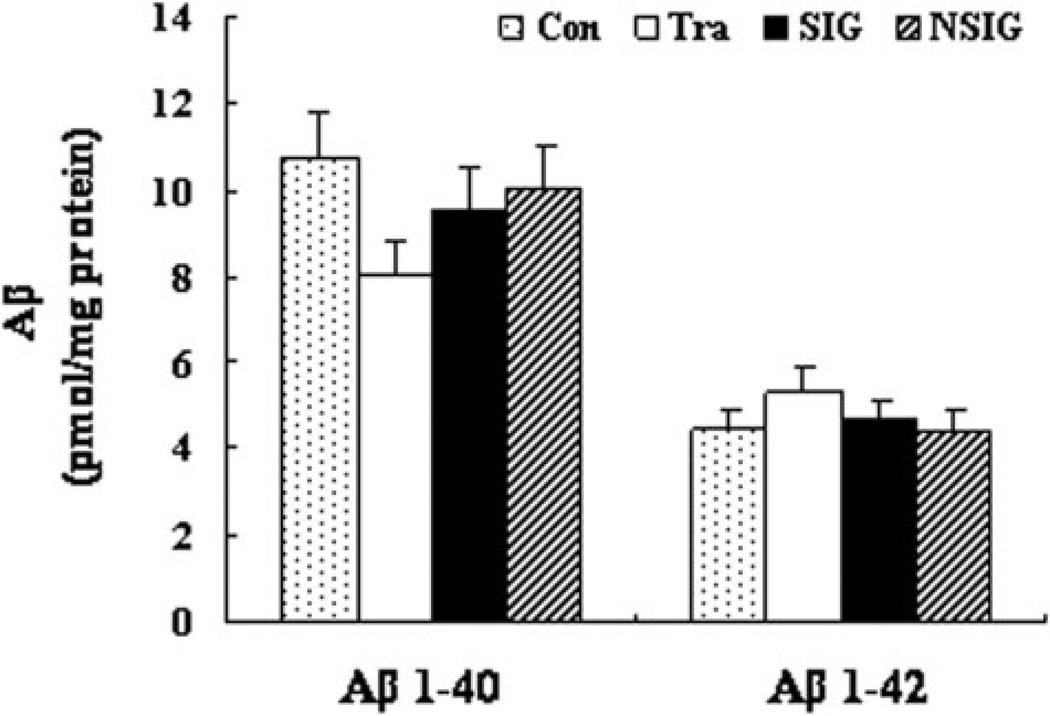
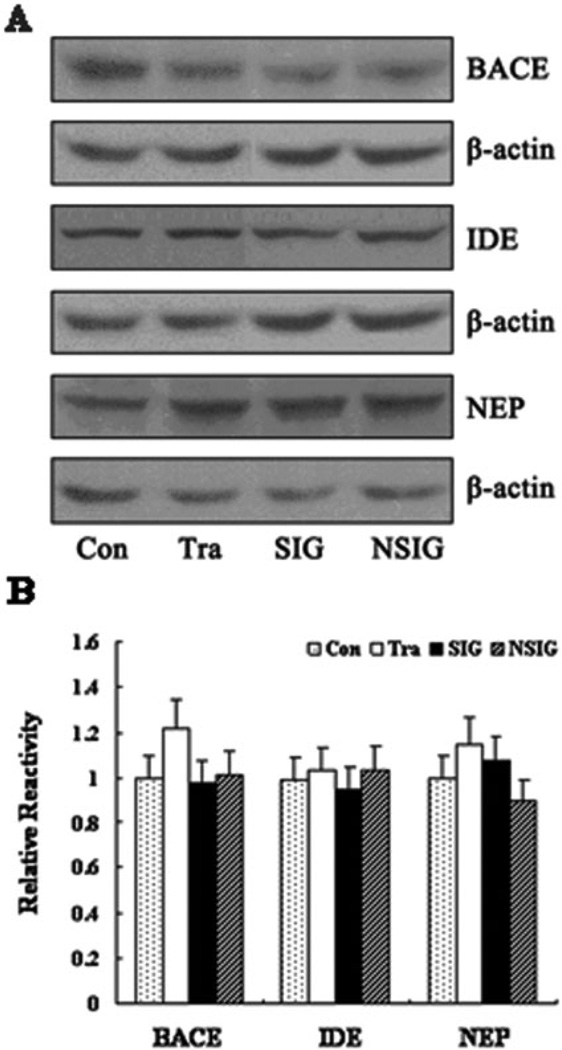
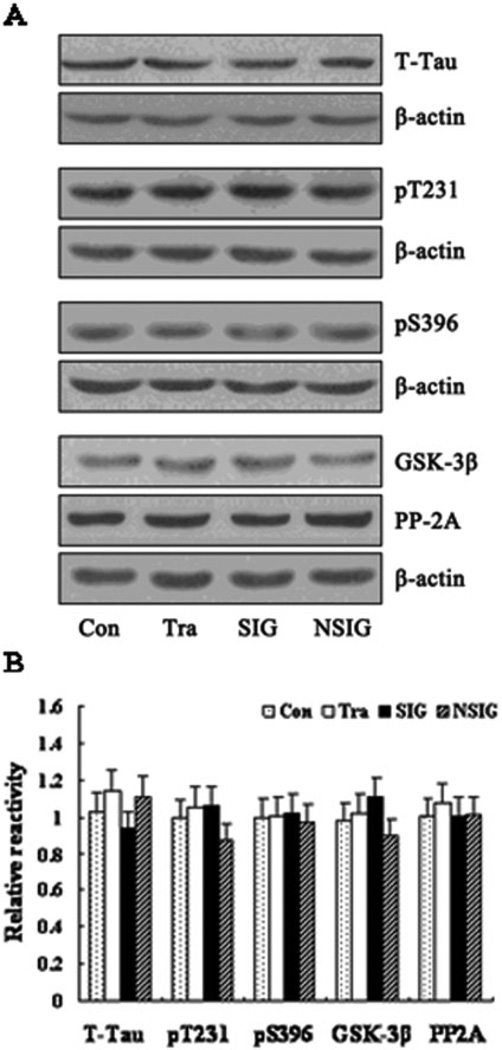
We first measured A-beta 1–40 and A-beta 1–42 levels in the hippocampus by ELISA and found that there was no significant difference in A-beta levels among the control, training, NSIG, and SIG groups (Fig. 4). Then, we assessed the effects of the isoflurane anesthesia on the protein levels of IDE, NEP, and BACE by quantitative western blot analysis. We found that the isoflurane anesthesia did not change the levels of IDE, NEP, and BACE, as evidenced that there was no significant difference in the levels of IDE, NEP, and BACE among the control, training, NSIG, and SIG groups (Fig. 5A and B). Finally, we found that there was no significant difference in the protein levels of T-Tau, pT231, pS396, GSK-3-beta, and PP2A in the hippocampus among the control, training, NSIG, and SIG groups (Fig. 6A and B).
Figure 4.
Quantification of A-beta 1–40 and A-beta 1–42 in the hippocampus by ELISA. There were no changes in A-beta levels among groups post-exposure to isoflurane. Con: control group; Tra: training group; SIG: severe memory impairment group; NSIG: no severe memory impairment group; n = 6–8 for each experimental group. Data are