Abstract
Purpose
To quantify diabetic patients’ change in blood glucose levels after corticosteroid injection for common hand diseases and to assess which patient-level risk factors may predict an increase in blood glucose levels.
Methods
Patients were recruited for this case-crossover study in the clinic of fellowship-trained hand surgeons at a tertiary care center. Patients with diabetes mellitus type 1 or 2 receiving a corticosteroid injection recorded their morning fasting blood glucose levels for 14 days after their injection. Fasting glucose levels on days 1–7 after injection qualified as “case” data with levels on days 10–14 providing control data. A mixed model with a priori contrasts were used to compare post-injection blood glucose levels to baseline levels. A linear regression model was used to determine patient predictors of a post-injection rise in blood glucose levels.
Results
Forty of 67 patients (60%) recruited for the study returned completed blood glucose logs. There was a significant increase in fasting blood glucose levels following injection limited to post-injection days 1 and 2. Among patient risk factors in our linear regression model, type 1 diabetes and use of insulin each predicted a post-injection increase in blood glucose levels from baseline while higher HbA1c levels did not predict increases.
Discussion
Corticosteroid injections in the hand transiently increase blood glucose levels in diabetic patients. Patients with type 1 diabetes and insulin-dependent diabetics are more likely to experience this transient rise in blood glucose levels.
Level of Evidence
Therapeutic Level III
Keywords: Blood Glucose, Corticosteroid, Diabetes, Methylprednisolone
Introduction
Corticosteroid injections are commonly used to treat a variety of common hand and wrist conditions such as trigger finger, De Quervain tenosynovitis, and osteoarthritis. Local injection is often first-line therapy and is anticipated to provide temporary or lasting pain relief with the potential for a definitive cure as in the case of trigger fingers.1,2,3
Diabetic patients are at increased risk for developing trigger fingers.4 Previous studies have reported a 10 to 20% higher incidence of trigger finger in diabetic patients and a higher likelihood of multiple digit involvement than a non-diabetic patient.5,6,7,8 With prior data indicating only moderate long-term relief (36–60%) after corticosteroid injections for trigger finger in diabetic patients, the risks of injection must be weighed against potential benefits. 1,9,10
Corticosteroid injections in the knee, shoulder, and spine produce a short-term rise in blood glucose levels with no affect on long-term measures of blood glucose control (fructosamine or HbA1c).11,12,13 Two studies have reported transient increases in blood glucose after corticosteroid injection in the hand or wrist.10,14 Patient characteristics that distinguish an individual’s reaction to injection were not assessed. Some clinicians may be hesitant to administer corticosteroids due to the perceived risk of hyperglycemia following an injection coupled with potentially limited efficacy of injection in diabetic patients.
The purpose of this study was to quantify diabetic patients’ change in blood glucose levels after corticosteroid injection for common hand diseases and to assess which patient-level risk factors could predict an increase in blood glucose levels. We hypothesized that diabetic patients would show a significant but transient rise in blood glucose levels post-injection with greater increases in poorly controlled diabetics as measured by HbA1c.
Methods
This prospective case-crossover study evaluated the effect of corticosteroid injection in the hand on blood glucose levels in patients with diabetes. Diabetic patients treated with corticosteroid injections of methylprednisolone acetate completed a daily blood glucose log to determine changes in blood glucose levels following injection. This study was approved by our institutional review board.
Patients at least 18 years old undergoing clinically indicated corticosteroid injection for De Quervain disease, trigger finger, osteoarthritis, or carpal tunnel syndrome in the clinic of fellowship-trained hand surgeons at a tertiary care institution were considered for the study. Patients were included if they had a diagnosis of type 1 or 2 diabetes mellitus and checked blood glucose values daily. Patients were excluded if they were unable to provide consent, had received a corticosteroid injection in the previous 3 months, had been administered oral corticosteroid in the past 6 months, or had any contraindications to the administration of corticosteroids.
