Abstract
Studies of cognitive reappraisal have demonstrated that reinterpreting a stimulus can alter emotional responding, yet few studies have examined the durable effects associated with reinterpretation-based emotion regulation strategies. Evidence for the enduring effects of emotion regulation may be found in clinical studies that use cognitive restructuring (CR) techniques in cognitive–behavioral therapy (CBT) to alleviate anxiety. These techniques are based on cognitive theories of anxiety that suggest these disorders arise from biased cognitions; therefore, changing a person's thoughts will elicit durable changes in an individual's emotional responses. Despite the considerable success of CBT for anxiety disorders, durable effects associated with emotion regulation have not been thoroughly examined in the context of a laboratory paradigm. The goal of this study was to determine whether CR, a technique used in CBT and similar to cognitive reappraisal, could attenuate conditioned fear responses, and whether the effect would persist over time (24 hr). We conditioned participants using images of snakes or spiders that were occasionally paired with a mild shock to the wrist while we obtained subjective fear reports and electrodermal activity (EDA). After conditioning, half of the participants were randomly assigned to CR training aimed at decreasing their emotional response to the shock and the conditioned stimuli, while the other half received no such training. All participants returned 24 hr later to repeat the conditioning session. Compared with control participants, CR participants demonstrated a reduction in fear and EDA across sessions. These findings suggest that CR has durable effects on fear responding.
Keywords: emotion, cognitive restructuring, reappraisal, conditioned fear, electrodermal activity
It is often assumed that changing the way one thinks can have a lasting impact on one's emotional responses. The impact of thoughts on emotions has been demonstrated in laboratory studies of cognitive reappraisal that illustrate how reinterpreting a stimulus or event can effectively alter emotional responding, as assessed through subjective reports as well as autonomic arousal (e.g., Delgado, Gillis, & Phelps, 2008; Gross, 1998; Gross & Levenson, 1997). These reappraisal studies, however, typically focus on the immediate effects associated with this technique. Alternatively, clinical studies have examined lasting changes associated with a different technique known as cognitive–behavioral therapy (CBT). CBT is a complex clinical therapy comprising many component parts, one of which is cognitive restructuring. Cognitive restructuring is similar to cognitive reappraisal in that patients are asked to reinterpret negative stimuli. Clinical studies have examined the lasting changes of CBT in the context of mood and anxiety disorders, but it is unknown whether the mechanism of change and persistence over time is driven by cognitive restructuring or some other component of CBT. Here, in an empirical study, we examined the lasting effects of cognitive reinterpretation on emotional responses outside a therapeutic context.
Although studies of cognitive reappraisal typically examine the immediate effects associated with this technique, a recent study investigated the effects of an interpretative strategy similar to reappraisal up to 30 min after initial stimulus presentation (MacNamara, Ochsner, & Hajcak, 2011). In that study, the experimenter presented audio descriptions prior to the presentation of neutral or negative images. The audio description suggested an interpretation of the image that was either neutral or negative and served as a contextual “frame.” Participants were then tested on whether these contextual frames influenced the subsequent appraisal of the images 30 min later. It was found that the contextual frames had a sustained effect on the interpretation of such images later in the same session. It is important to note that these effects were measured only 30 min after training, and participants were given specific contextual frames rather than relying on self-generated reappraisals. Investigating the enduring effects of regulatory strategies over extended periods of time, as well as their influence on subsequent exposure, remains an important area of research yet to be thoroughly examined.
In contrast to the relatively brief influence that contextual framing may have on emotional responding, CBT has been demonstrated to have durable effects in the clinic. CBT has been established as a lasting and effective treatment for anxiety disorders, including generalized anxiety disorder (Gould, Safren, Washington, & Otto, 2004), social phobia (Brown, Heimberg, & Juster, 1995), and specific phobias (Ost, 1989). CBT aims to reduce anxiety and other negative emotions by encouraging patients to identify irrational thoughts and modify them with adaptive coping responses, specifically targeting each domain of dysfunction. CBT makes use of a range of techniques to help individuals transform their maladaptive cognitive appraisals into adaptive, evidence-based appraisals (Beck & Dozois, 2011). Some of the most common techniques used in CBT include (a) constructing a strong therapeutic relationship, (b) establishing behavioral change strategies, (c) using cognitive restructuring techniques, (d) altering core beliefs, and (e) preventing relapse (Beck & Dozois, 2011). Together, these strategies are implemented over an extended period of time, often between 12 and 24 weekly sessions.
