Abstract
Objective
Depression is a proposed risk factor for heart failure based largely on epidemiological data; little experimental data is available addressing this hypothesis.
Methods
Depression was evaluated in relation to cardiac structural and functional phenotypes assessed by transthoracic echocardiography in 42 adult female cynomolgus monkeys that consumed a Western-like diet for 3 years. Half of the monkeys were treated with the SSRI sertraline HCl for 18 months and depressive behavior was assessed for 12 months prior to echocardiography.
Results
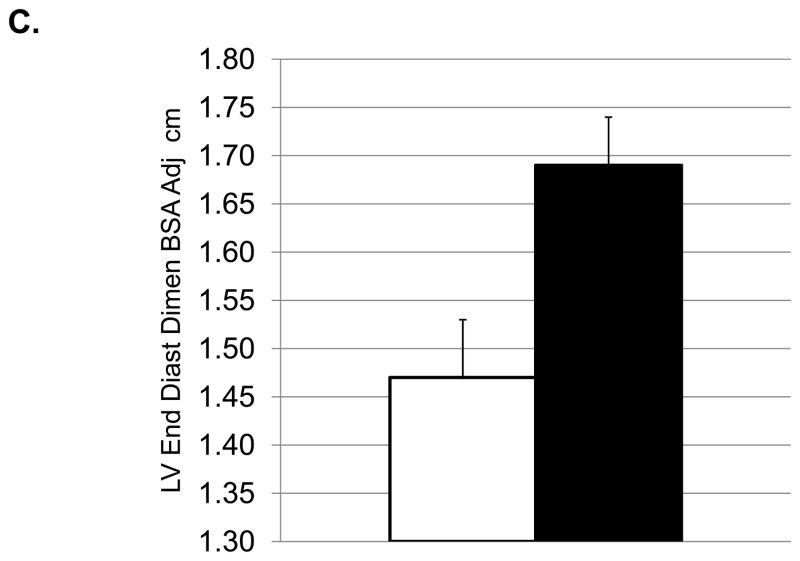
Depressed monkeys (the 19/42 with depressive behavior rates above the mean rate) had higher HRs (171±4.1 vs 152±6.1), and smaller BSA (0.13±0.003 vs 0.15±0.004). Cardiac measures showed lower LV end systolic dimension (0.75±0.05 vs 0.89±0.04), LV systolic (0.76±0.08 vs 1.2±0.11) and diastolic (2.4±0.23 vs 3.4±0.26) volumes, and left atrial volumes (1.15±0.14 vs 1.75±0.12) in depressed versus nondepressed monkeys (p’s <0.05). Doppler profiles of depressed monkeys indicated greater myocardial relaxation (higher e′ and higher e′/a′ ratio) and lower filling pressures (lower E/e′) compared to nondepressed monkeys (p’s<0.05). Although treatment with sertraline reduced HR (150±5.8 vs 171±4.8) and modestly increased chamber dimensions (left ventricular end systolic dimension: 0.91±0.05 vs 0.74±0.03; left ventricular end diastolic dimension, BSA adjusted 1.69±0.05 vs 1.47±0.06) (p’s<0.05), it did not overtly affect systolic or diastolic function (p’s >0.10).
Conclusions
These data suggest that behavioral depression in female primates is accompanied by differences in cardiac function, although not in ways classically associated with subclinical heart failure. SSRIs show promise in supporting heart function by reducing HR and perhaps improving LV filling, however further investigation is needed to confirm this hypothesis.
Keywords: Nonhuman primates, females, SSRI, sertraline, CSF, cardiac function
INTRODUCTION
The incidence of heart failure and depression are both increasing in the US. The prevalence of depression in heart failure patients is 22% and the condition greatly worsens the course, and increases the cost, of the disease (1; 2). Women have a higher rate of depression and worse cardiovascular outcomes than men (3). While it seems clear that heart failure promotes the occurrence of depression, whether depression increases the likelihood of heart failure is unknown.
Several physiological perturbations are common to heart failure and depression (4). These include cardiovascular autonomic dysregulation characterized by relatively high sympathetic tone resulting in increased HR, reduced HR variability, and elevated levels of circulating catecholamines (1; 4; 5; 6). The serotonergic system is perturbed in depression, and both heart failure and depression are also often accompanied by hypercortisolemia which elevates free fatty acid concentrations (1; 4; 5; 6). Depression is also accompanied by an increased production of inflammatory cytokines. All of these are characteristics of depression, and all are thought to increase heart failure (1; 4; 5; 6). Thus, there are biologically plausible pathways through which depression earlier in life might increase the risk of heart failure in later life.
However, data directly supporting the hypothesis that depression increases the risk of heart failure are sparse. Abramson et al. (7) studied 4538 patients 60 years of age and older with systolic hypertension and observed that depression at baseline was independently associated with a substantial increase in heart failure risk at a 4.5 year follow up. Likewise, May et al. (8) studied 13,708 coronary artery disease patients and found that 10% developed depression post-coronary artery disease which greatly increased heart failure risk after an average 5.6 year follow up. Little is known about whether antidepressant treatment affects heart failure. Recently, Leftheriotis et al. (9) studied the effects of sertraline HCl, a commonly prescribed selective serotonin reuptake inhibitor, in 62 nondepressive patients with ischemic heart failure and implantable cardioverter-defibrillators. Sertraline treatment reduced ventricular extrasystoles, improved quality of life, and seemed to have beneficial effects on some indices of HR variability. Thus, SSRIs could have a favorable clinical impact on heart failure patients which may be independent of their effects on depressive symptoms.
Animal models of comorbidity are rare. Cynomolgus monkeys (Macaca fascicularis) have been used as a model of coronary artery atherogenesis for 40 years. Over the last 20 years, we have developed an adult female cynomolgus monkey model of depression (10; 11; 12). Depressive behavior is observed in adult female cynomolgus macaques consuming a Western-type diet, and closely resembles human depression in physiological, neurobiological, and behavioral characteristics, including reduced body mass, hypothalamic–pituitary–adrenal axis perturbations, autonomic dysfunction, reduced hippocampal volume, altered serotonergic function, decreased activity levels, increased mortality, and exacerbated coronary artery atherosclerosis (10; 11). Also like human beings, there is an inverse relationship between social status and depression; although many subordinates never exhibit depressive behavior and some dominants do, and subordinates and depressed monkeys are different in terms of neurobiology, behavior, and extent of pathology found in other organ systems (12; 13). Depressive behavior in female cynomolgus monkeys spontaneously occurs, and is not induced by experimental manipulation (10; 11). Here we examine the heart function of depressed versus nondepressed monkeys and the effects of sertraline HCl, a commonly prescribed antidepressant, on cardiac structural and functional phenotypes. An SSRI was chosen because this is the most common class of antidepressant currently prescribed, and sertraline was chosen because most was known about the effects of this SSRI on the cardiovascular system at the onset of the study.