Patients were consented after electing to proceed with a corticosteroid injection following their discussion of treatment options and risks and benefits with their surgeon. Following the injection, we recorded the location (i.e., tendon sheath, joint) and amount of methylprednisolone acetate administered (range: 20–120 mg), patient demographics, diabetes type, most recent HbA1c result, and diabetes control regimen (diet, oral medication, and/or insulin).
Participants were provided a daily log to record their morning fasting blood glucose levels for 14 days after injection. All participants used personal glucometers to record their readings. Upon completion of the 14-day post- injection period, patients returned their logs via a self-addressed stamped envelope provided at the time of their corticosteroid injection.
Data Analysis
In this case-crossover design, participants’ first 7 days of blood glucose readings provided “case” outcome data. Days 8 and 9 represented the washout period, while the average of days 10–14 were used to determine baseline blood glucose levels. This design was based on prior literature of corticosteroid injections in diabetic patients’ knees, shoulders, spine, and hands that showed no statistically significant elevation in blood glucose levels after 5 days following injection.10,11,13,15,16 To assure a proper washout period, we doubled this time and did not consider participant blood glucose values to be at baseline until 10 days after injection. Recording blood glucose levels prospectively prior to a corticosteroid injection would have required withholding pharmacologic therapy for a painful condition and a second visit to the clinic for treatment, which would increase participant burden.
Our sample size analysis determined 40 patients were needed to detect a difference in 20 mg/dl in blood glucose level with a power of 0.90 using a paired data design with a 2-tailed alpha of 0.05. To analyze our data, we used a mixed model and a priori contrasts of paired t-tests to compare the calculated baseline fasting blood glucose levels to the blood glucose levels attained on each day of the first week post-injection. This model took into account each patient’s repeated contribution to the data. All 95% confidence intervals underwent a Bonferroni adjustment to account for multiple comparisons.
To determine which risk factors were associated with a rise in blood glucose levels a Fisher exact test was used. For this univariate analysis, we compared patients with a >50 mg/dl rise in fasting post-injection day 1 blood glucose levels with different patient risk factors (diabetes type, diabetes control regimen, sex, and location of injection). A rise in >50 mg/dl was chosen as it would represent an 80% change relative to the normal standard deviation of fasting glucose levels.17 For each risk factor we reported Mantel-Haenszel adjusted odd’s ratios to account for differences in the amount of methylprednisolone acetate received. The Spearman rho was used to determine if overall glucose control (HbA1c) correlated with changes in fasting blood glucose levels on post-injection day 1. To determine if type of diabetes, diabetes control regimen, or amount of injection predicted the level of change in fasting blood glucose levels (as a continuous variable without cut point) on post-injection day 1 after controlling for the other variables, we constructed a linear regression model.
Results
Sixty-seven patients were recruited to participate in our study, of which 40 participants (60%) returned their completed daily blood glucose logs. Patient demographics, location and amount of methylprednisolone acetate, baseline fasting blood glucose values, diabetes type, and medication type of both study completers and those failing to finish our study are documented in Table 1. All 7 patients with type 1 diabetes and 10 (30%) with type 2 diabetes were dependent on insulin.
Table 1.