CBT has been demonstrated to be an effective technique for treating a variety of disorders, but the mechanisms underlying the success of CBT are not well understood. Breaking CBT into its component parts and examining each technique on its own would be useful to understand why CBT has lasting effects on the regulation of anxiety. One technique worthy of examination is cognitive restructuring. In cognitive restructuring, anxious individuals are taught to actively reinterpret the anxiety-inducing aspects of a stimulus, thereby reducing negative emotional responses when the stimulus is encountered (Beck & Emery, 1985). Cognitive restructuring uses cognitive reappraisal to reinterpret stimuli in a less negative way. However, cognitive restructuring differs from reappraisal in that it is individually tailored to help patients understand their own experience of anxiety with the goal of alleviating fear and worry while forming more rational appraisals. Cognitive restructuring is a collaborative process that relies on the interaction between the patient and therapist to alter core beliefs and reduce negative affect. It is unclear whether cognitive restructuring is effective at altering emotional processing because of its focus on reinterpreting negative stimuli, or if other issues, such as practice, individual variability, and the strong social connection between patient and therapist, contribute to these changes. Given that cognitive restructuring shares important similarities with reappraisal, it is useful to investigate the durable changes associated with reinterpretive emotion regulation techniques as applied to a laboratory paradigm.
The goal of the present study was to examine the enduring effects of an emotion regulation technique based on the reinter-pretation of an emotionally salient stimulus. Specifically, we examined whether cognitive restructuring techniques, such as those used in CBT, could provide enduring emotional change without the other components of CBT, which may or may not play a key role. To this effect, we determined whether cognitive restructuring could attenuate conditioned fear responses 24 hr after exposure under controlled laboratory conditions. Based on the basic and clinical literatures, we hypothesized that cognitive restructuring would lead to durable changes in experiential and autonomic components of the emotional response.
Method
Participants
Seventy-nine individuals (27 men; M age = 22.67 years, SD = 4.69 years) were recruited from New York University and surrounding communities. Participants were initially selected on the basis of responses to the snake phobic questionnaire (SNAQ; Klorman, Weerts, Hastings, Melamed, & Lang, 1974) and spider phobic questionnaire (SPQ; Klorman et al., 1974). Because our goal was to induce fear in healthy participants, those who scored above 15 on both of the questionnaires were not selected for the study to prevent the inclusion of snake and spider phobic individuals. Our selected criteria was lower than previously established means for phobic individuals (24.44 ± 2.95 for the SNAQ and 23.76 ± 3.8 for the SPQ; Fredrikson, 1983). The means for our participants for the SPQ and SNAQ were 8.39 ± 5.32 and 7.12 ± 4.77, respectively, which are similar to means for healthy populations (see Klorman et al., 1974).
Four participants were excluded from our final analysis because of experimenter error, and five participants were excluded because they did not follow experimental instructions. Fourteen participants were excluded on the first day of the experiment because they failed to display variable electrodermal activity (EDA), and another four were excluded because they failed to demonstrate adequate levels of fear acquisition (see the Psychophysiological Assessment section for detailed exclusion criteria). The final sample included 52 participants (19 men; M age = 22.73 years, SD = 5.24 years). The University Committee on Activities Involving Human Subjects at New York University approved this study. All participants were compensated $30 for 2 hr of participation.
Procedure
The experiment was divided into two sessions. In the first session, participants completed a classic Pavlovian fear-conditioning paradigm followed by a cognitive restructuring manipulation (CR group) or a card-sorting task (control group). In the second session, participants returned 24 hr later to repeat the conditioning paradigm.
In Session 1, participants underwent a Pavlovian fear-conditioning paradigm with partial reinforcement. The conditioned stimuli (CS+ and CS−) were two different images of snakes or spiders taken from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008), and each participant viewed either two snakes or two spiders. The images of snakes and spiders were matched for valence and arousal and counterbalanced across participants. Stimulus selection was based on participants' scores on the SPQ and SNAQ; the prepared stimulus with the higher score served as the stimuli during conditioning to encourage arousal. If participants had equal scores for both questionnaires, they were randomly assigned to a set of stimuli. The unconditioned stimulus (US) was a mild shock on the right wrist (200 ms) coterminating with the final 200 ms presentation of the CS + . All CSs were presented for 4 s with a variable intertrial interval of 8–10 s. Each participant viewed 15–17 presentations of the CS− (never paired with shock), 15–17 presentations of the CS + , and eight presentations of the CS + that coterminated with the US. Two trial orders were counterbalanced across participants, and images were never repeated more than three times sequentially. EDA was simultaneously collected.