expressed as mean ± standard deviation. A-beta 1–40, Con: 10.72 ± 3.53, Tra: 8.06 ± 1.30, SIG: 9.57 ± 4.64, NSIG: 10.02 ± 2.02, F = 0.69, df = 3, P = 0.57; A-beta 1–42, Con: 4.46 ± 0.94, Tra: 5.28 ± 0.49, SIG: 4.57 ± 0.88, NSIG: 4.42 ± 0.88, F = 1.90, df = 3, P = 0.16.
Figure 5.
Expression of BACE, IDE, and NEP in the hippocampus in aged rats. (A) Western blot analysis of the expression of beta-secretase (BACE), insulin degrading enzyme (IDE), and neprilysin (NEP). The rows are BACE, IDE, NEP, and beta-actin. (B) Band density analysis of the western blot study. The BACE, IDE, and NEP levels in the hippocampus in aged rats from the different groups did not show significant differences. Beta-actin was used as an internal reference. Con: control group; Tra: training group; SIG: severe memory impairment group; NSIG: no severe memory impairment group; n = 3–5 for each experimental group. Data are expressed as mean ± standard deviation. BACE, Con: 1.00 ± 0.15, Tra: 1.10 ± 0.09, SIG: 0.98 ± 0.25, NSIG: 1.02 ± 0.10, F = 0.47, df = 3, P = 0.71; IDE, Con: 0.99 ± 0.10, Tra: 1.23 ± 0.12, SIG: 0.85 ± 0.21, NSIG: 1.23 ± 0.50, F = 1.62, df = 3, P = 0.24; NEP, Con: 0.71 ± 0.09, Tra: 1.15 ± 0.11, SIG: 1.08 ± 0.19, NSIG: 1.00 ± 0.10, F = 1.15, df = 3, P = 0.37.