Demographic and Baseline Values
| Completed Study | Failed to Complete Study* | ||
|---|---|---|---|
| Age | 62 (±10) | 57 (±9) | |
| Sex (female) | 22 (55%) | 16 (67%) | |
| Amount Injected (mg/ml)† | 40 (20–120) | 40 (40–120) | |
| Fasting BG Level (mg/dl) | 138 (43) | Not available | |
| Diabetes Mellitus | |||
| Type 1 | 7 (18%) | 7 (29%) | |
| Type 2 | 33 (82%) | 18 (71%) | |
| HbA1C | 7.1 (6-10) | 7.5 (6-11) | |
| Medication Type | |||
| Insulin | 17 (43%) | 11 (46%) | |
| Oral Meds Only | 19 (47%) | 12 (50%) | |
| Diet Only | 4 (10%) | 1 (4%) | |
| Injection location‡ | |||
| Tendon Sheath | 28 (70%) | 23 (96%) | |
| Joint | 10 (25%) | 1 (4%) | |
| Other§ | 2 (5%) | -- | |
Data available for 24 of 27 excluded participants
Data not normal, mode value reported
23 Trigger finger, 3 DeQuervain’s, 1 CTS, 1 Unknown; all 10 joints were injected for OA
Injections in both Tendon Sheath and Joint
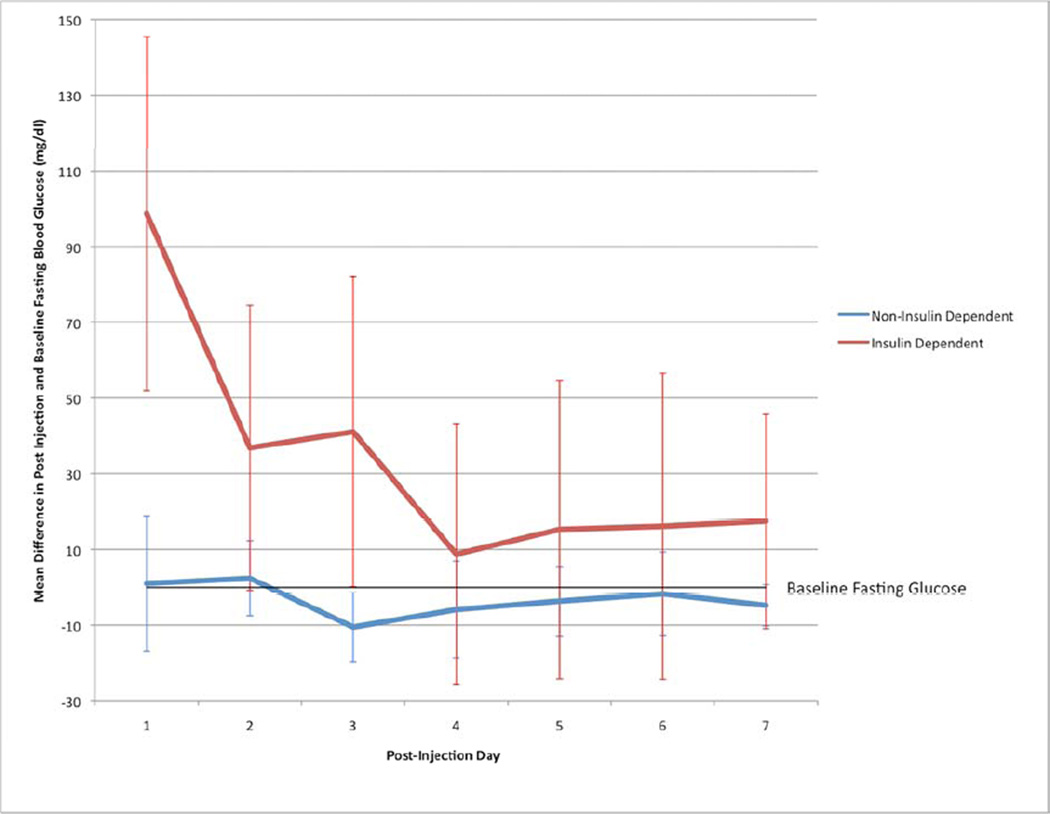
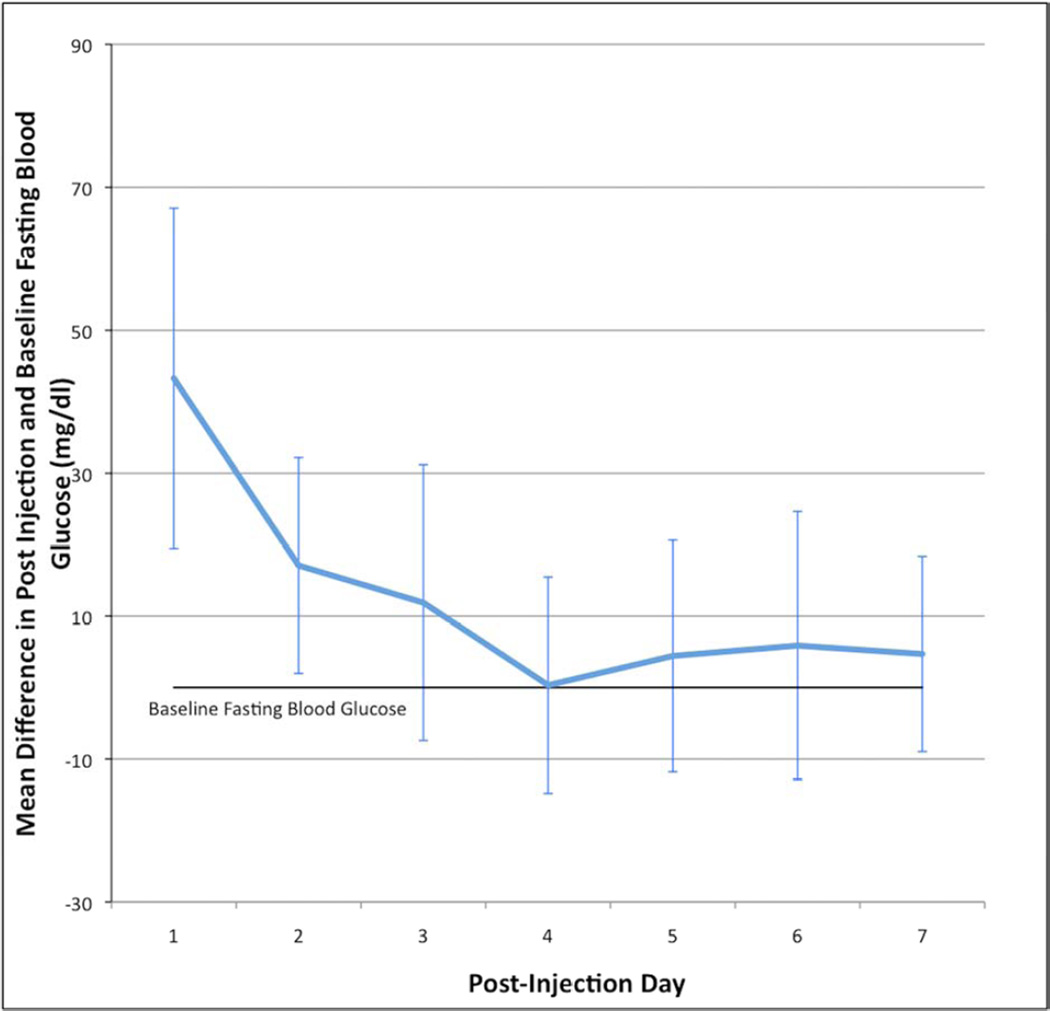
The number of days after injection was a significant predictor of the difference in blood glucose values from baseline levels (df=51.8, F=3.09, P=0.01). Specifically, there was a statistically significant increase in fasting blood glucose levels on post-injection day 1 (43.3, 95% CI: 19.4–67.1), and post-injection day 2 (17.1, 95% CI: 2.0–32.2), with a non-significant increase in mean glucose on day 3 (11.9, 95% CI: −7.4–31.2). There was no statistically significant difference between post-injection days 4–7 and baseline fasting blood glucose levels (Figure 2).
Figure 2.
Change in blood glucose level from baseline for 7 days following injection in insulin and non-insulin dependent diabetic patients
Patients with type 1 diabetes and patients taking insulin were more likely to experience a rise >50 mg/dl on post-injection day 1. Patient sex and location of injection were not statistically significantly associated with a >50 mg/dl increase on post-injection day 1 despite a 3-fold (36% vs 10%) incidence of this following extra-articular injection. These results remained the same after controlling for amount of methylprednisolone administered using the Mantel-Haenszel odds ratio (Table 2). Thirty patients had available HbA1c data. In these patients, HbA1c levels were not significantly correlated with a rise in fasting blood glucose levels 1 day following injection (Spearman’ correlation coefficient = 0.15, P =0.42).