After the conditioning experiment, the shock bar was removed and the conditioned stimuli were presented on a computer screen. Participants were asked to report at least three emotions and explain any thoughts they had while viewing the images. Each participant was asked to rate the intensity of any reported emotion on a scale of 1–100, with 1 being the least intense and 100 being the most intense. The experimenter recorded all responses.
Participants in the CR condition were then asked to further discuss the relationship between thoughts and feelings. Participants were presented with a simple cartoon (see Supplemental Materials) and asked to explain how the thoughts of the cats in the cartoon influenced their feelings toward the dog (Kendall & Hedtke, 2006). The purpose of this exercise was to introduce the relationship between thoughts and feelings and explain how different thoughts about an emotionally salient event may shape one's emotional reactions to the event. Participants were then asked to describe their thoughts and feelings about two vague but not aesthetically unpleasant images (HIV and penicillin). The purpose of this task was to demonstrate that adding new information may change how an image is perceived, which may change the associated thought and feeling toward that image.
The experimenter went on to explain how participants often “catastrophize” and focus exclusively on the shock when viewing the CS + , attributing their feelings of fear and anxiety to the image itself rather than considering the image and the shock separately. The experimenter highlighted how being stressed and anxious may make the experiment seem longer and more uncomfortable, and explained the importance of changing the participant's thinking. The participant was invited to brainstorm alternative ways of thinking about the CS + , specifically focusing on aspects of the stimuli the participant found less negative. After the manipulation, participants were again shown the CS + and asked to rerate their degree of fear, anxiety, and belief in any other emotions they had previously listed in the beginning of the experiment. Because the focus of our study was feelings of fear, only self-reported ratings for the CSs that were categorized under “fear” were subsequently analyzed. Participants were asked to describe any additional thoughts and emotions they had when viewing the CS +.
Participants in the control condition completed a cartoon rearrangement task from the Wechsler Adult Intelligence Scale—Revised, which took approximately the same amount of time to complete as the CR condition (12–15 min). The experimenter presented participants with a series of cartoon picture sets one set at a time, with each set depicting an event. For each picture set, participants rearranged the cartoons in the correct order for the event depicted. This task was chosen because it required limited social interaction yet was matched in social proximity to the CR manipulation, and used cartoons that were similar to the ones used in the CR manipulation. After the rearrangement task, participants were asked to rerate their degree of fear and belief in other emotions they previously held for the CS + , and asked to describe any additional thoughts and emotions they had when viewing the CS + .
In Session 2, which took place 24 hr after Session 1, all participants were asked to view the CS + and CS− and write down any thoughts and emotions they had while viewing the images. The CR group was also asked to write down any alternative thoughts and feelings for the CS + and CS−. The fear-conditioning task was then repeated.
Electrical Stimulation
Shocks were delivered to the right inner wrist by a Grass Medical Instruments (Manchester, NH) shock bar stimulator attached with a Velcro strap. Participants determined the level of shock individually, starting at a level of 20 V and gradually increasing until they reached a shock level that was “uncomfortable but not painful,” with a maximum voltage of 60 V. All shocks were delivered at 50 pulses/s for 200 ms.
Psychophysiological Assessment
EDA was measured using two Ag-AgCl electrodes connected to a Biopac Systems (Goleta, CA) galvanic skin response module. Each participant was fitted with two Ag-AgCl electrodes attached to the left distal interphalangeal joint of the index and middle finger. Samples were recorded at a rate of 200 samples/s.
EDA was analyzed offline using AcqKnowledge 3.9 software (Biopac Systems, Inc., Goleta, CA). The EDA was calculated by taking the base-to-peak difference for all waveforms (in microsiemens, μs) in the 0.5-s to 4.5-s window after the stimulus onset, with a minimal response criteria of 0.02 μs. EDA scores were normalized by a square root transformation, then divided by each participant's mean square-root-transformed US response (see Schiller et al., 2010).
Because our index of fear arousal was EDA, a variable EDA response was required for subjects to participate. Consistent with previous research, participants who failed to show variable EDA were excluded prior to analysis (Dunsmoor, Mitroff, & LaBar, 2009; Hartley et al., 2012; Phelps, Delgado, Nearing, & LeDoux, 2004). To measure fear acquisition, we calculated differential fear response scores by subtracting responses to the CS− from those to the CS + ; this difference score is commonly used in the human fear-conditioning literature (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Schiller, Levy, Niv, LeDoux, & Phelps, 2008; Schiller et al., 2010). Any subject who failed to show adequate fear learning (CS + minus CS− > 0.02 μs) during the latter half of the acquisition session was subsequently excluded from the experiment. This criterion was chosen to ensure that subjects demonstrated adequate discrimination between the two stimuli and was essential to assess whether they could then regulate arousal responses to stimuli that were threatening versus those that were safe, although other criteria have been used (Dunsmoor, Mitroff, & LaBar, 2009; Hartley et al., 2012; Schiller et al., 2010).