Since the first hemodynamic abnormality seen in all cardiac disease is a slower rate of LV relaxation (14), a general understanding of the assessment of diastolic function is warranted. The LV inflow Doppler is probably the most commonly used measurement in diastolic echocardiographic examination because the various patterns of early and late LV filling represent increasing degrees of LV diastolic impairment. In brief, early filling velocity, or E, is due to LV relaxation. Late filling velocity, or A, represents the atrial contraction at the end of diastole. Normally, the majority of LV filling occurs during the early phase corresponding to a taller E than A wave. The early-to-late LV filling (E/A ratio) is an established marker of global diastolic function. Indeed, conventional or pulsed Doppler inflow measures of diastolic function are load (e.g., preload and afterload) and heart rate dependent (15; 16; 17; 18). Reductions in preload can lead to reductions in early filling velocity and in the E/A ratio. Conversely, increases in preload entail a transition towards a pseudonormal pattern (e.g., E>A) or reduced LV compliance. Heart rate and rhythm are other factors that affect LV diastolic filling, independent of diastolic function. For instance, at higher heart rates (shorter diastolic filling time), the late inflow velocity (A) may be increased as it becomes superimposed on the E wave. When the diastolic interval is very short during high heart rates there could be fusion of the E and A velocities. Thus, the inclusion of tissue Doppler facilitates the assessment of diastolic function since it is less affected by loading conditions than conventional pulsed Doppler (19), and the tissue Doppler surrogate of LV filling pressure, E/e′, has been shown to be relatively independent of heart rate and rhythm abnormalities (20; 21). Therefore, we used pulsed and tissue Doppler modalities in the assessment of diastolic function in order to identify subtle impairments in LV function. We hypothesized that depression would be associated with less efficient heart function, and that sertraline may have some beneficial effects.
METHODS
Subjects
Forty-five adult, reproductive-aged female cynomolgus monkeys were imported directly from Indonesia (Institut Pertanian Bogor, Bogor, Indonesia) and quarantined in single cages for one month. Following quarantine, the monkeys were randomly assigned to social groups of n=4–5, in indoor pens (3.05m × 3.05m × 3.05m), in a climate controlled building, with 12/12 light/dark, and water ad libitum. All monkeys were fed a Western-like diet containing 44% of calories from fat and 0.29 mg/Cal cholesterol, comparable to a human consumption of 500 mg cholesterol/2000 calories, roughly comparable to 2 eggs per day (Table S1, Supplemental Digital Content 1). The monkeys were approximately 15.7 ± 0.3 years of age, estimated from dentition, which approximates a human age of 50 years. Over the course of the 3.5 year study, three animals died of causes unrelated to the experiment resulting in a final sample size of 42. All procedures involving primates were conducted using protocols approved by the Animal Care and Use Committee of Wake Forest University Health Sciences and were in compliance with all institutional, state, and federal laws for the usage of primates in laboratory settings.
Experimental Design
The monkeys consumed the Western-like diet for an 18 month Pretreatment Phase following formation of social groups. During this time depressive behavior was recorded. At the end of the 18 months, the iliac artery was biopsied and the extent of atherosclerosis measured. All monkeys were trained to comply with an oral dosing regimen. The monkeys were trained to run out of their social group pens into a dosing cage where they were individually offered fat-free vanilla pudding orally from a metal syringe. After consuming the pudding orally the monkeys were immediately released into their home cage. This initial dose-training was completed in about two weeks. The monkeys were assigned by social group to either placebo (n=20) or sertraline (n=22) treatment balanced on the rate of depressive behavior during the pretreatment period, BW, and iliac artery atherosclerosis extent using a method of stratified randomization. Thus, all animals in a social group had the same treatment. Sertraline HCl (Zoloft®) was in introduced by gradually increasing doses over a 4 week period until the final dose of 20 mg/kg was attained. One animal had an adverse reaction (hemorrhaging) to the sertraline during week 3 and was switched to the placebo group leaving final treatment group sizes of n=21 each. Throughout the treatment phase sertraline was administered orally in the pudding at about 0800 each day. Monkeys in the placebo group were given pudding alone.
Circulating sertraline and desmethylsertraline concentrations
After 1 week of dosing with placebo, and 4 weeks later at the end of the first week of the 20 mg/kg dose, the monkeys were sedated with 10–15 mg/kg ketamine HCl and blood samples were taken to measure circulating sertraline and desmethylsertraline. During placebo administration sertraline/desmethylsertraline was undetectable. After 1 week at the 20 mg/kg dose, circulating concentrations of sertraline/desmethylsertraline rose to average levels of 78 and 88 ng/ml, respectively (22) which are comparable to circulating levels observed in patients (23).
CSF Monoamine and Metabolite Determinations
After 1 week of placebo, and 4 weeks later, after 1 week of the 20 mg/kg sertraline dose, cerebrospinal fluid samples were taken for determination of central monoamine and metabolite concentrations. CSF samples were taken by inserting a 22-gauge needle percutaneously into the cisternal space, after sedation (ketamine HCl 10 mg/kg), while the animal was restrained in lateral recumbency. Approximately 1–1.5 cc of spinal fluid was obtained and frozen at −70°C until metabolite determinations were made. 5 HIAA was analyzed using HPLC with electrochemical detection as previously described (24). The intra- and interassay coefficients of variation were <10%. After a week at the 20 mg/kg dose CSF 5-HIAA decreased 33% in the monkeys compared to 1 week of placebo, a decrease similar to that observed in patients treated with sertraline (25; 22).
Behavior Observations
The definition of depressive behavior includes three components: 1) exhibition of a slumped or collapsed body posture; 2) a relative lack of responsiveness to environmental stimuli to which other monkeys are attending; and 3) open eyes to distinguish this behavior from resting (26; Figure 1). The frequency with which the monkeys were observed in the depressed posture was recorded for 10 minutes, twice/week, counterbalanced for time of day, for 12 months during the Pretreatment Phase, and 12 months during the Treatment Phase (an average of 33.3 hours/monkey total), using a focal animal technique that has been described in detail previously (27; 28). Depressive behavior is easily recognizable; and inter-rater reliability, determined biannually, was ≥ 0.92 throughout the experiment. The average frequency/hour that the monkeys exhibited this behavior each month was calculated from these observations. Monkeys with depressive behavior rates below the mean depression rate were classified as nondepressed, and those with depressive behavior rates above the mean were classified as depressed.
Figure 1.

A depressed monkey (left) and an alert monkey (right).
Anthropometrics
BW, and trunk length were measured at the end of the Pretreatment Phase, and 16 months after the onset of the Treatment Phase. BMI was estimated as the ratio of BW to the square of trunk length measured from the suprasternal notch to the pubic symphysis (kg/m2) (29). BSA was calculated using the formula of DuBois and DuBois (BSA = [W 0.425 × H 0.725] × 0.007184) with weight in kilograms and height (trunk length) in centimeters, a formula which has been previously used in monkeys (30; 31).
Iliac Artery Atherosclerosis Extent
Premenopausal iliac artery atherosclerosis extent has been shown to predict postmenopausal coronary artery atherosclerosis extent late in life in this species (32). At the end of the 18 month Pretreatment Phase, a segment of the left common iliac artery (approximately 3 cm in length) was removed, fixed in 4% paraformaldehyde, sectioned, and then stained for measurement of atherosclerosis extent as previously described (33). The extent of atherosclerosis in each arterial section was measured as the area (in mm2) between the internal elastic lamina and the lumen.