Table 2.
Factors Associated with >50 mg/dl change in Blood Glucose Levels
| >50mg/dl Change in Glucose |
Fisher's Exact Test |
Odds Ratio† (95% CI) |
|||
|---|---|---|---|---|---|
| No (28) | Yes (12) | ||||
| Sex | |||||
| Male | 14 (78%) | 4 (22%) | P = 0.49 | 0.23 (0.35 to 1.6) | |
| Female | 14 (64%) | 8 (36%) | |||
| Diabetes Mellitus | P = 0.001 | 0.04 (0.003 to 0.51) | |||
| Type 1 | 1 (14.3%) | 6 (86%) | |||
| Type 2 | 27 (82%) | 6 (18%) | |||
| Medication Type | P < 0.001 | 50.0 (4 to 620) | |||
| Insulin | 6 (35%) | 11 (65%) | |||
| Oral or Diet Only | 22 (96%) | 1 (4%) | |||
| Injection Location* | P = 0.23 | 0.235 (0.02 to 2.4) | |||
| Tendon Sheath | 18 (64%) | 10 (36%) | |||
| Joint | 9 (90%) | 1 (10%) | |||
Two patients with shots in both locations excluded (N = 38)
Mantel-Haenszel Odds Ratio Controlling for Amount of Methylprednisolone Injected
In our linear regression model, the amount of corticosteroid injected did not predict a change in blood glucose (β= 0.49, P=0.43) level after accounting for diabetes type or medication used. Both type 1 diabetes (β= 72.2, P =0.03) and insulin use (β= 62.5, P =0.02) predicted a rise in fasting blood glucose from baseline fasting values on post-injection day 1 after accounting for amount of methylprednisolone administered. The patterns of the change in fasting blood glucose levels during the week after injection in diabetics taking insulin vs. those on oral medications/diet are seen in Figure 3.
Discussion
Diabetic patients are at increased risk for common hand diseases for which a corticosteroid injection may provide lasting relief.4 The current study showed an increase in blood glucose levels 1 and 2 days following injection with return to baseline on day 3. Those most likely to experience an increase in blood glucose levels were patients with type 1 diabetes and patients dependent on insulin.
Prior studies have shown varying impact of corticosteroid injections on diabetic glucose control. Habib and Ahmad evaluated the effect on18 type 2 diabetic patients (6 using insulin) undergoing intra-articular corticosteroid injections in the shoulder.15 They found no significant effect on blood glucose levels after injection or on long-term measures of glycemic control (fructosamine). They also studied 24 patients (10 using insulin) undergoing intra-articular corticosteroid injections for osteoarthritis of the knee.12 Unlike their previous study on shoulder injections, this study showed an increase in blood glucose values for 2–4 days following injection. It is unclear whether injection location, study population, or difference in type of corticosteroid may have accounted for the difference in results. Our study suggests, however, that the higher number of insulin users among patients undergoing intra-articular knee injections may have resulted in a change in post-injection blood glucose levels. As seen in Figure 3, there was minimal change in post-injection blood glucose levels from baseline values in patients not taking insulin.
Two studies have evaluated the effect of corticosteroid injections in the hand. Wang and Hutchison followed 18 patients receiving 10 mg of methylprednisolone acetate for trigger finger.10 These patients showed an average increase of 73% over baseline values on post-injection day 1 with no statistically significant difference in values on \ days 2–5. Possibly due to their small population, they found no difference in change in blood glucose levels between type 1 and type 2 diabetic patients. Patient baseline blood glucose values were also not recorded prospectively. Patients were simply asked to recall average morning levels. Catalano et al. evaluated morning blood glucose levels in 23 diabetic patients receiving an extra-articular injection of 10 mg of triamcinolone acetonide in the hand.14 Their data revealed a small, statistically significant increase on post- injection days 1 and 5 of 14.2 and 9.7 mg/dl, respectively. Unlike Wang et al, insulin-dependent diabetics did experience statistically significant higher post- injection blood glucose levels than non-insulin-dependent diabetics. The authors, however, were not certain they obtained pre-breakfast morning data; some patients may have recorded post-prandial morning blood glucose levels. This likely contributed to the variation of their data.