Results
Manipulation Check
To confirm that participants in the CR group had similar shock levels compared with those in the control group, we performed a repeated measures analysis of variance (ANOVA). As expected, there was no Session × Group interaction, F(1, 49) = .0000672, p = .879. In addition, shock levels were not significantly different between groups during Session 1, t(49) = −0.291, p = .772, or Session 2, t(48.79) = −0.319, p = .751.
Effects of Cognitive Restructuring
Electrodermal activity
As shown in Figure 1, a Session × Group mixed-model ANOVA on mean differential EDA with subject modeled as a random effect revealed a significant Session × Group interaction, F(1, 50) = 12.959, p = .001. The CR group demonstrated a significant reduction in mean differential EDA across sessions, t(25) = 3.504, p = .002, whereas this effect was not seen in the control group, t(25) = −1.746, p = .093. The CR group demonstrated significantly lower differential EDA compared with the control group during Session 2, t(40.25) = 2.467, p = .018, whereas there were no differences during Session 1, t(50) = −0.314, p = .755, as expected. In addition, a mixed-model ANOVA using stimulus type and session as within-subjects factors and group as a between-subjects factor and controlling for the random effect of subject revealed a marginally significant interaction of session, stimulus type, and group, F(1, 112.736) = 3.652, p = .059.
Figure 1.
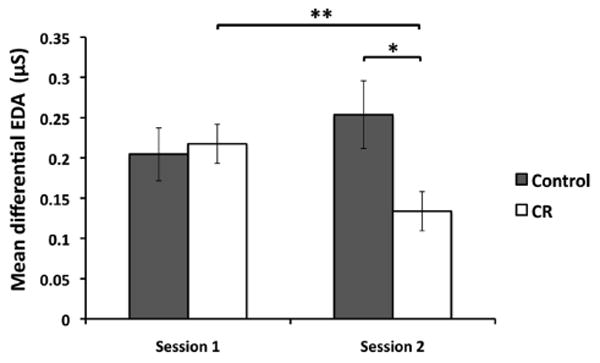
The average differential electrodermal activity (EDA) for each group across sessions (CS+ minus CS−). The two groups showed roughly equivalent fear acquisition in Session 1. The cognitive restructuring (CR) group demonstrated a significant reduction in differential EDA across sessions, whereas the control group did not show this effect. During Session 2, the CR group had significantly lower differential EDA compared with the control group. * p < .05; ** p < .01. Error bars indicate standard error of the mean.
Self-reported fear
We calculated a differential fear response rating to account for the affective nature of negative images in general (CS+ minus CS−). To assess how these differential fear ratings changed over time, we calculated a Session × Group repeated measures ANOVA on mean differential fear ratings, which revealed no significant Session × Group interaction, F(1, 46) = 1.741, p = .194 (see Figure 2). However, there was a main effect of session, F(1, 46) = 24.412, p = .0000110, and a trend level effect of group, F(1, 46) = 3.10, p = .085. Planned comparisons revealed a significant reduction in the subjective experience of fear for both the control and CR groups across sessions, t(22) = 2.852, p = .009, and t(24) = 4.116, p = .000393, respectively. The CR group had significantly lower differential fear ratings compared with the control group during Session 2, t(45.90) = 2.031, p = .048, but not during Session 1, t(46) = 0.351, p = .727, as expected.
Figure 2.
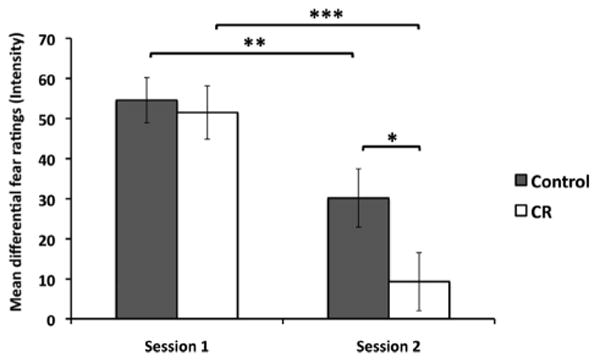
The average differential fear ratings for each group across sessions (CS+ minus CS−). The two groups showed roughly equivalent fear responses in Session 1. Both the control and cognitive restructuring (CR) groups demonstrated a significant reduction in differential fear ratings across sessions. During Session 2, the CR group had significantly lower differential fear ratings compared with the control group. * p < .05; ** p < .01; *** p < .001. Error bars indicate standard error of the mean.