Transthoracic Echocardiography
Doppler-echocardiography was performed 14 months after onset of the Treatment Phase, following sedation with 15 mg/kg ketamine HCl. Table 1 lists the variables measured. A single, highly experienced echosonographer, who was masked to behavior and treatment class, performed all examinations using a neonatal cardiac phased array probe (5–12 MHz) and Philips IE33 sector scanner (Philips Medical Systems, Andover, MA) with the animal in a shallow left lateral decubitus position. Images were obtained and analyzed according to American Society of Echocardiography recommendations (34; 35). LVIDD and LVISD, LV posterior wall thickness, and interventricular septal wall thickness measurements were acquired and measured from the parasternal long-axis view using 2D-guided M-mode echocardiography using the leading edge-to-leading edge. Relative wall thickness was calculated as (2 × posterior wall thickness)/LV end diastolic dimension. LV mass was calculated by the Devereaux formula modified for the smaller monkey heart (LV Mass=1.04((LVIDD + interventricular septal wall thickness + posterior wall thickness)3 –LVIDD3). The %FS, an index of contractile function, was calculated as FS (%) = [(LVIDD –LVISD)/LVIDD] × 100. Apical 4-chamber and 2-chamber views were obtained while optimizing endocardial border definition and avoiding foreshortening of the cavity. 2-D echocardiographic calculation of ventricular volume was based on endocardial border tracing at end-diastole and end-systole using biplane apical views with a modified Simpson’s rule (34; 35). Ejection fraction was calculated as EF(%) = [(end diastolic volume –end systolic volume/end diastolic volume)] × 100. HR was derived from the R-R interval, or the time elapsing between two consecutive R waves in the electrocardiogram (heart rate = 60/R-R).
Table 1.
Baseline Characteristics of Study Population
| Variables | Placebo Non Depressed n=12 | Placebo Depressed n=9 | Sertraline Non Depressed n=11 | Sertraline Depressed n=10 | Sertraline | Depression |
|---|---|---|---|---|---|---|
| Depressive behavior freq/hour | 0.25 (0.08) | 4.53 (0.89) | 0.11 (0.05) | 6.44 (1.91) | F[1,38]=0.92, p=0.34 | F[1,38]=33.2, p<0.001 |
| BW kg | 3.86 (0.29) | 2.96 (0.22) | 3.92 (0.26) | 3.12 (0.07) | F[1,38]=0.22, p=0.64 | F[1,38]=12.13, p=0.001 |
| Trunk Length cm | 27.4 (0.51) | 26.5 (0.31) | 29.7 (0.38) | 28.0 (0.56) | F[1,38]=17.6, p<0.001 | F[1,38]=8.62, p=0.006 |
| BMI kg/m2 | 50.8 (2.75) | 41.7 (2.54) | 44.1 (2.30) | 40.0 (1.31) | F[1,38]=3.12, p=0.09 | F[1,38]=7.54, p=0.009 |
| BSA m2 | 0.14(0.006) | 0.12 (0.004) | .15 (0.005) | 0.13 (0.003) | F[1,38]=3.18, p=0.08 | F[1,38]=13.9, p=0.0006 |
| Iliac artery atherosclerosis mm2 | 0.16 (0.06) | 0.06 (0.03) | 0.07 (0.04) | 0.06 (0.05) | F[1,38]=1.08, p=0.30 | F[1,38]=1.51, p=0.23 |
| Age years | 17.3 (0.45) | 16.4 (0.56) | 18.6 (0.68) | 17.1 (0.65) | F[1,38]=2.83, p=0.10 | F[1,38]=3.81, p=0.058 |
Baseline characteristics were analyzed by one-way analysis of variance.
The atria transfer blood to the ventricles in 2 steps: in the first step, the weight of the collected blood causes it to fall into the ventricles below when the valve opens. The speed at which the blood moves during this initial action is called the early or “E” filling velocity. Preload, estimated here by LVIDD, promotes E. But some blood always remains, so toward the end of the atrial emptying cycle (diastole), the second step occurs in which the atria contract to complete emptying. The speed of the blood filling the ventricle in this step is the “A” (for atrial) filling velocity. Mitral inflow measurements of early (Emax orTransmitral E velocity) and late filling velocities (Amax or Transmitral A velocity) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Due to relatively high HRs and fusion of the early and late Doppler profiles, the late transmitral filling velocity was not recorded. Wall velocities can also be assessed by Doppler imaging. The following measurements were made from the septal and lateral mitral annular walls: early mitral annular descent (e′) and late mitral annular descent (a′). The Doppler index of LV filling pressure (E/e′), or transmitral early filling (Emax) to tissue Doppler mitral annular descent (e′), was calculated. All measurements were performed with an off-line analysis system (Xcelera 3.1; Koninklijke Philips Electronics, Amsterdam, The Netherlands) by one experienced echocardiographer (LG) who was blinded to experimental groups. Intra-rater variability, expressed as the mean percent error (absolute difference between two measurements divided by the mean of the two measurements) ranged from 7 +/− 6% to 13+/− 10% (36). An average of at least five consecutive cardiac cycles was used to minimize beat-to-beat variability for all measured and calculated systolic and diastolic indices.
Statistical Analysis
Baseline characteristics were analyzed by one-way analysis of variance (ANOVA). Characteristics of the animals during the Treatment Phase were analyzed by 2 (placebo, sertraline) × 2 (depressed, not depressed) ANOVA. All measurements from the echocardiograms were analyzed by 2 (placebo, sertraline) × 2 (depressed, not depressed). Variables for which heart rate might have an influence were also analyzed by ANCOVA using heart rate as a covariate, and variables that might be influenced by body size were also analyzed by ANCOVA using BSA as a covariate. The covariate used in analysis is identified in Table 3. The results of the ANCOVA are reported in addition to the ANOVA only for variables for which covariates accounted for a significant proportion of the variance. For all other variables, only the results of the simpler ANOVA model are reported. The level of significance was set at p=0.05.
Table 3.