Figure 6.
Expression of tau protein, GSK-3-beta, and PP2A in the hippocampus in aged rats. (A) Western blot analysis of the expression of total tau protein (T-Tau), p-Tau-Thr231 (pT231), p-Tau-Ser396 (pS396), glycogen synthase kinase 3-beta (GSK-3-beta), and protein phophatase-2A (PP2A). The rows are T-Tau, pS396, pT231, GSK-3-beta, PP2A, and beta-actin. (B) Band density analysis of the western blot. There were no significant differences in T-Tau, pS396, pT231, GSK-3-beta, and PP2A levels in the hippocampus between groups. Beta-actin was used as an internal reference. Con: control group; Tra: training group; SIG: severe memory impairment group; NSIG: no severe memory impairment group; n = 3–5 for each experimental group. Data are expressed as mean ± standard deviation. T-Tau, Con: 1.02 ± 0.05, Tra: 1.14 ± 0.24, SIG: 0.94 ± 0.10, NSIG: 1.11 ± 0.11, F = 1.54, df = 3, P = 0.26; pS396, Con: 1.00 ± 0.10, Tra: 1.01 ± 0.08, SIG: 1.02 ± 0.14, NSIG: 0.97 ± 0.10, F = 0.13, df = 3, P = 0.94; pT231, Con: 1.00 ± 0.14, Tra: 1.06 ± 0.14, SIG: 1.06 ± 0.09, NSIG: 0.88 ± 0.11, F = 1.69, df = 3, P = 0.22; GSK-3-beta, Con: 0.98 ± 0.14, Tra: 1.02 ± 0.07, SIG: 1.11 ± 0.09, NSIG: 0.98 ± 0.04, F = 1.65, df = 3, P = 0.23; PP2A, Con: 1.00 ± 0.13, Tra: 1.08 ± 0.10, SIG: 1.01 ± 0.10, NSIG: 1.01 ± 0.15, F = 0.34, df = 3, P = 0.79.
Discussion
In the current study, we found that anesthesia with 1.4% isoflurane for 2 hours caused learning and memory impairment in old rats. Since the prolonged escape latencies showed individual differences, the rats in the anesthesia group were divided according to whether prolonged escape latency after anesthesia was more than 1.96 SD of the mean escape latency of the training group.3 However, we did not find that the behavioral changes induced by isoflurane were related to A-beta levels or tau protein metabolism in the hippocampus of the aged rats.
Culley and colleagues have shown that anesthesia with 1.2% isoflurane plus 70% nitrous oxide for 2 hours could induce long-term impairment in the acquisition of a spatial memory task in aged rats.18,19 Bianchi’s study also indicated that the anesthesia with 0.9–1% isoflurane for 2 hours daily for 5 days would impair learning and memory in rats.15 In the present study, we found that a single exposure to a clinically relevant concentration of isoflurane anesthesia impaired learning and memory in aged (e.g. 18–19 months old) rats. These results suggest that isoflurane affects memory for a longer time and it could not be explained by its pharmacology. The isoflurane anesthesia did not alter the swimming speed of rats in MWM, suggesting that the impairment in MWM performance following the isoflurane anesthesia was indeed due to the changes in learning and memory, rather than motor function.
A-beta and tau protein exist in normal brains. A proteome study of the rat hippocampus showed that isoflurane may affect up to 12 proteins or their respective genes, which are associated with AD neuropathogenesis.20 Moreover, isoflurane has been shown to promote AD neuropathogenesis, e.g. apoptosis, A-beta accumulation, and tau protein hyperphosphorylation in cultured cells and in the brain tissues of wild-type rats.8,21 In current study, we sought to determine whether isoflurane-induced spatial memory decline was related to changes in A-beta levels and tau protein phosphorylation in aged wild-type rats.