The current study enrolled twice the number of participants and used 5 days of patient control data for comparison of post-injection blood glucose values. Our data showed similar results with a sharp rise in blood glucose levels in post-injection days 1 and 2 with a return to baseline values on day 3. With our sample size, we were able to evaluate factors associated with increases in post-injection blood glucose levels. The presence of type 1 diabetes and insulin-dependent diabetes predicted greater changes in post-injection blood glucose levels when accounting for amount of injection in our regression model. However, chronic glucose control (HbA1c) was not associated with changes in post-injection blood glucose values. This suggests that chronically elevated blood glucose does not heighten the elevation of post-injection glucose levels in insulin-dependent diabetics.
There were several limitations to our study. Sixty percent of participants returned fully completed blood glucose logs despite being provided self-addressed envelopes and phone call reminders. Despite similar baseline data, we cannot be certain that those either not returning or returning incomplete logs were similar to the patients returning fully completed logs, thus, possibly introducing differential bias. Similarly 75% of the patients reported HbA1c levels, and those not reporting their value may have differed systematically from those who did. Additionally, injections in this study were not uniformly administered to either joint spaces or tendon sheaths.
In this study design, we did not collect pre-injection baseline blood glucose values. We believe, however, our 14-day follow-up was sufficient to ensure patient blood glucose levels from days 10–14 represented baseline values. If in fact blood glucose levels were elevated during the control period, it would affect results towards the null hypothesis. The non-significant but potentially relevant finding that more patients receiving extra-articular injection had an increase in glucose of >50 mg/dl when compared to those with intra-articular injection deserves further study as we were underpowered for this subgroup analysis. Lastly, the Hawthorne effect may lead to a change in patient behavior (e.g. improved diabetic management). Despite these potential biases toward the null hypothesis, our data still revealed an increase in post-injection blood glucose values.
The case-cross over design strengthened our study by allowing us to compare each patient’s post-injection blood glucose levels to their own baseline values without withholding treatment. This was not a controlled study as we asked patients to continue to manage their diabetes in their typical fashion. We believe this also strengthens the results as conditions more closely resemble the typical clinical scenario.
Our results add to the evidence that corticosteroid injections for common hand conditions should not be withheld from diabetic patients. Previously, we advised all diabetic patients there may be a transient rise in blood glucose levels after an injection. Based on our data, we now tell patients there is likely to be a transient rise in blood glucose levels potentiated in type 1 diabetics and those taking insulin. These patients should maintain vigilant glucose monitoring and adjust their medications accordingly. We cannot provide a specific glucose value above which injections should be avoided. We do, however, advise caution in type I diabetic patients and those on insulin when considering steroid injection if glucose levels have been acutely unstable in the days preceding injection.
Figure 1.
Change in blood glucose level from baseline over 7 days following injection
Acknowledgements
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448, sub-award TL1 TR000449, from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
This work was supported by a grant from the Doris Duke Charitable Foundation to Washington University to fund Doris Duke Clinical Research Fellow Daniel London.