Sertraline, Depression, and Heart Function1
| ANOVA Results | n | Placebo Non Depressed | Placebo Depressed | Sertraline Non Depressed | Sertraline Depressed | Effect of Sertraline | Effect of Depression |
|---|---|---|---|---|---|---|---|
| Heart rate (beats/min) | 38 | 168 (8.0) | 174 (5.0) | 137 (6.7) | 167 (6.4) | F[1,34]=7.7, p=0.009 | F[1,34]=6.87, p=0.013 |
| Interventricular septal wall thickness2 (cm) | 38 | 0.41 (0.02) | 0.38 (0.03) | 0.41 (0.04) | 0.37 (0.02) | F[1,34]=0.01, P=0.91 | F[1,34]=1.22, P=0.27 |
| Posterior wall thickness2 (cm) | 38 | 0.43 (0.02) | 0.41 (0.03) | 0.42 (0.02) | 0.41(0.02) | F[1,34]=0.06, P=0.81 | F[1,34]=0.19, P=0.66 |
| LV end diastolic dimension2,4 (cm) | 38 | 1.58 (.08) | 1.34 (0.08) | 1.76 (0.06) | 1.60 (0.09) | F[1,34]=7.7, p=0.009 | F[1,34]=6.52, p=0.01 |
| LV end systolic dimension2 (cm) | 38 | 0.79 (0.05) | 0.68 (0.05) | 0.97 (0.06) | 0.83 (0.08) | F[1,34]=7.42, p=0.01 | F[1,34]=4.60, p=0.04 |
| Relative wall thickness2 | 38 | 0.55 (0.11) | 0.62 (0.05) | 0.48 (0.02) | 0.53 (0.05) | F[1,34]=4.48, P=0.04 | F[1,34]=2.81, P=0.10 |
| LV mass2,4 (g/m2) | 38 | 10.7 (0.95) | 8.05 (1.48) | 12.7 (1.27) | 10.0 (1.23) | F[1,34]=0.2.4, P=0.13 | F[1,34]=4.49, P=0.04 |
| Fractional shortening2 (%) | 38 | 49.53 (1.74) | 48.4 (2.86) | 45.0(2.21) | 48.4 (3.48) | F[1,34]=0.74, p=0.40 | F[1,34]=0.18, p=0.67 |
| LV diastolic volume2 (ml) | 37 | 3.12 (0.36) | 2.17 (0.30) | 3.63 (0.39) | 2.79 (0.32) | F[1,33]=2.48, p=0.12 | F[1,33]=6.14, p=0.02 |
| LV systolic volume2 (ml) | 37 | 1.03(0.08) | 0.69 (0.11) | 1.41 (0.18) | 0.86 (0.12) | F[1,33]=4.08, p=0.05 | F[1,33]=10.41, p=0.003 |
| Left atrial volume2 (ml) | 34 | 1.51 (0.17) | 1.00 (0.12) | 1.95 (0.16) | 1.29 (0.25) | F[1,30]=4.01, p=0.05 | F[1,30]=10.3, p=0.003 |
| Ejection fraction 2,3 (%) | 37 | 64.66 (2.85) | 67.4 (3.38) | 61.45 (2.21) | 69.3 (2.79) | F[1,33]=0.05, p=0.83 | F[1,33]=3.44, p=0.07 |
| Transmitral E velocity (Emax)3 (cm/sec) | 31 | 73.23 (4.45) | 51.4 (10.1) | 76.71 (3.03) | 69.1 (3.36) | F[1,27]=3.84, p=0.06 | F[1,27]=7.63, p=0.01 |
| Transmitral A velocity (Amax)3 (cm/sec) | 30 | 67.47 (2.49) | 59.1 (10.5) | 59.44 (3.21) | 59.6 (6.09) | F[1,26]=0.43, p=0.52 | F[1,26]=0.56, p=0.46 |
| Emax/Amax3,4 | 31 | 1.12 (0.12) | 0.91 (0.12) | 1.33 (0.09) | 1.18 (0.14) | F[1,27]=4.0, p=0.056 | F[1,27]=2.31, p=0.141 |
| Emax / e′ septal annulus3 | 29 | 12.93 (0.80) | 9.1 (2.1) | 13.62 (1.08) | 9.41 (0.8) | F[1,25]=0.16, p=0.69 | F[1,25]=10.6, p=0.003 |
| Emax / e′ lateral annulus3 | 26 | 10.0 (0.71) | 8.1 (1.65) | 9.54 (1.07) | 7.29 (0.56) | F[1,22]=0.44, 0.51 | F[1,22]=3.72, 0.067 |
| Early mitral annular descent (e′) lateral annulus3 (cm/sec) | 33 | 7.42 (0.42) | 8.91 (0.61) | 9.05 (0.80) | 10.1 (0.79) | F[1,29]=3.85, p=.059 | F[1,29]=3.18, p=.08 |
| Late mitral annular descent (a′) lateral annulus3 (cm/sec) | 32 | 6.66 (0.51) | 7.48 (0.63) | 6.38 (0.58) | 6.65 (0.81) | F[1,28]=0.75, p=0.39 | F[1,28]=0.73, p=0.40 |
| Systolic annular motion- lateral 3 (cm/sec) | 34 | 4.83 (0.39) | 5.28 (0.76) | 5.22 (0.30) | 5.25 (0.46) | F[1,30]=0.18, p=0.67 | F[1,30]=0.31, p=0.58 |
| Early mitral annular descent (e′) septal annulus 3 (cm/sec) | 36 | 5.59(0.27) | 7.49 (0.71) | 6.24 (0.51) | 8.12 (0.52) | F[1.32]=1.55, p=0.22 | F[1.32]=13.41, p<0.001 |
| Late mitral annular descent (a′) septal annulus3 (cm/sec) | 35 | 6.17 (0.32) | 6.67 (0.80) | 5.3 (0.40) | 6.91 (1.03) | F[1.31]=0.26, p=0.61 | F[1.31]=2.85, p=0.10 |
| e′/a′ septal annulus3,4 | 35 | 0.92 (0.06) | 1.22 (0.15) | 1.24 (0.11) | 1.3 (0.18) | F[1.31]=2.63, p=0.11 | F[1.31]=2.43, p=0.13 |
| Systolic annular motion (s′) - septal 3 (cm/sec) | 36 | 4.73 (0.20) | 5.21 (0.31) | 4.68 (0.24) | 4.66 (0.23) | F[1,32]=1.44, p=0.24 | F[1,32]=0.82, p=0.37 |
| ANCOVA Results: Adjusted Means of Variables with significant covariates | |||||||
| LV end diastolic dimension2 (cm) | 38 | 1.58 (0.07) | 1.34 (0.08) | 1.76 (0.07) | 1.60 (0.08) | F[1,33]=7.34, p=0.01 | F[1,33]=1.13, p=0.30 |
| Emax/Amax3 | 31 | 1.18 (0.10) | 0.96 (0.12) | 1.21 (0.11) | 1.19 (0.13) | F[1,25]=1.07, p=0.31 | F[1,25]=1.07, p=0.31 |
| e′/a′ septal annulus3 | 35 | 0.98 (0.11) | 1.33 (0.12) | 1.05 (0.12) | 1.37 (0.12) | F[1,30]=0.27, p=0.60 | F[1,30]=7.28, p=0.01 |
| LV mass2 | 38 | 10.1 (1.23) | 8.92 (1.33) | 11.9 (1.20) | 10.6 (1.36) | F[1,33]=0.2.07, P=0.16 | F[1,33]=0.68, P=0.42 |
RESULTS
Characteristics of the Study Population at the Time of Randomization to Treatment (Table 2) and During Treatment (Table 2)
Table 2.