Many studies have suggested that the steady levels of A-beta are determined by a balance between production and degradation. In the AD brain, BACE is elevated while IDE and NEP are decreased. Recent studies showed that anesthetics altered amyloid-precursor protein (APP) processing, increased aggregation of A-beta, and induced cytotoxicity in vitro.10 A follow-up study indicated that isoflurane increased levels of BACE and A-beta in rat brain tissue and the isoflurane-induced A-beta increase was most likely dose- and time-dependent.21 However, it is still largely unknown whether the isoflurane-induced learning and memory impairment is associated with the isoflurane-induced AD neuropathogenesis. In the current study, we measured the levels of A-beta 1–40, A-beta 1–42, and their regulating enzymes. We found that the isoflurane anesthesia did not change them, which are consistent with recent reports that general anesthetics did not affect cell death, APP metabolism, and A-beta levels in vivo.15,22,23 Our results suggest that isoflurane-induced spatial memory impairment may not be related to the A-beta pathway in aged rats. There is a report24 that rat carrying presenilin and APP mutations seemed especially vulnerable to some anesthetics. They found that APP transgenic rats treated with isoflurane have increased apoptotic cells and A-beta aggregation. However, these effects were not present in wide-type rats. Therefore, our future studies may include assessing the effects of isoflurane on learning, memory, and AD neuropathogenesis in the AD transgenic rats.
Tau hyperphosphorylation can be induced by a shift in the phophorylation equilibrium as a result of kinase activation or phosphatase inhibition. Hypothermia has een shown to cause tau protein hyperphosphorylation.25 Recent reports demonstrated that anesthesia induced hypothermia, which led to tau hyperphosphorylation by direct inhibition of phosphatase activity.14,15 In our study, core body temperature was precisely controlled and we measured phophorylation of two sites found in tau pathology in AD brain, pS396 and pT231. We found that the isoflurane anesthesia did not affect tau protein hyperphosphorylation in the brain tissues of aged rats. In addition, we did not detect any changes in PP2A or GSK levels 6 days after isoflurane anesthesia. Thus, the isoflurane anesthesia-induced spatial memory impairment might not be associated with tau hyperphosphorylation in old rats.
We set out to study the relationship between cognitive function and changes in A-beta and tau protein. We did not assess the time course of the isoflurane’s effects on A-beta, BACE, and tau protein hyperphosphorylation. These levels were only determined 7 days after the isoflurane anesthesia. It is possible that the isoflurane anesthesia changed these levels at earlier times (e.g. 12–24 hours) after the anesthesia as described by other studies.21 The future experiments will include a through time course study to determine the effects of isoflurane on the levels of A-beta, BACE, and tau protein hyperphosphorylation.
In conclusion, anesthesia with 1.4% isoflurane for 2 hours induces spatial memory impairment in aged rats. However, isoflurane-induced spatial memory impairment may be independent of the changes in A-beta levels and tau phosphorylation in wild-type aged rats. Other mechanisms, such as apoptosis, calcium dysregulation, and inflammatory reaction, may be responsible for the isoflurane-induced learning and memory impairment.8,20 These findings would lead to more studies to investigate the potential mechanisms by which isoflurane induced learning and memory impairment.
Table 3.
The analysis results of escape latency in reference memory testing after subdivision
| Day | Group | Mean ± SD | F value | P value |
|---|---|---|---|---|
| D1 | Tra | 51.02 ± 9.18 | 0.22 | 0.81 |
| SIG | 49.66 ± 25.63 | |||
| NSIG | 47.69 ± 19.29 | |||
| D2 | Tra | 27.39 ± 11.54 | 0.35 | 0.71 |
| SIG | 24.27 ± 11.98 | |||
| NSIG | 24.00 ± 15.90 | |||
| D3 | Tra | 13.86 ± 4.69 | 1.40 | 0.26 |
| SIG | 19.38 ± 7.44 | |||
| NSIG | 14.60 ± 8.61 | |||
| D4 | Tra | 12.31 ± 3.41 | 1.03 | 0.36 |
| SIG | 14.40 ± 5.131 | |||
| NSIG | 11.41 ± 5.35 | |||
| D5 | Tra | 10.58 ± 3.57 | 0.09 | 0.91 |
| SIG | 11.32 ± 4.36 | |||
| NSIG | 11.04 ± 4.95 | |||
| D8 | Tra | 10.59 ± 2.98 | 91.67 | <0.01 |
| SIG | 37.64 ± 11.52 | |||
| NSIG | 8.21 ± 3.54 | |||
| D9 | Tra | 7.55 ± 3.46 | 29.93 | <0.01 |
| SIG | 17.35 ± 1.90 | |||
| NSIG | 6.86 ± 2.88 | |||
| D10 | Tra | 6.84 ± 3.29 | 12.01 | <0.01 |
| SIG | 12.79 ± 2.23 | |||
| NSIG | 6.36 ± 2.77 | |||
| D11 | Tra | 5.98 ± 2.34 | 27.23 | <0.01 |
| SIG | 14.71 ± 6.14 | |||
| NSIG | 5.47 ± 1.93 | |||
| D12 | Tra | 5.73 ± 3.16 | 14.22 | <0.01 |
| SIG | 12.39 ± 4.57 | |||
| NSIG | 5.69 ± 2.09 |
Note: NSIG, no severe memory impairment group; SIG, severe memory impairment group; Tra, training group; SD, standard deviation.