The authors of the paper would like to thank and acknowledge the work of Andre Guthrie whose help in collecting and organizing the data was key to this project’s success
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nimigan AS, Ross DC, Gan BS. Steroid Injections in the Management of Trigger Fingers. Am J Phys Med Rehabil. 2006 Jan;85(1):36–43. doi: 10.1097/01.phm.0000184236.81774.b5. [DOI] [PubMed] [Google Scholar]
- 2.Shakeel H, Ahmad TS. Steroid injection versus NSAID injection for trigger finger: a comparative study of early outcomes. J Hand Surg. Elsevier Inc. 2012 Jul;37(7):1319–1323. doi: 10.1016/j.jhsa.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Dala-Ali BM, Nakhdjevani A, Lloyd Ma, Schreuder FB. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012 Dec;4(4):263–268. doi: 10.4055/cios.2012.4.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg. 1995 Jan;20(1):109–114. doi: 10.1016/S0363-5023(05)80068-1. [DOI] [PubMed] [Google Scholar]
- 5.Koh S, Nakamura S, Hattori T, Hirata H. Trigger digits in diabetes: their incidence and characteristics. J Bone Joint Surg Eu. 2010 May;35(4):302–305. doi: 10.1177/1753193409341103. [DOI] [PubMed] [Google Scholar]
- 6.Kameyama M, Meguro S, Funae O, Atsumi Y, Ikegami H. The presence of limited joint mobility is significantly associated with multiple digit involvement by stenosing flexor tenosynovitis in diabetics. J Rheumatol. 2009 Aug;36(8):1686–1690. doi: 10.3899/jrheum.081024. [DOI] [PubMed] [Google Scholar]
- 7.Wessel LE, Fufa DT, Boyer MI, Calfee RP. Epidemiology of carpal tunnel syndrome in patients with single versus multiple trigger digits. J Hand Surg. 2013 Jan;38(1):49–55. doi: 10.1016/j.jhsa.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Kameyama M, Chen K-R, Kiyoshi M, Shimada A, Atsumi Y, Yanagimoto S. Histopathological Characteristics of Stenosing Flexor Tenosynovitis in Diabetic Patients and Possible Associations With Diabetes-Related Variables. J Hand Surg Am. 2013;38(7):1331–1339. doi: 10.1016/j.jhsa.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Baumgarten KM, Gerlach D, Boyer MI. Corticosteroid injection in diabetic patients with trigger finger. A prospective, randomized, controlled double-blinded study. J Bone Joint Surg. 2007 Dec;89(12):2604–2611. doi: 10.2106/JBJS.G.00230. [DOI] [PubMed] [Google Scholar]
- 10.Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg. 2006;31(6):979–981. doi: 10.1016/j.jhsa.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural steroid injections on blood glucose levels in patients with diabetes mellitus. Spine. 2012 Jan 1;37(1):E46–E50. doi: 10.1097/BRS.0b013e31821fd21f. [DOI] [PubMed] [Google Scholar]
- 12.Habib GS, Miari W. The effect of intra-articular triamcinolone preparations on blood glucose levels in diabetic patients: a controlled study. J Clin Rheumatol. 2011 Sep;17(6):302–305. doi: 10.1097/RHU.0b013e31822acd7c. [DOI] [PubMed] [Google Scholar]
- 13.Habib G, Safia A. The effect of intra-articular injection of betamethasone acetate/betamethasone sodium phosphate on blood glucose levels in controlled diabetic patients with symptomatic osteoarthritis of the knee. Clin Rheumatol. 2009 Jan;28(1):85–87. doi: 10.1007/s10067-008-1023-9. [DOI] [PubMed] [Google Scholar]
- 14.Catalano LW, Glickel SZ, Barron OA, Harrison R, Marshall A, Purcelli-Lafer M. Effect of local corticosteroid injection of the hand and wrist on blood glucose in patients with diabetes mellitus. Orthopedics. 2012 Dec;35(12):e1754–e1758. doi: 10.3928/01477447-20121120-20. [DOI] [PubMed] [Google Scholar]
- 15.Habib GS, Abu-Ahmad R. Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clinical rheumatology. 2007 Apr;26(4):566–568. doi: 10.1007/s10067-006-0353-8. [DOI] [PubMed] [Google Scholar]
- 16.Habib GS. Systemic effects of intra-articular corticosteroids. Clinical rheumatology. 2009 Jul;28(7):749–756. doi: 10.1007/s10067-009-1135-x. [DOI] [PubMed] [Google Scholar]
- 17.Barzilay JI, Kronmal Ra, Gottdiener JS, Smith NL, Burke GL, Tracy R, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004 Jun 16;43(12):2236–2241. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]