Characteristics of Study Population During the Treatment Phase1
| Variables | Placebo Non Depressed n=11 | Placebo Depressed n=10 | Sertraline Non Depressed n=12 | Sertraline Depressed n=9 | Sertraline | Depression |
|---|---|---|---|---|---|---|
| Depressive Behavior freq/hr | 0.25±0.08 | 4.53±0.89 | 0.12±0.05 | 6.44±1.91 | F[1,38]=0.93, p=0.34 | F[1,38]=33.2, p<0.001 |
| BW kg | 4.18 ±0.35 | 3.06±0.24 | 3.89±0.28 | 3.03±0.08 | F=[1,38]=0.34 p=0.56 | F=[1,38]=13.0, p<0.009 |
| Trunk Length cm | 28.40±0.36 | 26.8±0.43 | 29.4±0.26 | 27.7±0.43 | F=[1,38]=9.4, p<0.004 | F=[1,38]=15.4, p<0.001 |
| BMI kg/m2 | 52.8±3.53 | 42.3±2.62 | 44.6±2.39 | 39.7±1.32 | F=[1,38]=3.9, p=0.056 | F=[1,38]=8.1, p=0.007 |
| BSA m2 | 0.15±0.006 | 0.12±0.005 | 0.148±0.005 | 0.13±0.002 | F=[1,38]=0.14, p=0.71 | F=[1,38]=16.2, p<0.001 |
determined by analysis of variance
Monkeys assigned to the placebo and sertraline treatment groups were similar in age, BW, BMI, depressive behavior rate, and extent of iliac artery atherosclerosis at the time of randomization and during the treatment phase. Monkeys that were classified as depressed during the pretreatment phase had lower BW, trunk length, BMI, and BSA, as well as higher rates of depressive behavior.
Characteristics of Cardiac Structure and Function (Table 3 and Figures 2 and 3)
Figure 2.
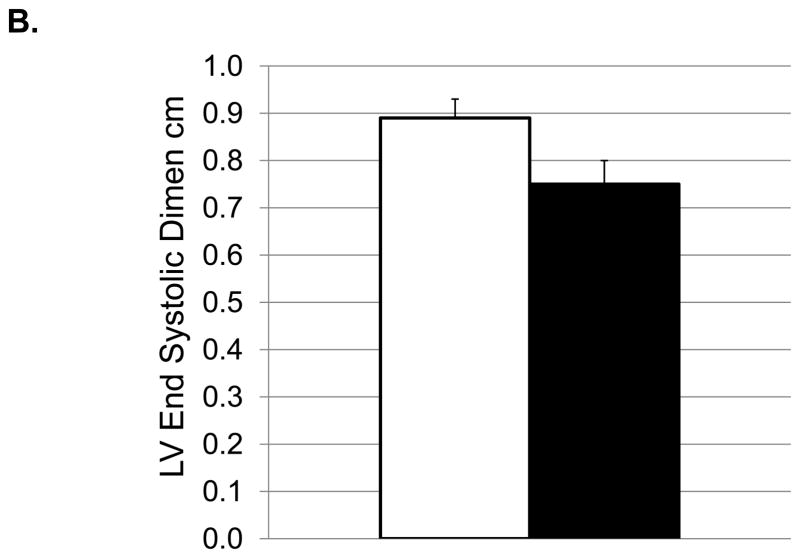
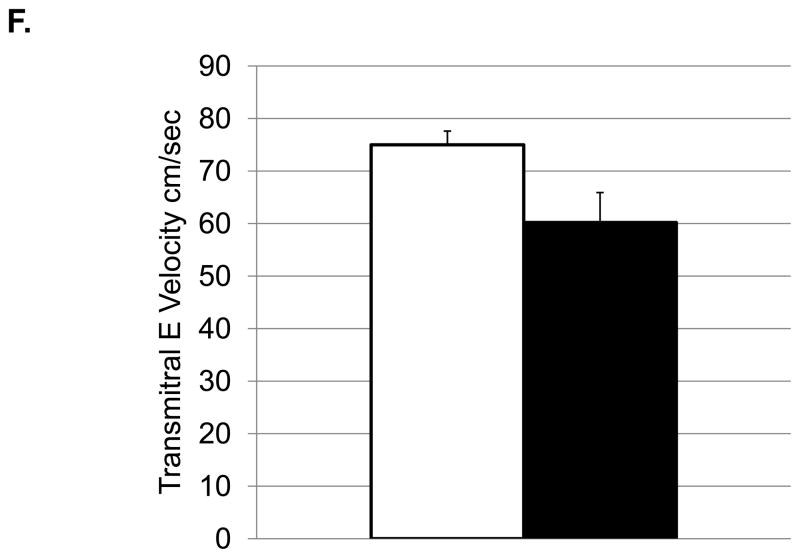
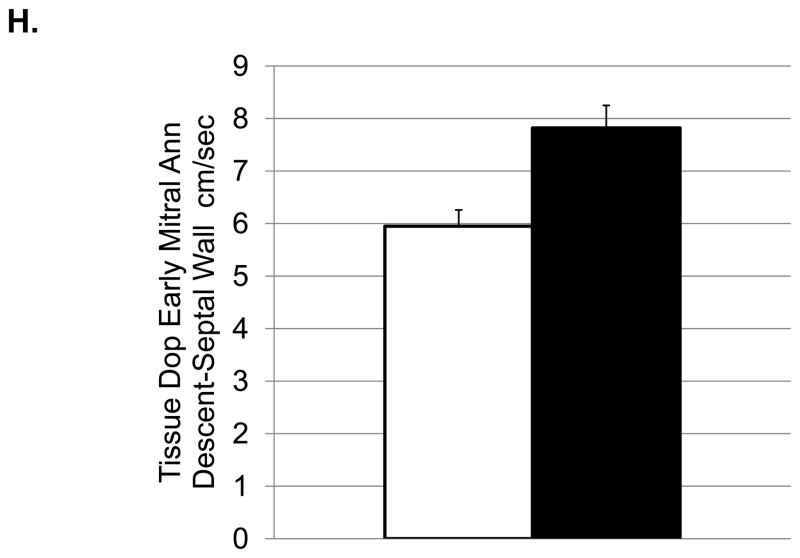
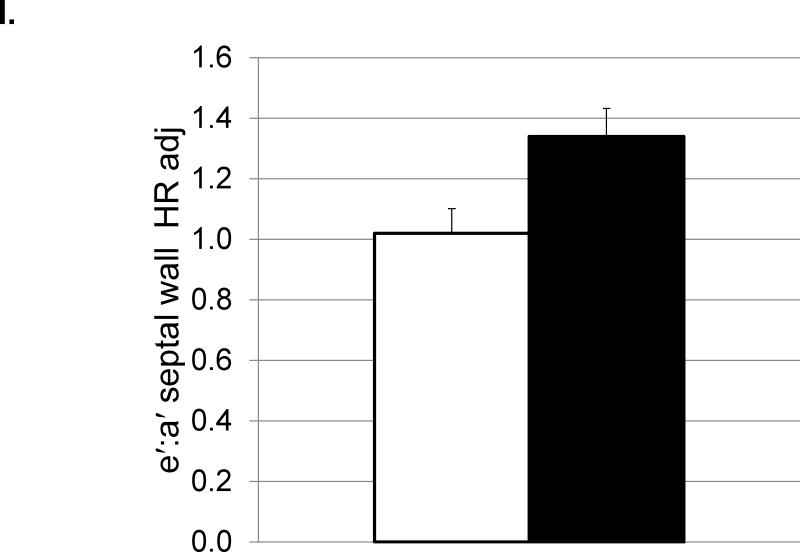
Depressive behavior and cardiac function (means and SEMs). A: Heart rate (beats/minute); B: Left ventricular end systolic dimension (cm); C: Left ventricular diastolic volume (ml); D) Left ventricular systolic volume (ml); E: Left atrial volume (ml); F:Transmitral E Velocity or Emax (cm/sec); G: Transmitral E velocity/e′ lateral annulus (cm/sec); H: Early mitral annular descent (e′) septal annulus (cm/sec); I: e′/a′, early-to-late mitral annular (septal) descent adjusted (adj) for heart rate.