Acknowledgement
Our study was supported by National Nature Science Foundation of China (grant no. 30872425).
References
- 1.Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–263. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS. Long-term post-operative cognitive dysfunction in the elderly ISPOCD1 study: ISPOCD investigators international study of post operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvin CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Perouansky M. General anesthetics and long-term neurotoxicity. Handb Exp Pharmacol. 2008;182:147–157. doi: 10.1007/978-3-540-74806-9_7. [DOI] [PubMed] [Google Scholar]
- 5.Perouansky M. Liaisons dangereuses? General anaesthetics and long-term toxicity in the CNS. Eur J Anaesthesiol. 2007;24:107–115. doi: 10.1017/S0265021506001165. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin ML, de Roquefeuil G, Ledesert B, Bonnel F, Cheminal JC, Ritchie K. Exposure to anaesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry. 2001;178:360–366. doi: 10.1192/bjp.178.4.360. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, Tanzi RE. Alzheimer’s disease and postoperative cognitive dysfunction. Exp Gerontol. 2006;41:346–359. doi: 10.1016/j.exger.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid β-protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc AC. The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- 10.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, et al. Inhaled anesthetic enhancement of amyloid-β oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Ghirlanda G, Hilcove SA, Pidikiti R, Johansson JS, Lear JD, DeGrado WF, et al. Volatile anesthetic modulation of oligomerization equilibria in a hexameric model peptide. FEBS Lett. 2004;578:140–144. doi: 10.1016/j.febslet.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 12.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid β-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Run X, Liang Z, Zhang L, Iqbal K, Grundke-Iqbal I, Gong CX. Anesthesia induces phosphorylation of tau. J Alzheimers Dis. 2009;16:619–626. doi: 10.3233/JAD-2009-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi SL, Tran T, Liu C, Lina S, Li Y, Keller JM, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Neurosci Methods. 1984;1:47–64. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Amaya V, Balderas I, Sandoval J, Escobar ML, Bermudez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. J Neurosci. 2001;21:7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–1397. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid β-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stratmann G, Sall JW, Bell JS, Alvi RS, May LDV, Ku B, et al. Isoflurane does not affect brain cell death, hippocampal neurogenesis, or Long-term neurocognitive outcome in aged rats. Anesthesiology. 2010;112:305–315. doi: 10.1097/ALN.0b013e3181ca33a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palotás M, Palotás A, Bjelik A, Pákáski M, Hugyecz M, Janka Z, et al. Effect of general anesthetics on amyloid precursor protein and mRNA levels in the rat brain. Neurochem Res. 2005;30:1021–1026. doi: 10.1007/s11064-005-6786-7. [DOI] [PubMed] [Google Scholar]
- 24.Perucho J, Rubio I, Casarejos MJ, Gomez A, Rodriguez-Navarro JA, Solano RM, et al. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1245–1257. doi: 10.3233/JAD-2010-1318. [DOI] [PubMed] [Google Scholar]
- 25.Tan W, Cao X, Wang J, Lv H, Wu B, Ma H. Tau hyperphosphorylation is associated with memory impairment after exposure to 1.5% isoflurane without temperature maintenance in rats. Eur J Anaesthesiol. 2001;27:835–841. doi: 10.1097/EJA.0b013e32833a6561. [DOI] [PubMed] [Google Scholar]