Discussion
The present study examined whether an emotion regulation technique, CR, would have enduring effects on emotional responses, specifically learned fear. Results demonstrated a significant reduction in experiential and autonomic fear responses 24 hr after participants received cognitive restructuring. This study shows that cognitive restructuring can lead to lasting changes in emotional responding, demonstrating the durable effects of this regulatory technique.
Recent research has demonstrated that when subjects are given a specific contextual frame, they will apply it when subsequently presented with the same image even without being cued (MacNamara et al., 2011). Our study extends these findings in several ways. First, our study is unique in that it induced fear and then trained participants to use individually generated reappraisals to change the meaning associated with the fear-inducing stimulus. When participants were later reexposed to the stimulus, they applied these techniques to reduce fear. Second, our study examined durability over a longer time window (24 hr) than prior studies (30 min). Finally, our study employed both a self-reported measure of emotion experience and an objective measure of physiological responding. Taken together with the results of MacNamara et al. (2011), these findings suggest that altering cognitions may have durable effects. Given that as many as 85% of psychological disorders involve disturbances in emotional responding (Thoits, 1985), this technique has important implications for how clinical populations may attenuate emotional responses, and how this attenuation may persist over an extended period of time.
Although this study provides important new evidence regarding the durability of the effects of CR, it has a few limitations. The effects of CR were examined 24 hr after initial exposure, but adding additional time points are necessary to determine whether there are temporal limits to this technique. In addition, CR and cognitive reappraisal both involve reinterpreting emotion-eliciting stimuli to change their emotional impact. However, CR has important distinctive features that may enhance its efficacy, such as social interaction with the experimenter during the development of the restructuring technique, an emphasis on individual variability for the strategy itself, and practicing the cognitive restructuring strategies. It is unclear whether these factors contribute to the efficacy of cognitive restructuring, or whether it is driven by a reinterpretation of negative stimuli. Finally, this study focused on psychophysiology and subjective experience as dependent measures of emotional response. One important future direction will be to examine the neural mechanisms underlying CR to assess whether they overlap with traditional reappraisal techniques and mechanisms of extinction.
Supplementary Material
Footnotes
Contributor Information
Ashley A. Shurick, Department of Psychology, Stanford University
Jeffrey R. Hamilton, Department of Psychology, New York University
Lasana T. Harris, Department of Psychology and Neuroscience, Duke University
Amy K. Roy, Department of Psychology, Fordham University
James J. Gross, Department of Psychology, Stanford University
Elizabeth A. Phelps, Department of Psychology, New York University
References
- Beck AT, Dozois DJA. Cognitive therapy: Current status and future directions. Annual Review of Medicine. 2011;62:397–409. doi: 10.1146/annurev-med-052209-100032. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G. Anxiety disorders and phobias: A cognitive perspective. New York, NY: Basic Books; 1985. [Google Scholar]
- Brown EJ, Heimberg RG, Juster HR. Social phobia subtype and avoidant personality disorder: Effect on severity of social phobia, impairment, and outcome of cognitive behavioral treatment. Behavior Therapy. 1995;26:467–486. [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nature Neuroscience. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M. Reliability and validity of some specific fear questionnaires. Scandinavian Journal of Psychology. 1983;24:331–334. doi: 10.1111/j.1467-9450.1983.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Gould RA, Safren SA, Washington DO, Otto MW. A meta-analytic review of cognitive–behavioral treatments. In: Turk CL, Heimberg RG, Mennin DS, editors. Generalized anxiety disorder: Advances in research and practice. New York, NY: Guilford Press; 2004. pp. 248–264. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hartley CA, McKenna MC, Salman R, Holmes A, Casey BJ, Phelps EA, Glatt CE. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proceedings of the National Academy of Sciences, USA. 2012;109:5493–5498. doi: 10.1073/pnas.1202044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Hedtke KA. Coping cat workbook. 2nd. Ardmore, PA: Workbook Publishing; 2006. [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specific-fear questionnaires. Behavior Therapy. 1974;5:401–409. [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Tech Rep No A-8) University of Florida; Gainesville, FL: 2008. [Google Scholar]
- MacNamara A, Ochsner KN, Hajcak G. Previously reappraised: The lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience. 2011;6:348–358. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost LG. One-session treatment for specific phobias. Behaviour Research and Therapy. 1989;27:1–7. doi: 10.1016/0005-7967(89)90113-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. The Journal of Neuroscience. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits PA. Self-labeling processes in mental illness—The role of emotional deviance. American Journal of Sociology. 1985;91:221–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