Figure 3.
Effects of Sertraline Treatment on Cardiac Function (means and SEMs). A. Heart rate (beats/minute); B: Left ventricular end systolic dimension (cm); C: Left ventricular diastolic dimension adjusted (adj) for body surface area (BSA); D: Relative Wall thickness; E: Left ventricular systolic volume (ml); F: Left atrial volume (ml).
All statistical outcomes, means and standard errors for echocardiographic endpoints in the Placebo (depressed, nondepressed) and Sertraline (depressed, nondepressed) groups are shown in Table 3. No significant interactions of depressive behavior and sertraline treatment were observed, thus only the main effects of depressive behavior, and sertraline treatment are reported here. Significant main effects are graphed in Figure 2 (differences by depression status) and Figure 3 (sertraline effects).
Depressed versus Nondepressed Monkeys (Figure 2)
Transthoracic echocardiography revealed differences in cardiac structure in depressed versus nondepressed monkeys. Depressed monkeys had smaller LV end systolic dimension (Figure 2B), smaller LV systolic (Figure 2D) and diastolic (Figure 2C) volumes, and smaller left atrial volumes (Figure 2E). Because cardiac dimensions can be associated with body size, the analyses of cardiac dimensions were adjusted for BSA and the findings reexamined. ANCOVA revealed that BSA accounted for a significant proportion of the variance in LV end diastolic dimension only (F [1, 33] =4.45, p=0.04), and eliminated the effect of depression observed in the ANOVA (Table 3). No differences in interventricular septal thickness, posterior wall thickness, or relative wall thickness were observed between depressed and nondepressed monkeys in ANOVAs or ANCOVAs (Table 3). Despite differences in LV geometry, there were no significant differences in systolic function between groups, as determined by endocardial fractional shortening and ejection fraction (Table 3).
Depressed monkeys also had higher heart rates (Figure 2A), slower transmitral E velocity (Figure 2F), a lower ratio of transmitral E velocity: early mitral annular descent in the septal region (Figure 2G), and faster early mitral annular descent in the septal wall as indicated by tissue Doppler (Figure 2H). Since HRs were different between the depressed and nondepressed groups, and HR can affect these aspects of cardiac function, these analyses were adjusted for HR and the results reexamined. ANCOVA revealed that HR accounted for a significant portion of the variance in the ratio of the transmitral E velocity: transmitral A velocity (F[1,25]=4.65, p=0.04), but did not change the outcome of the ANOVA (Table 3). HR accounted for a significant portion of the variance in the ratio of early mitral annular descent septal wall: late mitral annular descent septal wall (F [1, 30] =7.91, p=0.008) and revealed significantly higher ratios in depressed monkeys compared to their nondepressed counterparts (Figure 2I).
Sertraline Treatment
The sertraline decreased heart rate (Figure 3A), increased the LV end systolic (Figure 3B) and diastolic (Figure 3C) dimensions, increased LV systolic (Figure 3E) and left atrial (Figure 3G) volumes, and decreased relative wall thickness (Figure 3D). Transmitral E velocity, the ratio of transmitral E: transmitral A velocities and the ratio of E velocity: early mitral annular descent from the lateral annulus was increased, on average, in the sertraline group although these effects did not reach significance (all p’s≥0.06) (Table 3). In ANCOVA, BSA accounted for a significant proportion of the variance in LV end diastolic dimension, but this adjustment did not change the outcome of these analyses. Likewise, ANCOVA adjusting for HR accounted for a significant proportion of the variance in the ratio of transmitral E: transmitral A velocities and the ratio of early to late mitral annular descent measured at the septal wall, but these adjustments did not change the outcome of these analyses.
DISCUSSION
Elucidation of the mechanisms that underlie the relationship between major depression and heart disease is problematic due to the bidirectional nature of the association. Depression is a major risk factor for new and recurrent cardiac events in medically healthy individuals and cardiac patients (37; 38; 39; 40). Similarly, heart disease may contribute to affective states – major depression is frequently reported among patients with coronary artery disease and heart failure (41; 42; 43). Therefore, it is important to perform objective studies in reliable animal models that can focus on core behavioral features of depression and cardiac structure and function early in the atherogenic process, and prior to clinical cardiovascular disease. The cynomolgus monkey used in this study was selected because it has been useful in the study of the comorbidity of diet-induced coronary artery atherogenesis and depression. This is the first study to our knowledge of differences in heart function in depressed nonhuman primates consuming a Western-like diet.
The primary findings of this study is that depressed monkeys had smaller body sizes, higher resting HRs, smaller chamber sizes, and smaller left ventricles without evidence of systolic or diastolic dysfunction compared to nondepressed monkeys. Furthermore, the Doppler profiles of depressed monkeys indicated greater myocardial relaxation (higher e′ and higher e′/a′ ratio) and lower filling pressures (lower E/e′) than their nondepressed counterparts. While treatment with sertraline reduced heart rate and modestly increased chamber dimensions including LVIDD, an indirect measure of preload, it did not alter systolic or diastolic function.
The higher HRs and lower BWs among depressed monkeys are common biological markers of clinical depression and anxiety disorders. Elevated HRs have been observed in depressed patients both with and without cardiovascular disease and hypertension (44; 45). Indeed, autonomic modulation with major affective disorders is characterized by sympathetic predominance and vagal withdrawal and this is often represented by reductions in heart rate variability in depressed patients (46). Similarly, high 24 hour heart rates are characteristic of depressed monkeys suggesting chronic sympathetic hyperactivity (47). At high heart rates, diastolic filling time is significantly shortened, and therefore there is less time to fill the ventricle which may account for smaller LV volumes, smaller end diastolic dimensions and lower filling pressures. Heart muscle may not be as efficiently perfused, and chronic myocardial ischemia may eventually result in damaged heart muscle. Here covariate adjustments were made for HR and BSA in the present study in an attempt to understand the potential influence of heart rate and body size on echocardiographic measurements (48; 49).
The results reported here are similar to those reported from a sample of 1020 patients with stable coronary heart disease enrolled in the Heart and Soul study (50). No differences in systolic function, diastolic function, inducible ischemia, or wall motion abnormalities were found between those individuals with or without current or recent symptoms of major depression. Not only was there no relationship between global cardiovascular function impairments and depression, like the nonhuman primate findings reported here, better diastolic function was observed in female patients with current or recent depression compared to those with no history of depression (50). The results reported here replicated and extend these observations to a widely used NHP model of coronary artery atherogenesis, with no clinical signs of coronary heart disease, studied under controlled experimental conditions. Moreover, in addition to conventional transmitral Doppler measures of global LV filling dynamics, regional LV diastolic function was determined by tissue Doppler. Indeed, detection of regional diastolic dysfunction might represent an early change in LV relaxation preceding global diastolic dysfunction and systolic dysfunction. The higher velocities of early mitral annular descent (e′), higher e′/a′ ratios, and lower Doppler-derived filling pressures (E/e′) observed in depressed monkeys even after adjusting for heart rate are not consistent with subclinical heart failure. Whether this seemingly better regional diastolic function among depressed monkeys was due to a sympathetic arousal-induced LV suction effect remains speculative (51; 52). Sympathetic activation can influence the rate of LV untwisting, which is a key determinant of LV suction, facilitating LV filling without an increase in left atrial pressure (53). Since this apparently healthier diastolic profile has been observed both in patients and in a controlled NHP model, the potential mechanisms and clinical relevance of this observation warrants further study, particularly in females.
Oral administration of sertraline achieved therapeutic levels of plasma drug concentrations and modified cerebrospinal fluid monoamine and metabolite determinations in all treated monkeys to that observed in clinical patients (22). Sertraline did not significantly reduce depressive behavior. This was expected, as SSRIs have limited efficacy in human depression (54), and based on reported effect sizes in human beings, this experiment was not adequately powered to test the efficacy of sertraline for depression. In fact, it is important to note that no SSRI has ever been shown to reduce depression in a nonhuman primate model. Sertraline reduced resting HR. The SSRIs, including sertraline, are known to have cardiac effects, the best known of those being a mild bradycardia (54; 55). Despite case reports of various arrhythmias and syncopal episodes associated with the use of SSRIs, no overt rhythm disturbances were detected during the echocardiograms nor were differences in noninvasive blood pressure measurements (data not shown) noted among treatment groups. LV ejection fraction and fractional shortening were also not affected by sertraline, further reducing potential concerns for a drug-induced myocardial depressant effect (56; 57). Indeed, sertraline lowered heart rate, and may have enhanced filling of the heart chambers, as evidenced by the modest increases in atrial and LV chamber dimensions in treated versus untreated monkeys. These effects reduce workload on the heart, suggesting that SSRI treatment may reduce the long term risk of developing heart failure subsequent to depression. This possibility requires further investigation.
Depression is associated with high heart rate, low heart rate variability, platelet dysfunction, and inflammation (4; 5; 6). There are several mechanisms through which SSRIs may improve heart function particularly in depression. For example they reduce heart rate, increase heart rate variability (55; 56; 58; this report), inhibit serotonin-mediated and collagen-mediated platelet aggregation, reduce inflammatory mediator levels, and improve endothelial function (59).
Depressed females were smaller than their nondepressed counterparts. However, adjustment for current body size did not appreciably account for differences in heart function. Alternatively, it may be that early decrements in growth may contribute to later life neurobehavioral and heart dysfunction, a hypothesis that requires further investigation. Depressed females also had higher HRs, and higher HR could account for some of the differences in heart function. Adjustment for HR in the statistical analysis did little to account for the observed differences between depressed and nondepressed females in heart function. While statistical approaches were taken to control for body size and HR in this study, it remains possible that heart function was affected by both of these. Finally, a number of statistical comparisons were made, increasing the possibility of Type 1 error.
Relative to human hearts, monkey hearts are small and heart rates are fast, thus we were not able to measure some endpoints in all monkeys as movement of specific anatomic landmarks could not be clearly delineated. Specifically, there were missing data from 10% or more of the monkeys in transmitral E and A velocity, and measures of mitral annular descent. Thus, conclusions involving these endpoints should be made cautiously. The anesthetic agent used to sedate the monkeys prior to echocardiography, ketamine HCl, is known to reduce myocardial contractility thus it likely affected heart function in this study (60). However, all animals were subject to the same dose of ketamine on a per kg BW basis, so effects should be uniform across groups.
Taken together, the data presented here suggest that depression is accompanied by small body size, increased heart rate, smaller systolic, diastolic and atrial volumes, greater myocardial relaxation and lower filling pressures. In spite of statistical attempts to control for body size and heart rate effects it remains that these may be influencing heart structure and function in depressed monkeys. Sertraline treatment decreased heart rate and increased volumes, had no significant effects on relaxation (e′ or e′/a′) or filling pressures as indicated by Doppler measures (E/e′). In spite of statistical attempts to control for heart rate effects, the SSRI may be resulting in increased chamber dimensions because of lower heart rate. Thus, while it seems clear that the hearts of depressed and nondepressed monkeys were functioning differently, and that sertraline affected heart function, the differences in function may be due to different underlying physiological reasons. Furthermore, since these observations were made on a subclinical population showing no overt signs of heart disease, it is difficult to predict what their eventual clinical significance might be. Nonetheless, SSRIs show some promise in supporting heart function and should be investigated further for efficacy in reducing long term risk of developing heart failure in depressed patients. Finally, these results suggest that adult female cynomolgus monkeys consuming a Western diet may be a useful model in which to study factors that promote as well as ameliorate subclinical indicators of heart dysfunction in female primates.
Supplementary Material
Acknowledgments
Source of Funding
This research was supported by the National Institutes of Health RO1HL087103 (to CAS) R01-AG033727-03 (to LG), and the Pepper Older Americans for Independence Center (P30 AG21332). We would like to thank Ms. Kathy Stewart for her expert sonography in this project.
Acronyms
- HR
heart rate
- BSA
body surface area
- SSRI
selective serotonin reuptake inhibitor
- BW
body weight
- CSF
cerebrospinal fluid
- 5-HIAA
5-hydroxy indole acetic acid
- HVA
homovanillic acid
- HPLC
high performance liquid chromatography
- BMI
body mass index
- LV
left ventricular
- LVIDD
left ventricular end-diastolic dimensions
- LVISD
left ventricular end-systolic dimensions
- EF
ejection fraction
- %FS
percentage of LV fractional shortening
Footnotes
Conflicts of Interest
Drs. Groban, Kitzman, Register and Shively reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Leanne Groban, Department of Anesthesiology
Dalane W. Kitzman, Department of Internal Medicine, Section on Cardiology
Thomas C. Register, Department of Pathology, Section on Comparative Medicine
Carol A. Shively, Department of Pathology, Section on Comparative Medicine
References
- 1.Gnanasekaran G. Epidemiology of depression in heart failure. Heart Fail Clin. 2011;7:1–10. doi: 10.1016/j.hfc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Freedland KE, Carney RM, Rich MW. Effect of depression on prognosis in heart failure. Heart Fail Clin. 2011;7:11–21. doi: 10.1016/j.hfc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta LS. Cardiovascular disease and depression in women. Heart Fail Clin. 2011;7:39–45. doi: 10.1016/j.hfc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 5.Silver MA. Depression and heart failure: an overview of what we know and don’t know. Cleve Clin J Med. 2010;77 (Suppl 3):S7–S11. doi: 10.3949/ccjm.77.s3.02. [DOI] [PubMed] [Google Scholar]
- 6.Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: A review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166:30–7. doi: 10.1016/j.ijcard.2012.03.165. [DOI] [PubMed] [Google Scholar]
- 7.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;23;161:1725–30. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 8.May HT, Horne BD, Carlquist JF, Sheng X, Joy E, Catinella AP. Depression after coronary artery disease is associated with heart failure. J Am Coll Cardiol. 2009;21;53:1440–1447. doi: 10.1016/j.jacc.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Leftheriotis D, Flevari P, Ikonomidis I, Douzenis A, Liapis C, Paraskevaidis I, Iliodromitis E, Lykouras L, Kremastinos DT. The role of the selective serotonin re-uptake inhibitor sertraline in nondepressive patients with chronic ischemic heart failure: a preliminary study. Pacing Clin Electrophysiol. 2010;33(10):1217–1223. doi: 10.1111/j.1540-8159.2010.02792.x. [DOI] [PubMed] [Google Scholar]
- 10.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74:528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- 12.Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;15;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Housmans PR, Hatle LK, Tajik AJ. Assessment of diastolic function of the heart: Background and current applications of Doppler echocardiography. Part I. Physiologic and pathophysiologic features. Mayo Clin Proc. 1989;64:71–81. doi: 10.1016/s0025-6196(12)65305-1. [DOI] [PubMed] [Google Scholar]
- 15.Appleton CP. Influence of incremental changes in heart rate on mitral flow velocity: assessment in lightly sedated, conscious dogs. J Am Coll Cardiol. 1991;17:227–236. doi: 10.1016/0735-1097(91)90731-n. [DOI] [PubMed] [Google Scholar]
- 16.Choong CY, Herrmann HC, Weyman AE, Fifer MA. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith SA, Stoner JE, Russell AE, Sheppard JM, Aylward PE. Transmitral velocities measured by pulsed Doppler in healthy volunteers: effects of acute changes in blood pressure and heart rate. Br Heart J. 1989;61:344–347. doi: 10.1136/hrt.61.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, et al. Influence of alteration in preload on the pattern of left ventricular diastolic filling as assessed by Doppler echocardiography in humans. Circulation. 1989;79:1226–1236. doi: 10.1161/01.cir.79.6.1226. [DOI] [PubMed] [Google Scholar]
- 19.Dumesnil JG, Paulin C, Pibarot P, Coulombe D, Arsenault M. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–1231. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Kopelen HA, Quiñones MA. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–2145. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quiñones MA, et al. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 22.Shively CA, Register TC, Higley JD, Willard SL. Sertraline Effects on Cerebrospinal Fluid Monoamines and Species-typical Socioemotional Behavior of Female Cynomolgus Monkeys. Psychopharmacology. doi: 10.1007/s00213-013-3329-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis M, Aberg-Wistedt A, Agren H, Höglund P, Akerblad AC, Bengtsson F. Serum disposition of sertraline, N-desmethylsertraline and paroxetine: a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum Psychopharmacol. 2004;19:283–291. doi: 10.1002/hup.599. [DOI] [PubMed] [Google Scholar]
- 24.Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, Sitruk-Ware RL, Tsong YY, Cline JM. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Shively CA, Laber-Laird K, Anton RF. The behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;15,41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 27.Shively CA, Register TC, Friedman D, Morgan T, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 29.Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring) 2009;17:1513–20. doi: 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- 30.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Medicine. 1916;17:863–71. [Google Scholar]
- 31.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–8. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 33.Walker SE, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008;196:106–113. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 35.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 37.Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 38.Wulsin LR, Singal BM. Do depressive symtoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 39.Carney RM, Freedland KE, Sheline YI, Weiss ES. Depression and coronary heart disease: A review for cardiologists. Clin Cardiol. 1997;20:196–200. doi: 10.1002/clc.4960200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherwood A, Blumenthal JA, Hinderliter AL, Koch GG, Adams KF, Jr, Dupree CS, Bensimhon DR, Johnson KS, Trivedi R, Bowers M, Christenson RH, O’Connor CM. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2011;57:418–423. doi: 10.1016/j.jacc.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guck TP, Elsasser GN, Kavan MG, Barone EJ. Depression and congestive heart failure. Congestive Heart Failure. 2003;9:163–169. doi: 10.1111/j.1527-5299.2003.01356.x. [DOI] [PubMed] [Google Scholar]
- 42.Frasure-Smith N, Lesparance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 43.Schleifer SJ, Macari-Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, Zucker HD. The nature and course of depression following myocardial infarction. Arch Intern Med. 1989;149:1785–1789. [PubMed] [Google Scholar]
- 44.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 45.Moser M, Lehofer M, Hoehn-Saric R, McLeod DR, Hildebrandt G, Steinbrenner B, Voica M, Liebmann P, Zapotoczky HG. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? J Affect Disord. 1998;48:115–24. doi: 10.1016/s0165-0327(97)00164-x. [DOI] [PubMed] [Google Scholar]
- 46.Brown AD, Barton DA, Lambert GW. Cardiovascular abnormalities in patients with major depressive disorder: autonomic mechanisms and implications for treatment. CNS Drugs. 2009;23:583–602. doi: 10.2165/00023210-200923070-00004. [DOI] [PubMed] [Google Scholar]
- 47.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 48.Burns AT, Connelly KA, La Gerche A, Mooney DJ, Chan J, MacIsaac AI, Prior DL. Effect of heart rate on tissue Doppler measures of diastolic function. Echocardiography. 2007;24:697–701. doi: 10.1111/j.1540-8175.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 49.Huwez FU, Houston AB, Watson J, McLaughlin S, Macfarlane PW. Age and body surface area related normal upper and lower limits of M mode echocardiographic measurements and left ventricular volume and mass from infancy to early adulthood. Br Heart J. 1994:276–280. doi: 10.1136/hrt.72.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lett H, Ali S, Whooley M. Depression and cardiac function in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2008;70:444–449. doi: 10.1097/PSY.0b013e31816c3c5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Udelson JE, Bacharach SL, Cannon RO, 3rd, Bonow RO. Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects. Evidence for elastic recoil and diastolic “suction” in the normal heart. Circulation. 1990;82:1174–1182. doi: 10.1161/01.cir.82.4.1174. [DOI] [PubMed] [Google Scholar]
- 52.Courtois M, Mechem CJ, Barzilai B, Ludbrook PA. Factors related to end-systolic volume are important determinants of peak early diastolic transmitral flow velocity. Circulation. 1992;85:1132–8. doi: 10.1161/01.cir.85.3.1132. [DOI] [PubMed] [Google Scholar]
- 53.Beladan CC, Calin A, Rosca M, Ginghina C, Popescu BA. Left ventricular twist dynamics: principles and applications. Heart. 2013 May 9; doi: 10.1136/heartjnl-2012-302064. [DOI] [PubMed] [Google Scholar]
- 54.Daws LC, Koek W, Mitchell NC. Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders. ACS Chem Neurosci. 2013;4:16–21. doi: 10.1021/cn3001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001:433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 56.Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 2003:293–298. doi: 10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- 57.Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: New drugs, old concerns? Curr Pham Des. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J. 2001;142:617–623. doi: 10.1067/mhj.2001.116766. [DOI] [PubMed] [Google Scholar]
- 59.Chittaranjan A, Chethan KB, Sandarsh S. Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. Int Clin Psychopharmacol. 2013;28:145–155. doi: 10.1097/YIC.0b013e32835d735d. [DOI] [PubMed] [Google Scholar]
- 60.Kawakubo A, Fujigaki T, Uresino H, Zang S, Sumikawa K. Comparative effects of etomidate, ketamine, propofol, and fentanyl on myocardial contractility in dogs. J Anesth. 1999;13:77–82. doi: 10.1007/s005400050030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.